CD33/CD123 NANOBODY TCE kills single and double-positive cells, outperforming single-targeting TCE in a subset of AML samples.
CD33/CD123-TCE efficiently kills AML cells in a mouse model and targets cells in nonhuman primates without signs of cytokine release.
Visual Abstract
Novel therapies are needed for effective treatment of acute myeloid leukemia (AML). Relapse is common and salvage treatment with cytotoxic chemotherapy is rarely curative. CD123 and CD33, 2 clinically validated targets in AML, are jointly expressed on blasts and leukemic stem cells in >95% of patients with AML. However, their expression is heterogenous between subclones and between patients, which may affect the efficacy of single-targeting agents in certain patient populations. We present here a dual-targeting CD33/CD123 NANOBODY T-cell engager (CD33/CD123-TCE) that was designed to decrease the risk of relapse from possible single antigen-negative clones and to increase coverage within and across patients. CD33/CD123-TCE killed AML tumor cells expressing 1 or both antigens in vitro. Compared with single-targeting control compounds, CD33/CD123-TCE conferred equal or better ex vivo killing of AML blasts in most primary AML samples tested, suggesting a broader effectiveness across patients. In a disseminated cell-line–derived xenograft mouse model of AML, CD33/CD123-TCE cleared cancer cells in long bones and in soft tissues. As cytokine release syndrome is a well-documented adverse effect of TCE, the compound was tested in a cytokine release assay and shown to induce less cytokines compared to a CD123 single-targeting control. In an exploratory single-dose nonhuman primate study, CD33/CD123-TCE revealed a favorable PK profile. Depletion of CD123 and CD33 expressing cells was observed, but there were neither signs of cytokine release syndrome nor clinical signs of toxicity. Taken together, the CD33/CD123 dual-targeting NANOBODY TCE exhibits potent and safe anti-AML activity and promises a broad patient coverage.
Introduction
Acute myeloid leukemia (AML) is the most common adult hematologic malignancy. It comprises a variety of subsets defined by various genetic mutations in hematopoietic stem and progenitor cells linked to clonal expansion of abnormally differentiated myeloid cells in bone marrow and blood.1,2 Traditional cytotoxic chemotherapy followed by stem cell transplantation can cure ∼40% of younger patients with AML. However, due to drug resistance, the long-term survival of patients with AML remains poor.3-5 Novel targeted agents and differentiation inducers are only suitable for subsets of patients with targetable mutations.6,7 Immunotherapy targeting surface antigens expressed in leukemia cells has emerged as an effective approach for AML treatment for patients who are older/unfit for or relapsed from/refractory to current regimens.8
The use of bispecific antibodies for redirecting the cytotoxic activity of T cells in a non-major histocompatibility complex restricted fashion has expanded in recent years, with a particular thrust by promising clinical results obtained with blinatumomab, a CD19/CD3 bispecific T-cell engager (TCE) in B-cell neoplasias9-11 and more recently by Tecvayli (B-cell maturation antigen-TCE in multiple myeloma) and Kimmtrak (HLA/gp100peptide-TCE in uveal melanoma). Such TCE compounds mediate redirected tumor cell killing by clustering of T cells with tumor cells, resulting in an immune synapse, T-cell activation and release of perforins and granzymes ultimately leading to tumor cell killing.
The success of blinatumomab (overall response rates of 35%-50% across studies) has thus far not translated to similar responses in the AML setting. One obvious explanation is the lack of specific tumor antigens universally expressed on AML cells, similar to CD19 and CD20 in the lymphoma setting. The most-studied tumor antigens in AML to date are CD33 and CD123.9-12
CD123, the alpha subunit of the interleukin-3 receptor (IL-3Ra) is significantly expressed on AML blasts and AML leukemic stem cells (AML-LSCs) in patients with AML.13-15 Elevated expression of CD123 in AML is associated with higher blast counts at diagnosis and a lower complete remission rate.16 Although it is also expressed on healthy bone marrow cells including hematopoietic stem cells, monocytes, and plasmacytoid dendric cells, its expression on AML blasts is at least 10-fold higher,17 making it an attractive tumor target.
CD33, a member of the sialic-acid–binding immunoglobulin-like lectin (siglec) family, is likewise expressed on most AML blasts, a subset of AML-LSCs and normal monocytes, and lower levels on activated T and natural killer (NK) cells, and its expression is correlated with inferior disease outcomes.18,19 Ehninger et al reported that most AML blasts express surface CD33 and/or CD123 with 95% of patients expressing at least 1 of both.20
Effectiveness of targeting CD33 or CD123 by TCE antibodies and chimeric antigen receptor (CAR)-T cells has been validated in preclinical studies. Several of them including AMG 330 (CD33-CD3) and MGD006 (CD123-CD3 = flotetuzumab) were also investigated in clinical trials demonstrating response rates of 19% and 30%, respectively.21-24 Limitations to TCE therapies both in B-cell malignancies and AML, are toxicities related to cytokine release syndrome (CRS) and drug resistance due to antigen loss and/or T-cell exhaustion. Cytokine release happens as a result of massive T-cell activation by the drug but can be managed clinically by step-up dosing and premedications to dampen the cytokine storm.10,24 CD19 antigen-loss has been described in as much as 10% to 20% of patients following treatment with blinatumomab or CD19-CAR-T cells.25-27 Loss of CD33 or CD123 has been suggested to occur in AML, but there is limited evidence in support.28 Nevertheless, any therapy targeting a single antigen may eventually lead to antigen-loss variants and disease relapse.
With these considerations, we designed a dual-targeting CD33/CD123-T-cell receptor (TCR) NANOBODY TCE. NANOBODY compounds are single-domain antibodies, derived from stable and fully functional heavy-chain-only camelid antibodies. The NANOBODY platform offers flexibility for combining 5 or more building blocks in a beads-on-a-string fashion with good biophysical properties. The CD33/CD123-TCE was specifically designed to eliminate target cells expressing 1 or both tumor targets and thus minimize antigen-loss and increase coverage within and between patients with AML. We herein provide evidence of its antileukemia effects on AML cell lines and primary AML samples in vitro as well as in a disseminated cell-line–derived xenograft mouse model of AML and in nonhuman primates in vivo.
Material and methods
Cell lines and human material
Information on cell lines, primary healthy human cells, and patient material is described in the online supplemental Methods and supplemental Table 1.
Primary patient samples were collected during routine diagnostic procedures at The University of Texas MD Anderson Cancer Center, Houston, TX, in accordance with protocols approved by the Institutional Review Board. All human samples were obtained after written consent and in accordance with the Declaration of Helsinki. All in vivo animal experiments were approved by the Sanofi Ethical Committee and conducted in accordance with local and institutional laws, ethics, and guidance in Association for the Assessment and Accreditation of Laboratory Animal Care accredited facilities.
