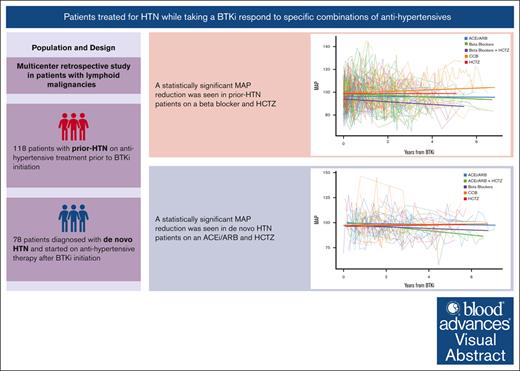
Patients treated for hypertension while taking ibrutinib benefit from combination therapy.
Regimens that combine HCTZ and β blockers benefit patients with prior-HTN, whereas HCTZ and ACEi/ARBs benefit patients with de novo HTN.
Visual Abstract
Although Bruton tyrosine kinase inhibitors (BTKis) are generally well tolerated and less toxic than chemotherapy alternatives used to treat lymphoid malignancies, BTKis like ibrutinib have the potential to cause new or worsening hypertension (HTN). Little is known about the optimal treatment of BTKi-associated HTN. Randomly selected patients with lymphoid malignancies on a BTKi and antihypertensive drug(s) and with at least 3 months of follow-up data were sorted into 2 groups: those diagnosed with HTN before BTKi initiation (prior-HTN), and those diagnosed with HTN after BTKi initiation (de novo HTN). Generalized estimating equations assessed associations between time varying mean arterial pressures (MAPs) and individual anti-HTN drug categories. Of 196 patients included in the study, 118 had prior-HTN, and 78 developed de novo HTN. Statistically significant mean MAP reductions were observed in patients with prior-HTN who took β blockers (BBs) with hydrochlorothiazide (HCTZ), (−5.05 mmHg; 95% confidence interval [CI], 10.0 to −0.0596; P = .047), and patients diagnosed with de novo HTN who took either an angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) with HCTZ (−5.47 mmHg; 95% CI, 10.9 to −0.001; P = .05). These regimens also correlated with the greatest percentages of normotensive MAPs. Treatment of HTN in patients taking a BTKi is challenging and may require multiple antihypertensives. Patients with prior-HTN appear to benefit from combination regimens with BBs and HCTZ, whereas patients with de novo HTN appear to benefit from ACEi/ARBs with HCTZ. These results should be confirmed in prospective studies.
Introduction
Bruton tyrosine kinase inhibitors (BTKis) fulfill a critical role in the treatment of numerous lymphoid malignancies, including chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), marginal zone lymphoma, Waldenström macroglobulinemia, and a subset of patients with diffuse large B-cell lymphoma.1-9 For all indications, BTKis are administered with the hope of providing long-term disease control with limited toxicity, although prolonged exposure confers a legitimate risk of adverse events.10 Hypertension (HTN) is a widely recognized adverse effect of BTKi use that often occurs late in the span of therapy and has the potential to cause major adverse cardiovascular events (MACEs) if not treated appropriately.11-15 Although standard antihypertensive (anti-HTN) medications are commonly used to treat HTN in patients taking a BTKi, guidance on the optimal class of anti-HTN medication is lacking, with some experts suggesting that these drugs should be chosen according to patients’ comorbidities or so as to avoid pharmacokinetic interactions between medications.16,17
Ibrutinib was the first covalent inhibitor of BTK to be approved for the treatment of patients with CLL, Waldenström macroglobulinemia, marginal zone lymphoma, and MCL. Its widespread use was accompanied by real-world experience with drug-associated HTN, corroborated by data from both clinical trials and retrospective studies. New or worsened HTN was identified in 78.3% of ibrutinib users over a median of 30 months in a series of 562 patients treated between 2009 and 2016. This same study reported high-grade HTN (blood pressure [BP] >160/100 mm Hg) in 17.7% of patients who were diagnosed with de novo HTN after BTKi initiation.14 Roeker et al examined BP data in 247 patients on ibrutinib and found that incident HTN occurred in 34.8% of patients, whereas 49.5% of patients with preexisting HTN experienced grade ≥3 systolic HTN.18 Of these patients with HTN before starting ibrutinib, 20.6% had a change in their cardiovascular medication regimen in the first year after ibrutinib exposure. The median time to peak BP was 6 months, suggesting a need for ongoing BP monitoring.
