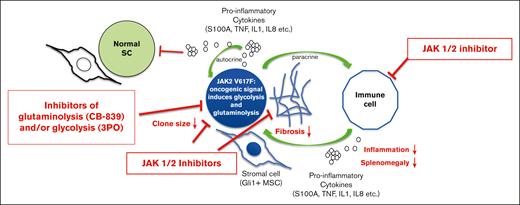
In this issue of Blood Advances, Usart et al1 provide an additional piece of information to the therapeutic potential of targeting metabolic vulnerabilities in myeloproliferative neoplasms (MPNs) driven by the gain-of-function mutation JAK2-V617F. The data complement a recent study from the same group demonstrating increased glucose consumption in JAK2-driven diseases, leading to (1) significant hypoglycemia in mice and (2) lipolysis, a process that can be reverted by the inhibition of JAK1/2.2 Both studies nicely complement the “hallmark of cancer” concept documented by D. Hanahan that exemplifies a “genome instability mutation” (ie, the JAK2 V617F mutation in MPN) and a “deregulating cellular metabolism,” which are not only known cancer characteristics but are also causally linked.3 The authors now focus on the pivotal role of glutamine metabolism for disease maintenance in JAK2-V617F–mutated MPN. The central question addressed by this article is whether inhibiting glutaminolysis (ie, the conversion of glutamine to glutamate) can disrupt the altered glutamine metabolism in MPN, thereby offering a potentially novel therapeutic strategy. Glutaminase (GLS) plays a vital role in cancer cell metabolism, particularly in cells that heavily rely on glutamine for their energy production as well as biosynthesis of macromolecules and maintenance of redox balance.4 By inhibiting GLS, CB-839 serves to starve cancer cells of necessary nutrients derived from glutamine, thereby inhibiting their survival and proliferation. In this study, the use of CB-839 provides compelling evidence of its therapeutic efficacy both in vitro and in vivo in preclinical murine models of JAK2-V617F–mutant MPN. The results demonstrate that CB-839 dramaticaly reduces mitochondrial respiration in bone marrow (BM) cells derived from JAK2-mutant mice, highlighting the dependency of these cells on glutamine-derived adenosine triphosphate (ATP) for energy consumption. Notably, treatment with CB-839 in vivo normalizes blood glucose levels, reduces splenomegaly, and decreases erythrocytosis, underscoring its potential as a therapeutic agent. When applying the same approach to human CD34+ hematopoietic stem and progenitor cells from patients with polycythemia vera (PV), CB-839 also inhibited proliferation at nanomolar concentrations. Based on the recent reports detailing the effects of 3-PO on glycolysis and the known anti-inflammatory effects of JAK inhibitors, the study further explores the synergistic effects of combining CB-839 with other inhibitors. In an earlier study, the hyperglycolytic phenotype of MPN was described, leading to hypoglycemia and hyperactive erythropoiesis, which can be reversed by 3PO combined with ruxolitinib.2 In this article, the triple combination (CB-839, 3PO, and ruxolitinib) yielded improved therapeutic outcomes (ie, spleen size reduction, normalization of metabolic abnormalities, and reduction of reticulin fibrosis in the BM), supporting the concept of a multitargeted approach to more effectively treat MPN (see figure). Importantly, the research also demonstrated that CB-839 preferentially targets JAK2-mutant hematopoietic stem cells (HSCs), leading to their reduced numbers in the BM. This selective effect on mutant HSCs, particularly those in the G1 phase of the cell cycle, also points to the potential of CB-839 to treat MPN symptoms. In addition, this study points to its potential to be used as a “disease-modifying” agent targeting the disease by selectively reducing mutant stem cells.
Synergistic action of JAK 1/2, glutaminolysis, and glycolysis inhibitors in JAK2-mutated MPN. The figure highlights that combined treatment of JAK2-V617F–mutated MPN with inhibitors of metabolic dependencies (ie, CB-839 and/or 3PO) together with JAK 1/2 inhibitors (ie, ruxolitinib) synergistically acts in reducing malignant clone size, BM fibrosis, inflammation, and splenomegaly.
Synergistic action of JAK 1/2, glutaminolysis, and glycolysis inhibitors in JAK2-mutated MPN. The figure highlights that combined treatment of JAK2-V617F–mutated MPN with inhibitors of metabolic dependencies (ie, CB-839 and/or 3PO) together with JAK 1/2 inhibitors (ie, ruxolitinib) synergistically acts in reducing malignant clone size, BM fibrosis, inflammation, and splenomegaly.
The study is novel and nicely complements recent work on metabolic alterations driven by the JAK2 V617F mutation. In addition, it provides a rational for clinical testing of compounds that revert the metabolic rewiring of JAK2–mutated hematopoietic stem and progenitor cells. However, several issues remain to be addressed. Specifically, future research should explore the long-term efficacy and safety of CB-839, particularly when used in combination with other inhibitors. Furthermore, its potential toxicity profile in clinical settings needs to be evaluated. Inhibition of glycolysis is hard to tackle due to the ubiquitous role of this process in each cell. In addition, understanding the mechanisms underlying the selective targeting of JAK2-mutant HSCs by CB-839 could provide further insights into the metabolic dependencies of cancer stem cells.
In conclusion, inhibition of glutaminolysis with CB-839, alone or in combination with other metabolic and/or oncogenic pathway inhibitors, offers a promising new strategy for the treatment of JAK2-V61F–positive MPN. This study not only sheds light on the altered metabolism of mutant MPN cells but also opens the door to onco-metabolism–targeted therapies that could transform the treatment landscape for patients with MPN.
Conflict-of-interest disclosure: D.W. received research support by Novartis, Pfizer, AOP, Ariad, Roche, Bristol Myers Squibb, MSD, and Celgene; and honoraria from Novartis, BMS, Pfizer, Ariad/Incyte, Amgen, Roche, AOP, Sanofi, Bexalta, BeiGene, and Gilead/Kite. A.P. declares no competing financial interests.