Engineering and production of NANOBODY and reference compounds
Single-targeting TCE were made as reference compounds based on published sequences (WO2010/037835A2; WO2015/026892A1). CD3-CD33 was made as bispecific TCE (BiTE) format and CD3-CD123 as a dual affinity retargeting antibody (DART) (details in supplemental Materials and Methods). TCRαβ-, CD33-, and CD123-targeting NANOBODY sequences were obtained after immunization of llamas according to local Ethical Committee approval, as previously described in29,30 and in supplemental Materials. The half-life extended dual-targeting CD33/CD123 NANOBODY TCE was designed as described in Figure 2A and supplemental Materials and Methods.
Primary AML samples are positive for CD33 and CD123. (A) Bar graph summarizing the percentage of positivity for CD33 and CD123 in all patient samples examined (mean ± standard error of the mean [SEM]). (B) Waterfall bar graph showing individual distribution of percentage positivity of CD33, CD123, and dual positivity and negativity for CD123 and CD33 of 55 patient samples.
Primary AML samples are positive for CD33 and CD123. (A) Bar graph summarizing the percentage of positivity for CD33 and CD123 in all patient samples examined (mean ± standard error of the mean [SEM]). (B) Waterfall bar graph showing individual distribution of percentage positivity of CD33, CD123, and dual positivity and negativity for CD123 and CD33 of 55 patient samples.
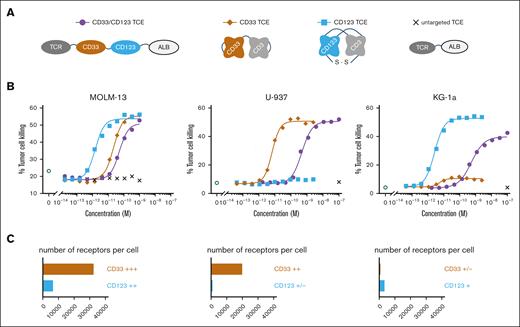
CD33/CD123 NANOBODY TCE kills double- and single-target–expressing tumor cell lines. (A) Structural design of the CD33/CD123 NANOBODY TCE and control compounds. (B) Dose dependent T-cell–mediated MOLM-13; U-937 and KG-1a cell killing using an effector to target ratio of 10:1. The percentage of TO-PRO-3 positive target cells is plotted against the concentration of the CD33/CD123 NANOBODY TCE, CD33-CD3, CD123-CD3, or untargeted NANOBODY TCE. (C) CD33 and CD123 target receptor expression (number of receptors/cell): MOLM-13 (CD33: 32 448 antigens per cell; CD123: 6543); U-937 (CD33: 19 872; CD123: 945); and KG-1a (CD33: 800; CD123: 3353). One representative experiment is shown out of ≥4, global mean EC50s are shown in Table 2.
CD33/CD123 NANOBODY TCE kills double- and single-target–expressing tumor cell lines. (A) Structural design of the CD33/CD123 NANOBODY TCE and control compounds. (B) Dose dependent T-cell–mediated MOLM-13; U-937 and KG-1a cell killing using an effector to target ratio of 10:1. The percentage of TO-PRO-3 positive target cells is plotted against the concentration of the CD33/CD123 NANOBODY TCE, CD33-CD3, CD123-CD3, or untargeted NANOBODY TCE. (C) CD33 and CD123 target receptor expression (number of receptors/cell): MOLM-13 (CD33: 32 448 antigens per cell; CD123: 6543); U-937 (CD33: 19 872; CD123: 945); and KG-1a (CD33: 800; CD123: 3353). One representative experiment is shown out of ≥4, global mean EC50s are shown in Table 2.
Affinity determination
Kinetic (kon, koff) and affinity constants (KD) of CD33/CD123 TCE for recombinant human and cynomolgus CD33, CD123, and ALB proteins were evaluated at 37°C by means of surface plasmon resonance assay on ProteOn XPR36 (BioRad Laboratories); experimental details are described in the supplemental Materials and Methods. Affinity of CD33/CD123 NANOBODY TCE for human and cynomolgus primary T cells was evaluated using flow cytometry in a competition setup using a monovalent TCRαβ NANOBODY protein as competitor.
Cytotoxicity against AML cell lines
PKH26-labeled (Sigma) target cells (2.5 × 104 cells per well) were cultured with effector T cells (2.5 × 105 cells per well) ([E:T] = 10:1) and treated with serial dilutions of CD33/CD123 TCE or reference compounds for 18 hours. Cell killing was monitored by TO-PRO-3 Iodide (Thermo Fisher Scientific) on a MACSQuant XXX and specific lysis was determined using GraphPad Prism5 software.
Autologous killing of monocytes and cytokine release within healthy donor peripheral blood mononuclear cells
HD peripheral blood mononuclear cells (PBMCs) (2 × 106 cells per well) were incubated with CD33/CD123 TCE or reference compounds for 18 hours. Killing of monocytes was monitored on MACSQuant X after staining with anti-CD14 (Biolegend) and TO-PRO-3. In parallel, a panel of cytokines including IL-2, IL-6, interferon gamma (IFN-γ), and TNF-α was measured in supernatants using a multiplex bead assay (Bio-Rad) acquired on Luminex FlexMAP 3D.
Autologous killing of AML blasts within patient PBMC
Primary AML patient PBMC (1 × 106 cells per well) were cocultured with healthy donor stromal cells (0.25 × 106 cells per well) and treated with NANOBODY or reference compounds. For NANOBODY TCE 2.5 and 25 nM were selected as first concentration reaching max killing and 10× higher. Single controls were used at 2.5 nM (clearly saturating). Apoptosis induction defined by positivity of annexin V (Roche Diagnostics) and 4′,6-diamidino-2-phenylindole (Sigma) was measured by flow cytometry on day 7. The extent of drug-specific apoptosis (SP) was calculated as, percentage SP = (test − control) × 100/(100 − control).31 One-way analysis of variance followed by Tukey test with adjust P values was performed to identify significance.
Information of antibodies used is provided in supplemental Table 2. Colony-formation assays using primary AML PBMC were performed as described previously32 and in supplemental Material.
Mass cytometry analysis of AML blast killing and lymphocyte immunophenotype
Cytometry by time-of-flight mass spectrometry (CyTOF) analysis using 26 markers was performed on day 4 of primary AML blast killing as described previously.33,34 The panel of antibodies used is described in supplemental Material (supplemental Table 2). CyTOF data were normalized by bead-normalization and analyzed with FlowJo v10 with plugins supported by R v4.1 (or above) and GraphPad Prism v9. Two algorithms, uniform manifold approximation and projection35 followed by FlowSOM36 were applied for dimension reduction, clustering, and visualization on high-dimensional CyTOF data.
In vivo efficacy in a disseminated AML xenograft mouse model
Antitumor activity was evaluated after MOLM-13-luc AML engraftment in humanized NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ(NSG) mice (Charles River Laboratories, Saint-Germain-Nuelles, France) as described in Bonnevaux et al37 and Figure 6A. Whole body and long bones tumor growth was monitored via in-life bioluminescence. Terminal tumor signals were measured in dissected soft tissues upon experiment termination. More details are provided in supplemental Materials and Methods.
CD33/CD123 NANOBODY TCE mediates killing of AML blasts and expansion of T cells. Primary AML samples from relapsed patients (n = 18) were treated with indicated TCE for 7 days and specific apoptosis was calculated. (A) Violin plots displaying the percentage of SP in bulk (n = 18) and CD34+ AML cells (n = 14) and the fold change of CD3 cells to untreated control (n = 15). Lines in the violin plots indicating median and quartiles. (B) Treatment effects in 2 representative AML samples. Top row: baseline expression of CD33 and CD123 in histograms. Bar graphs displaying the ratio of mean florescent intensity (MFI) to isotype control and the baseline percentage positivity of CD33, CD123 and CD3; bottom row: bar graphs displaying SP apoptosis in bulk and CD34+ AML cells, and fold change of CD3+ T cells in day 7 samples. (C) Bar graph displaying the number of colonies formed in samples under different treatment, CD33/CD123-TCE at 25 nM, triplicate/treatment, mean ± standard deviation (SD). L: 2.5 nM and H: 25 nM. Plus signs (+) indicates the comparison between untargeted TCE and the indicated treatment with P < .001 (one-way analysis of variance [ANOVA] followed by Tukey test with adjust P values). CD3 fold change is not significant difference among the treatments.
CD33/CD123 NANOBODY TCE mediates killing of AML blasts and expansion of T cells. Primary AML samples from relapsed patients (n = 18) were treated with indicated TCE for 7 days and specific apoptosis was calculated. (A) Violin plots displaying the percentage of SP in bulk (n = 18) and CD34+ AML cells (n = 14) and the fold change of CD3 cells to untreated control (n = 15). Lines in the violin plots indicating median and quartiles. (B) Treatment effects in 2 representative AML samples. Top row: baseline expression of CD33 and CD123 in histograms. Bar graphs displaying the ratio of mean florescent intensity (MFI) to isotype control and the baseline percentage positivity of CD33, CD123 and CD3; bottom row: bar graphs displaying SP apoptosis in bulk and CD34+ AML cells, and fold change of CD3+ T cells in day 7 samples. (C) Bar graph displaying the number of colonies formed in samples under different treatment, CD33/CD123-TCE at 25 nM, triplicate/treatment, mean ± standard deviation (SD). L: 2.5 nM and H: 25 nM. Plus signs (+) indicates the comparison between untargeted TCE and the indicated treatment with P < .001 (one-way analysis of variance [ANOVA] followed by Tukey test with adjust P values). CD3 fold change is not significant difference among the treatments.
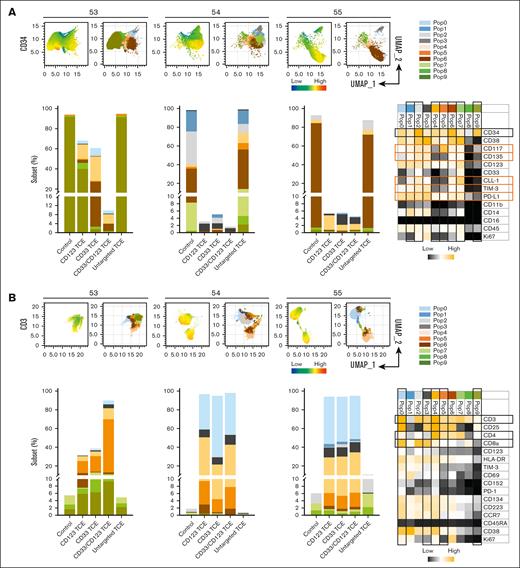
CyTOF immunophenotypic profiling revealing subsets of AML and T cells responding to CD33/CD123 NANOBODY TCE. CyTOF immunophenotyping was performed on primary AML PBMC samples. Uniform manifold approximation and projection (UMAP) plots displaying the expression (left) and clustering (right) of CD34+ (A) and CD3+ (B) in 3 samples examined. Bar graphs in panels A and B displaying the frequency of each cluster (subset) at the indicated treatment in 3 samples. Heat maps show the expression level of surface markers in each subset. Subsets and markers of interest are highlighted with rectangle boxes.
CyTOF immunophenotypic profiling revealing subsets of AML and T cells responding to CD33/CD123 NANOBODY TCE. CyTOF immunophenotyping was performed on primary AML PBMC samples. Uniform manifold approximation and projection (UMAP) plots displaying the expression (left) and clustering (right) of CD34+ (A) and CD3+ (B) in 3 samples examined. Bar graphs in panels A and B displaying the frequency of each cluster (subset) at the indicated treatment in 3 samples. Heat maps show the expression level of surface markers in each subset. Subsets and markers of interest are highlighted with rectangle boxes.
CD33/CD123 NANOBODY TCE kills monocytes and induces cytokine production in an autologous PBMC setup. Flow cytometry plot depicts the expression of CD33 and CD123 within human PBMC (A). Dose dependent monocyte depletion in PBMC by tested mono- and bispecific TCE is shown as CD14% determined in flow cytometry (B, 1 representative donor out of 8 is shown). The global geometric EC50 (half-effective dose) derived from the dose response curves of 8 different donors is shown to the right of the graph. Dose dependent cytokine release within human PBMC is shown for the same representative donor (C).
CD33/CD123 NANOBODY TCE kills monocytes and induces cytokine production in an autologous PBMC setup. Flow cytometry plot depicts the expression of CD33 and CD123 within human PBMC (A). Dose dependent monocyte depletion in PBMC by tested mono- and bispecific TCE is shown as CD14% determined in flow cytometry (B, 1 representative donor out of 8 is shown). The global geometric EC50 (half-effective dose) derived from the dose response curves of 8 different donors is shown to the right of the graph. Dose dependent cytokine release within human PBMC is shown for the same representative donor (C).
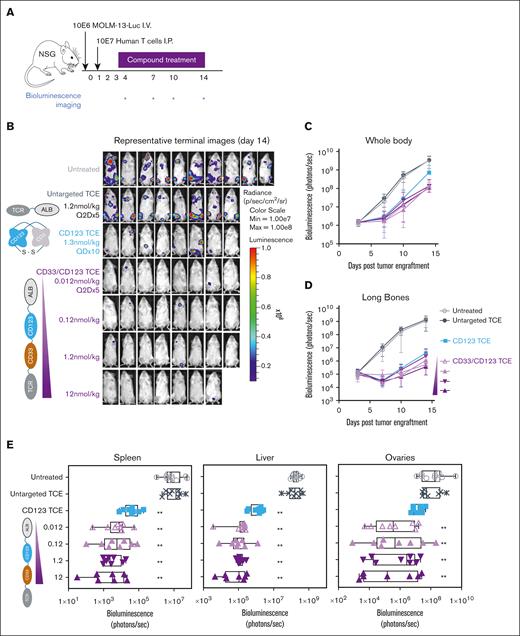
CD33/CD123 NANOBODY TCE exhibits antileukemic effects in a MOLM-13 AML xenograft mouse model. NSG mice were engrafted with 10E6 MOLM-13-Luc AML cells followed by 10E7 human T cells 1 day later. Mice were treated with TCE compounds at the indicated doses between days 2 and 13 of the experiment (QDx10 for CD123 TCE and Q2Dx5 for CD33/CD123 TCE) (A). Panel B illustrates antitumor activity of indicated TCE and doses corroborated by longitudinal quantitation of photon flux using in vivo bioluminescence imaging for global (C) and long-bone (D) tumor growth. Terminal ex vivo bioluminescence imaging analyses were performed of soft tissues spleen, liver, and ovaries to detect tumor cells (E). ∗∗P < .005 in comparison to untreated animals obtained using a two-way ANOVA performed on bio-layer interferometry signal changes from baseline of log values followed by Tukey test.
CD33/CD123 NANOBODY TCE exhibits antileukemic effects in a MOLM-13 AML xenograft mouse model. NSG mice were engrafted with 10E6 MOLM-13-Luc AML cells followed by 10E7 human T cells 1 day later. Mice were treated with TCE compounds at the indicated doses between days 2 and 13 of the experiment (QDx10 for CD123 TCE and Q2Dx5 for CD33/CD123 TCE) (A). Panel B illustrates antitumor activity of indicated TCE and doses corroborated by longitudinal quantitation of photon flux using in vivo bioluminescence imaging for global (C) and long-bone (D) tumor growth. Terminal ex vivo bioluminescence imaging analyses were performed of soft tissues spleen, liver, and ovaries to detect tumor cells (E). ∗∗P < .005 in comparison to untreated animals obtained using a two-way ANOVA performed on bio-layer interferometry signal changes from baseline of log values followed by Tukey test.
In vivo characterization in nonhuman primates
CD33/CD123-TCE pharmacokinetics (PK), pharmacodynamics (PD), and toxicity were evaluated in cynomolgus monkeys upon a single dose followed by a 21-day observation period. Purpose-bred, naïve cynomolgus monkeys (Macaca fascicularis) of Mauritian origin received a single 1-hour intravenous infusion of CD33/CD123-TCE at 0.04 or 0.4 μg/kg (5 mL/kg) (2 male monkeys per group). Parameters evaluated included mortality, clinical signs, body temperature and body weight, hematology, cytokines, and peripheral blood immunophenotyping. Serial samples were taken to determine CD33/CD123-TCE and cytokine serum levels using MedoScale Discovery ligand binding (LLOQ = 50.0 pg/mL) and QuickPlex-SQ120 assays, respectively.
Results
CD33 and CD123 expression is highly heterogeneous on AML blasts
We performed expression profiling of CD123 and CD33 on 55 primary samples from patient with relapsed/refractory AML. Expression of tumor antigens on AML blasts was measured gated as 4′,6-diamidino-2-phenylindole-AnnexinV-CD3-live cells. As expected, their expression was highly variable between patients and between cells within a sample (Figure 1; supplemental Figure 1). Roughly half of the samples expressed both markers on >50% of their cells, whereas the other half showed variable patterns of double (<50%) and single positive cells, with CD123 single positive populations being the most prominent across patients (average 37% ± 3.8% [mean ± SE]) and CD33 single positive the least (7.7% ± 2.0%) (descriptive statistics in supplemental Table 3). Double negative cell populations represented a minority. Hence, we postulated that a TCE targeting both CD33 and CD123 would not just kill more blasts within a patient but also widen the range of patients who would benefit from such therapy.
Design and binding characteristics of a dual-targeting CD33/CD123 NANOBODY TCE
Although the majority of TCE described to date target CD3ε to engage T cells, we opted to use TCRαβ as TCE arm. TCRαβ and CD3 form a structural and functional octameric complex, with the TCR mediating antigen-recognition and CD3 providing the signaling module necessary for T-cell activation.38,39 Nevertheless, early work conducted with CD3 mAb OKT-3 and TCRαβ mAb BMA031 indicated that TCRαβ stimulation by BMA031 was able to induce T-cell activation in vitro but did not lead to systemic adverse reactions after its administration to patients, contrarily to OKT-3.40,41 The more limited expression of TCRαβ vs CD3ε and potential minor differences in signaling, may be responsible for observed differences, which prompted us to use TCRαβ as a potentially safer alternative antigen for T-cell engagement.
We designed a half-life extended dual-targeting CD33/CD123-TCE by linking NANOBODY building blocks specific for respectively TCRαβ, CD33, CD123 and human serum albumin (Figure 2A; supplemental Figure 2A). CD33/CD123-TCE exhibited similar binding affinities to human and cynomolgus monkey targets. The mean binding affinities to both soluble tumor antigens were in the (sub)nM range (Table 1), which is comparable to the target arms of the single-targeting CD33-TCE and CD123-TCE (supplemental Table 4). Apparent KD to primary T cells was in the sub-μM range (310 nM to human and 210 nM to cyno T cells), which was substantially higher than the single-targeting controls despite relatively comparable KDs to TCRαβ and CD3 protein respectively (supplemental Table 4). Based on literature,42 such lower T-cell affinity could translate to lower killing potency but also lower cytokine release, for a better safety profile.
Kinetic parameters for binding of CD33/CD123 NANOBODY TCE to human and cynomolgous target proteins
| Antigen . | Human . | Cynomolgus monkey . | ||||
|---|---|---|---|---|---|---|
| kon (1/Ms) . | koff (1/s) . | KD(M) . | kon (1/Ms) . | koff (1/s) . | KD(M) . | |
| CD33-Fc | 3.5E+05 | 2.7E−03 | 7.6E−09 | 2.8E+05 | 1.9E−03 | 6.9E−09 |
| CD123-Fc | 1.3E+06 | 7.4E−04 | 5.9E−10 | 1.2E+06 | 2.4E−03 | 2.0E−09 |
| Albumin | 2.2E+05 | 2.1E−03 | 9.7E−09 | 2.5E+05 | 2.0E−03 | 7.9E−09 |
| Antigen . | Human . | Cynomolgus monkey . | ||||
|---|---|---|---|---|---|---|
| kon (1/Ms) . | koff (1/s) . | KD(M) . | kon (1/Ms) . | koff (1/s) . | KD(M) . | |
| CD33-Fc | 3.5E+05 | 2.7E−03 | 7.6E−09 | 2.8E+05 | 1.9E−03 | 6.9E−09 |
| CD123-Fc | 1.3E+06 | 7.4E−04 | 5.9E−10 | 1.2E+06 | 2.4E−03 | 2.0E−09 |
| Albumin | 2.2E+05 | 2.1E−03 | 9.7E−09 | 2.5E+05 | 2.0E−03 | 7.9E−09 |
CD33/CD123 NANOBODY TCE mediates killing of single and double positive target cells
Three myeloid cell lines were selected with different expression levels of CD33 and CD123 as determined by Qifikit: MOLM-13 (CD33: 32 448 antigens per cell; CD123: 6543); U-937 (CD33: 19 872; CD123: 945) and KG-1a (CD33: 800; CD123: 3353) (Figure 2C).
CD33/CD123-TCE was tested for redirected T-cell–mediated killing in a flow cytometry-based cytotoxicity assay using HD T cells as effector cells and indicated tumor cell lines as target cells. CD3-directed antibody-based CD33 BiTE43 and CD123 DART44 TCE were taken along as clinically validated single-targeting reference compounds to serve as assay controls. The CD33/CD123-TCE and control compounds redirected human T cells to kill CD33/CD123 double positive MOLM-13 cells in a concentration-dependent manner with a mean 50% effective concentration (EC50) of 18 pM for CD33/CD123-TCE, 8.5 pM for the CD33-TCE and 1.9 pM for the CD123-TCE (Table 2). Similar killing potencies were obtained when cynomolgus T cells were used as effector cells. An untargeted NANOBODY TCE did not induce any killing.
Compound mediated cytotoxic activity of T cells against MOLM-13, U-937, and KG-1a target cells
| Sample ID . | Human T cells (n > 4) . | Cynomolgous T cells (n = 1) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MOLM-13 . | KG-1a . | U-937 . | MOLM-13 (n = 1) . | |||||
| EC50 (M) . | % lysis . | EC50 (M) . | % lysis . | EC50 (M) . | % lysis . | EC50 (M) . | % lysis . | |
| CD33/CD3 BiTE AMG 330 | 8.5E−12 | 32 | 1.3E−11 | <10 | 1.0E−11 | 54 | 1.9E−11 | 23 |
| CD123/CD3 DART MGD006 | 1.9E−12 | 37 | 3.7E−12 | 57 | No fit | No killing | 2.7E−12 | 37 |
| CD33/CD123 TCE | 1.8E−11 | 36 | 2.8E−10 | 52 | 6.7E−10 | 52 | 1.4E−11 | 37 |
| Sample ID . | Human T cells (n > 4) . | Cynomolgous T cells (n = 1) . | ||||||
|---|---|---|---|---|---|---|---|---|
| MOLM-13 . | KG-1a . | U-937 . | MOLM-13 (n = 1) . | |||||
| EC50 (M) . | % lysis . | EC50 (M) . | % lysis . | EC50 (M) . | % lysis . | EC50 (M) . | % lysis . | |
| CD33/CD3 BiTE AMG 330 | 8.5E−12 | 32 | 1.3E−11 | <10 | 1.0E−11 | 54 | 1.9E−11 | 23 |
| CD123/CD3 DART MGD006 | 1.9E−12 | 37 | 3.7E−12 | 57 | No fit | No killing | 2.7E−12 | 37 |
| CD33/CD123 TCE | 1.8E−11 | 36 | 2.8E−10 | 52 | 6.7E−10 | 52 | 1.4E−11 | 37 |
The global geometric EC50 was calculated from different dose response curves using T cells of different healthy donors. Data are from at least 4 experiments for human T cells, only 1 experiment with cynomolgous T cells.
Further, CD33/CD123-TCE induced effective killing of both U-937 and KG-1a cells, myeloid cell lines expressing only 1 of both targets. We observed a killing potency of 670 and 280 pM, respectively, lower than the potency observed in MOLM-13 cells that express higher levels of CD123 and CD33. Single-targeting CD123-TCE was only active on CD123+ KG-1a cells (at same extent as on double positive MOLM-13), whereas CD33-TCE was active on CD33++ U-937 and showed a marginal killing window on KG-1a, related to low CD33 expression (Figure 2B-C).
When either tumor antigen building blocks were replaced by a control building block in the CD33/CD123-TCE, killing of target cells expressing the remaining tumor target occurred at same potency. As anticipated, no killing was observed when the TCR building block was replaced by a control building block (supplemental Figure 2B-C).
In conclusion, inherent to its dual-targeting, CD33/CD123-TCE mediated killing of double- and single-positive target cells in vitro, thus promising a better coverage compared to single-targeting controls.
CD33/CD123 NANOBODY TCE kills AML blasts ex vivo and results in a diverse T-cell–activation across patients
To investigate the antileukemic effect of CD33/CD123-TCE, we treated primary AML samples for 7 days and assessed blast apoptosis. Patient clinical info is provided in supplemental Table 1; all samples were from relapsed patients (n = 18). Treatment of CD33/CD123-TCE at 2.5 and 25 nM induced 70.4% (median) and 71.5% SP in bulk AML blasts. Similar results were observed in samples treated with single-targeting controls at 2.5 nM: CD123-TCE of 76.5% and CD33-TCE of 65.2% (Figure 3A left, not statistically significant). Activity of CD33/CD123-TCE toward CD34+ cells, representing AML-LSC revealed 43.4% and 42.2% apoptosis at 2.5 and 25 nM, respectively. This was relatively less active compared to CD123-TCE (56.4%), but stronger than CD33-TCE (36.9%) (Figure 3A middle, not significant).
Although the average activity was comparable to single-targeting controls, in some patient samples, the dual-targeting CD33/CD123-TCE induced higher apoptosis in bulk AML and CD34+ cells as compared to single-targeting controls, the advantage being most pronounced with the CD33 single-targeting control (examples in Figure 3B; supplemental Figure 3). A colony-forming assay further confirmed that CD33/CD123-TCE effectively inhibited the clonogenicity of primary AML cells (Figure 3C, 2 random samples).
Concomitantly, CD3+ T-cell expansion was observed in all treatment groups, with a trend toward stronger amplification by CD33/CD123-TCE (Figure 3A right; supplemental Figure 3). No significant correlation was found between cytotoxicity and baseline E:T ratio, surface expression of CD123/CD33 or frequency of CD123+, CD33+, and CD3+ in all treatment groups (supplemental Table 5).
Next, we examined AML blast and T-cell phenotypes more deeply using mass cytometry to understand the effect of the TCE treatments (3 random samples). Uniform manifold approximation and projection combined with FlowSOM analysis revealed highly heterogenous subsets of LSC within samples and across patients. LSC subsets characterized by CD34+ are known to coexpress various surface markers associated with LSC-drug resistance and disease relapse: CD135 (FLT3),45 CD117 (c-kit),46 chronic lymphocytic leukemia-1,47 programmed death-ligand 1 (PD-L1),48,49 and −3.50 These subsets were found highly sensitive to CD33/CD123-TCE and single-targeting TCE, with populations 6 (CD34+CD38+CD117+PD-L1+CD123+) and 9 (CD34+CD38−) in sample 53 showing greater sensitivity to CD33/CD123-TCE (Figure 4A). Despite very diverse expression of leukemic markers, CD33/CD123-TCE depleted CD34+ AML-LSC in all 3 samples tested in CyTOF, whereas single-targeting controls were only effective in 2 out of 3 samples.
Analysis of T cells revealed that treatment with TCE compounds expanded CD4+ helper and CD8+ cytotoxic subsets in all samples compared to control (Figure 4B; supplemental Figure 4Ba-c, Ca-c). Activated T cells coexpressing CD69, TIM-3, or PD-1 were expanded by TCE: subset 0, 1, 3 or 5 (CD4) and 0, 1, 3, 4, or 7 (CD8) in all 3 samples (supplemental Figure 4Cb-c). Expansion of central and effector memory T cells defined by CCR7+CD45RA− and CCR7−CD45RA− in CD4+ (4, 5) and CD8+ subsets (2, 6) was seen in all treatment groups in 2 of 3 samples (supplemental Figure 4Cb), whereas sample 53 reveals the highest expansion of CCR7−CD45RA−CD4+ effector memory subsets by CD33/CD123-TCE (5, 9) (Figure 4B).
Collectively, immunophenotypic profiling confirmed the effectiveness of CD33/CD123-TCE on eliminating various blast- and LSC-subsets, activating CD4+ and CD8+ T cells and expanding central/effector memory CD4+ and CD8+ T cells.
CD33/CD123 NANOBODY TCE depletes monocytes and induces in vitro cytokine release in an autologous PBMC assay
As part of the preliminary in vitro safety evaluation, we evaluated CD33/CD123 NANOBODY TCE activity against circulating normal blood cells and accompanying cytokine secretion in an autologous HD PBMC assay (n = 6). We specifically monitored monocyte depletion as these cells express both CD123 (1400-3000 antigens per cell) and CD33 (3000-14 000 antigens per cell) in vitro (Figure 5A). Across 6 donors, CD33/CD123-TCE mediated killing of autologous monocytes with EC50 = 25 pM, which is 5-fold to 40-fold lower potency compared to single-targeting controls CD33-TCE (5.5 pM) and CD123-TCE (0.6 pM) but comparable to its activity on MOLM-13 (18 pM). Monocyte killing was paralleled by significant induction of cytokines including IL-2, IL-6, IFN-γ and TNF-α (Figure 5B-C). High variability among donors was noted, but this was consistent with observations in other human cell-based assays predicting CRS. All cytokines tested were most strongly induced by CD123-TCE, followed by our CD33/CD123-TCE with 10-fold to 20-fold lower potency. TNF-α induction by CD33-TCE was comparable to the other 2 compounds, whereas it induced less IL-6 and hardly any IL-2 and IFN-γ. We additionally compared the dual-targeting NANOBODY CD33/CD123-TCE with a single-targeting NANOBODY CD123-TCE containing exact same building blocks, except for missing CD33. As depicted in supplemental Figure 2D, the data reveal that similar killing efficacy and potency is paralleled with comparable cytokine release, demonstrating comparable in vitro safety.
Efficient AML cell killing in disseminated mouse model
In vivo antitumor activity was evaluated in an AML xenograft NSG mouse model using CD33/CD123− double positive MOLM-13 tumor cells as shown in Figure 6A. The half-life of CD33/CD123 TCE in these mice was determined at 1.34 days. Hence, CD33/CD123 TCE was administered IV every 2 days at 4 different doses after engraftment of MOLM-13 cells and adoptive transfer of human effector T cells (T cell/tumor ratio = 10). CD123-TCE single-targeting control was taken along for benchmarking. Given its short half-life,44 this compound was administered daily. CD33/CD123-TCE was well tolerated at all doses with no evidence of adverse events or alterations of body weight. CD33/CD123-TCE inhibited tumor growth in whole body and in long bones to the same extent (<90% of baseline tumor signal reduction) at all doses tested (0.012; 0.12; 1.2 and 12 nmol/kg), which was not significantly different from CD123-TCE at 1.3 nmol/kg (Figure 6B-D). Untargeted TCE was inactive on tumor growth at 1.2 nmol/kg. Based on terminal ex vivo bioluminescence imaging, all doses of CD33/CD123-TCE were able to significantly inhibit tumor growth in nonmyeloid soft tissues namely spleen (P < .0001), liver (P < .0001) and ovaries (P < .0001) but not in abdominal fat (not shown). CD123-TCE significantly inhibited tumor load in liver (P < .0001) and spleen (P < .0001) but not in ovaries nor abdominal fat tissues, whereas untargeted TCE was inactive in all tissues (Figure 6E).
Next, doses down to 0.0001 nmol/kg CD33/CD123-TCE were tested to determine minimal active dose, defined as the lowest dose inducing significant tumor inhibition (supplemental Figure 5). The minimal active dose was determined at 0.0012 nmol/kg (0.064 μg/kg) for CD33/CD123-TCE and 0.013 nmol/kg (0.7 μg/kg) for CD123-TCE non-half-life extended single-targeting control.
Favorable PK/PD relationship in nonhuman primates without toxicities
A single-dose exploratory study in healthy cynomolgous monkeys was performed to evaluate PK, PD, and toxicity of the CD33/CD123 NANOBODY TCE. Dose levels were determined based on earlier studies with CD123/CD3 TCE 37: 0.04 μg/kg and 0.4 μg/kg (2 animals per dose level). A favorable PK profile was observed with half-life determined at 1.1 to 1.4 days indicating some target-mediated drug disposition (Figure 7A; supplemental Table 6).
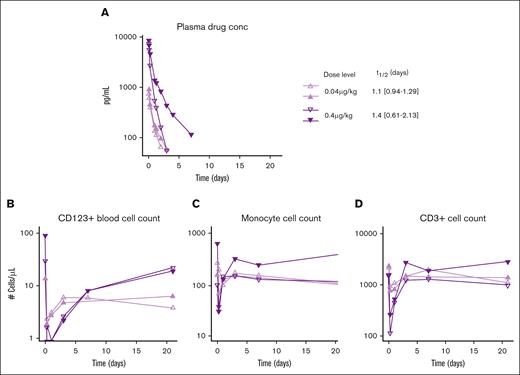
Favorable PK profile and effective target cell clearance by CD33/CD123 NANOBODY TCE in cynomolgous monkeys. A single dose CD33/CD123-TCE was administered at 0.4 or 0.04 μg/kg (2 animals per dose level). (A) Exposure of the NANOBODY compound was measured in plasma over time to determine half-life T1/2 (days) shown to the right. (B) Using flow cytometry, CD123+ cells, CD14+ monocytes (C), and CD3+ T cell counts (D) were determined in peripheral blood at the indicated time points. Individual animal values are shown.
Favorable PK profile and effective target cell clearance by CD33/CD123 NANOBODY TCE in cynomolgous monkeys. A single dose CD33/CD123-TCE was administered at 0.4 or 0.04 μg/kg (2 animals per dose level). (A) Exposure of the NANOBODY compound was measured in plasma over time to determine half-life T1/2 (days) shown to the right. (B) Using flow cytometry, CD123+ cells, CD14+ monocytes (C), and CD3+ T cell counts (D) were determined in peripheral blood at the indicated time points. Individual animal values are shown.
In parallel, a clear dose-dependent PD effect was observed as measured by depletion of CD123+ plasmacytoid dendric cells and CD33+ monocytes (Figure 7B-C). CD123+ cells came back to normal 3 to 7 days after treatment l, whereas monocytes normalized by day 2 to 4. This transient depletion of target cells was paralleled by a decrease in circulating T cells, presumably due to extravasation upon activation by the compound as suggested before.44
Finally, the single infusion of CD33/CD123-TCE to cynomolgus monkeys was well tolerated at both dose levels. No mortality, treatment-related clinical signs, body weight changes, or body temperature changes were observed. Cytokine evaluation showed minimal increases of IL-6 IL-2, IFN-γ and TNF-α at the highest dose only (supplemental Table 7). These changes were transient, peaking between 2 to 6 hours and returning to baseline within 24 hours. In summary, no signs of CRS were observed at doses providing a clear PD effect.
Discussion
Despite recent advances and successful combination therapies, a high unmet medical need remains for patients with relapsed/refractory AML. With the promising preliminary single-agent activity of flotetuzumab (CD123-CD3 = MGD006) in refractory AML,24 immunotherapy using TCE has gained much interest as treatment for relapsed/refractory AML. We designed a novel CD33/CD123 NANOBODY TCE for AML to overcome specific challenges identified with first generation TCE similar to flotetuzumab: (1) half-life extension for better PK-PD properties allowing patient-friendly (intermittent) dosing; (2) dual-antigen-targeting to mitigate disease heterogeneity and minimize the impact of potential antigen loss; and (3) optimized activity to reduce CRS severity.
Heterogeneous leukemia antigen expression arising from genetic heterogeneity in primary AML blasts poses an obstacle to the success of single-targeting immunotherapies. Furthermore, single-targeting treatments may favor the rise of antigen-loss variants resulting in relapse.11,25,51 We confirmed heterogeneity of CD123 and CD33 expression on ex vivo primary AML samples as described previously.12,20 We further demonstrated that cotargeting CD33 and CD123 not only confers coverage of both single and double positive cancer cell lines but also provides superior killing over single-targeting agents in a subset of primary AML samples. In-depth CyTOF phenotyping of AML blasts demonstrated a high heterogeneity of surface marker expression within AML samples and across patients and confirmed the superior cytolytic activity of the CD33/CD123-TCE over single-targeting TCE in certain subsets of patients. However, this cytotoxicity was neither directly correlated to expression levels of the respective antigens nor to E:T ratios always, indicating that other mechanisms may be at play in regulating sensitivity to TCE.11,52
In fact, CyTOF profiling revealed a couple of CD34+CD123+CD33 low-blast subsets that were remaining upon monotargeting or cotargeting TCE treatment. These cells expressed either PD-L1 alone (subset 4) or coexpressed PD-L1, TIM-3, and chronic lymphocytic leukemia-1 (subset 3); all 3 markers reportedly promote survival of AML cells and are associated with unfavorable prognosis.49,53,54 In parallel, T-cell functionality is key for the activity of TCEs. T-cell exhaustion has been reported in patients with AML and suggested to contribute to primary and secondary resistance to TCE.11,52 Our CyTOF analysis revealed expression of exhaustion markers TIM-3 and LAG-3 (CD223) on CD4 and CD8 subsets (0, 1, 2, 3, 4). Along with PD1, TIM-3, and LAG-3 are important T-cell–immune checkpoints that may mediate AML-induced immunosuppression, dampening TCE-triggered T-cell activation against AML.11,50,55,56
Although the data enable comparison of the NANOBODY-based dual-targeting CD33/CD123-TCR compound with clinically validated CD123-CD3 DART and CD33-CD3 BiTE reference molecules in specific assays, caution is advised when extrapolating beyond these experiments. As elucidated by Ellerman et al,42 numerous variables contribute to in vitro and in vivo potency and safety of bispecific TCE. Besides targeting 2 tumor antigens vs 1 and targeting TCR vs CD3, other criteria that differentiate the NANOBODY TCE from the references include format (distance between tumor arm and T-cell arm, rigidity), affinity and epitope of the tumor antigen arm and T-cell arm, and their respective half-lives. Hence, direct comparison of DART vs BiTE vs NANOBODY formats is not feasible from our study. However, inclusion of control constructs omitting 1 tumor arm in the NANOBODY TCE allowed us to infer that, in an in vitro setting, dual-targeting and monotargeting are equally safe.
On the comparison of CD3 vs TCRαβ, competitor data comparing CD19-targeted DART TCE to either TCRαβ or CD3 revealed equivalent in vitro killing potency between both.57 Our own unpublished in vitro findings on CD123 NANOBODY TCE, directed toward either CD3 or TCRαβ, indicate that affinity and epitope, irrespective of targeting CD3 or TCR, serve as primary determinants of efficacy and safety. It is crucial to emphasize that the ultimate assessment of safety and efficacy will be derived from clinical data. Currently, a phase 1 study is underway with a GPC3-TCR TCE,58 and we anticipate the release of results in the coming years.
Alternative CD33/CD123 cotargeting strategies, aimed at better coverage without increasing on-target–off-leukemia toxicity, are being developed using technologies as diverse as CAR-T cells,51,59,60 single chain variable fragment NK-cell engager61 and targeted liposomes.62 These preclinical data are very much in line with our own results, and clinical trials are currently recruiting for bispecific CAR-T cells (NCT04010877).
The second differentiation we aimed at with our CD33/CD123-TCE was tailored activity to minimize cytokine release. The TCE’s binding affinity to both CD3/TCR and the tumor anchor correlated with in vitro killing potency as well as cytokine release in vitro and in vivo.42,63 Several groups demonstrated that CD3 arm affinity-tuning could reduce in vitro killing potency and cytokine secretion with preservation of maximum killing64-67 and affinity-tuned TCE are currently being tested in the clinic, including an affinity-tuned, half-life extended version of flotetuzumab (NCT04740034, NCT05362773). Similar to those molecules, CD33/CD123 TCE exhibits medium affinity to the CD3/TCR complex (KD = 21 nM) with low apparent KD on primary T cells (≥300 nM). We demonstrated here that this translates into lower in vitro killing and cytokine potency whereas maintaining similar efficacy. Importantly, CD33/CD123-TCE exhibits potent tumor cell killing in vivo in a disseminated AML cell-line–derived xenograft model with a 10-fold lower minimal active dose and better tumor clearance in soft tissues compared with single-targeting non–half-life extended CD123-TCE. Furthermore, a single-dose exploratory non human primate study revealed a favorable PK/PD relationship with clear depletion of CD123+ and CD33+ cells in the absence of clinical signs or CRS.
Keeping in mind that CD33/CD123-TCE can bind and kill CD33+ and CD123+ target cells, we conclude that this novel dual-targeting NANOBODY TCE holds the promise of improving the therapeutic window by increasing coverage of heterogeneous AML clones, with a potentially safer profile compared to first-generation TCE. Such a compound would be a desirable treatment option for older and fragile patients with AML and could serve as a long-awaited “minimal residual disease eraser” in AML. We envision that patients achieving remission and minimal residual disease negativity with safe and effective TCE could proceed toward allogeneic stem cell transplantation or continue maintenance therapy, with the goal to extend depth of response, remission duration, and overall survival.
Acknowledgments
The authors thank Diane Van Hoorick, Anne Brigé, and all former and current Ablynx-Sanofi team members that contributed to the development of the molecule. The authors acknowledge that the term “NANOBODY” is a registered trademark of Ablynx-Sanofi.
This work was supported by Sanofi and in part by an NIH/NCI Cancer Center Support Grant to MD Anderson Cancer Center (P30 CA016672, used the Flow Cytometry and Cellular Imaging Core Facility).
Authorship
Contribution: Z.Z., A.R., G.D., H.B., A.V.-O., M.C., M.K., and M.D. focused on the conceptualization and design of the study and data interpretation; Z.Z., A.R., S.G., V.D.B., A.C., and E.D. contributed to the data acquisition, analysis, and interpretation; M.K. and M.D. supervised the overall study; M.K. was involved in the procurement of primary samples and funding acquisition; Z.Z., A.R., H.B., and M.D. wrote the original draft; and Z.Z., A.R., G.D., H.B., S.G., V.D.B., A.C., E.D., A.V.-O., M.C., M.K., and M.D. reviewed and edited the manuscript.
Conflict-of-interest disclosure: Z.Z. reports research support from Ablynx-Sanofi. M.K. reports research funding from Ablynx-Sanofi, Agios, Ascentage, AstraZeneca, CALITHERA, Cellectis, Eli Lilly, Rafael Pharmaceutical, and Sanofi; research funding and personal fees from AbbVie, F. Hoffmann La-Roche, Forty-Seven, Genentech, and Stemline Therapeutics; personal fees from Amgen and Kisoji; and patents and royalties from patent US 7,795,305 (B2 on CDDO-compounds and combination therapies) licensed to Reata Pharmaceutical, Inc, all outside the submitted work. A.R., G.D., H.B., V.D.B., A.C., E.D., A.V.-O., M.C., and M.D. are all Sanofi employees and may hold shares and/or stock options in the company.
Correspondence: Melissa Dullaers, Sanofi Ghent, Technologiepark 21, 9052 Zwijnaarde, Belgium; email: melissa.dullaers@sanofi.com.
References
Author notes
M.K. and M.D. are joint senior authors.
Data are available on request from either the first author, Zhihong Zeng (zzeng@mdanderson.org) or the corresponding author, Melissa Dullaers (melissa.dullaers@sanofi.com).
The full-text version of this article contains a data supplement.


![Primary AML samples are positive for CD33 and CD123. (A) Bar graph summarizing the percentage of positivity for CD33 and CD123 in all patient samples examined (mean ± standard error of the mean [SEM]). (B) Waterfall bar graph showing individual distribution of percentage positivity of CD33, CD123, and dual positivity and negativity for CD123 and CD33 of 55 patient samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/9/10.1182_bloodadvances.2023011858/2/m_blooda_adv-2023-011858-gr1.jpeg?Expires=1769103918&Signature=sNmWtd2mgZBXBDmCGT-PvH-DExYxTTSw3Xx6BeJZQOO2fhMyaNVS8EMAuFhwEfYPTD02cP6zFGIvJl5jL2oTOZPFBWL6~rgaSpG6UpeEgIhYAPAPlQwPVsKAXADuIZLYk89-GE7C-b8xAzC1dRSxG9mxbxoog4vYKHQ~2B~Svfo0Rtt9jvD~2N6BYm7ZBUXWjq2KpnUpbIvJeytShkofe34wgZpHPh4UeqDaXPcfUZfLKouF4fiWwbe-CveCbIXTonu0SiQutJgXTfGcjj5Gp4S0FbkSCVx2rFn4UL8jo3eIECdz~AUJ~7QU9EnP3ODpB43EA7iikHpHZIAsERKkng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD33/CD123 NANOBODY TCE mediates killing of AML blasts and expansion of T cells. Primary AML samples from relapsed patients (n = 18) were treated with indicated TCE for 7 days and specific apoptosis was calculated. (A) Violin plots displaying the percentage of SP in bulk (n = 18) and CD34+ AML cells (n = 14) and the fold change of CD3 cells to untreated control (n = 15). Lines in the violin plots indicating median and quartiles. (B) Treatment effects in 2 representative AML samples. Top row: baseline expression of CD33 and CD123 in histograms. Bar graphs displaying the ratio of mean florescent intensity (MFI) to isotype control and the baseline percentage positivity of CD33, CD123 and CD3; bottom row: bar graphs displaying SP apoptosis in bulk and CD34+ AML cells, and fold change of CD3+ T cells in day 7 samples. (C) Bar graph displaying the number of colonies formed in samples under different treatment, CD33/CD123-TCE at 25 nM, triplicate/treatment, mean ± standard deviation (SD). L: 2.5 nM and H: 25 nM. Plus signs (+) indicates the comparison between untargeted TCE and the indicated treatment with P < .001 (one-way analysis of variance [ANOVA] followed by Tukey test with adjust P values). CD3 fold change is not significant difference among the treatments.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/9/10.1182_bloodadvances.2023011858/2/m_blooda_adv-2023-011858-gr3.jpeg?Expires=1769103918&Signature=AShGPV4K4sW5q8d9beqY-pV7fk~l1WvEry4jcoTsbDjAR9UbZNxPt808~NQPFsM-DZ9MU2Spu6VHtHZhnJOpXyGGz6SPUga4~RHWk3BXyY50xiTKk~Fj09yk9n~uxR82mJjL~e~PV0FVQwMuygfYklv3NwiT4M1Qn0WzPesz1RGGFvfniFd~2ptp4DkpVg2quHMMAYTtVe0q0hPABOHx2ngO431qKfypFA1ykHRip0EP6lNISMFDIhzN7uP8T0mLUcIHIo7XqGIJAdhYSFUgB~Ue5oE9tHPPbGmQ3OLRT~C9Nd3apolxKyPWqxzLsPR5GHlW40SHSH0AyLmA3-JGYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)