Data from clinical trials have reported lower numbers of patients with new or worsening HTN on ibrutinib, although pooled analyses suggest it is a real problem. Long-term follow-up of the phase 3 RESONATE trial comparing ibrutinib with ofatumumab in patients with CLL found grade ≥3 HTN in only 4% to 9% of patients over the first 4 years of treatment.1 In contrast, extensive follow-up data from the phase 1b/11 PCYC-1102 study reported grade ≥3 HTN in 28% of patients.19 To better understand the association between ibrutinib and HTN, Caldeira et al conducted a meta-analysis of 8 randomized controlled trials (including RESONATE and RESONATE-2) to show that ibrutinib is associated with an increased risk of HTN as demonstrated by a risk ratio of 2.82 (95% confidence interval [CI], 1.52-5.23; P value < .001).13
Although plenty of data corroborate a relationship between BTKis and HTN, there are no formal guidelines on how to optimize the treatment of patients with new or worsening BTKi-associated HTN.10,15 Experts have recommended a variety of management strategies including referral to cardiology or cardio-oncology when >2 antihypertensives are needed.20 Using real-world, multicenter, retrospective data, we evaluated the antihypertensive effects of common medication classes and combinations in patients taking BTKis; although the vast majority of our patients were on ibrutinib and not 1 of the second-generation BTKis. During our evaluation, we distinguished between patients with HTN before ibrutinib initiation (prior-HTN), and those who developed incident HTN after starting ibrutinib (de novo HTN).
Methods
Study population
We included randomly selected patients with lymphoid malignancies and a diagnosis of HTN from 14 institutions in the United States. All patients were concurrently treated with a BTKi and an anti-HTN therapy for at least 3 months, between 2014 and 2018. They were then separated into 2 groups: those who were on anti-HTN mediations before starting a BTKi (prior-HTN) and those who started anti-HTN therapy after BTKi initiation (de novo HTN). Demographic variables such as lymphoid malignancy type and comorbidities were obtained, along with documentation of BTKi changes (dose reductions, medication discontinuation, and switching to an alternative BTKi). Anti-HTN medications were categorized into 4 major groups: angiotensin converting enzyme inhibitor (ACEi) and angiotensin receptor blockers (ARBs), β blockers (BBs), calcium channel blockers (CCBs), and hydrochlorothiazide (HCTZ). All time points for mean arterial pressures (MAP) measurements, medication start dates, and medication end dates were documented in relation to the index date, defined as the first date of concurrent BTKi and anti-HTN agent use. Retrospective MAP data were assessed for each patient in relation to the type of anti-HTN drug or combination of drugs prescribed.
Institutional review boards at each site approved of the study before data acquisition. This was an investigator-initiated study funded by AstraZeneca. The study design, data collection, and interpretation of data were conducted by all coinvestigators. Data management and statistical analysis was done at the Fred Hutchinson Cancer Center (Seattle, WA), which served as the coordinating site.
Outcomes
Our primary outcome was the clinical efficacy of different anti-HTN drug classes for the treatment of HTN in patients concurrently receiving a BTKi. Clinical efficacy, defined as effective anti-HTN treatment, was assessed by calculating mean MAP reductions in patients on various anti-HTN medications. We sought to identify whether a single anti-HTN drug class or a combination of classes would reduce HTN in 2 patient groups: those with prior-HTN and those with de novo HTN.
Statistical analysis
Generalized estimating equations (GEEs) were fit to assess the association of medication use with MAP, in which each medication was classified into 1 of several broad categories. Indicators for each category were included in the GEE model based on presence or absence of the relevant medication at the time of MAP measurement. The impact of a specific 2-drug combination was assessed by additionally including the following indicators in our regression model: an indicator for use of 1 of the 2 drugs alone, an indicator for use of the other drug in the combination alone, and an indicator for use of the 2 drugs at the same time. All 2-drug combinations were assessed; combinations of ≥3 were not feasible because of sample size restraints. Time from BTKi was included in the GEE models as a continuous linear variable, and race and sex were also included in the GEE models.
Summary lines on the figures were created using linear regression.
Results
Overall, 196 patients were included in the study: 118 received treatment for diagnosed HTN before starting a BTKi (prior-HTN), and 78 were diagnosed after BTKi initiation (de novo HTN). Of patients in the prior-HTN group, most had CLL/small lymphocytic lymphoma (n = 112, 94.9%) and were treated with ibrutinib (n = 101, 85.6%). Of patients with de novo HTN, most had CLL/small lymphocytic lymphoma (n = 72, 92.3%) on ibrutinib (n = 75, 96.2%). One patient (1.3%) in the de novo–HTN group was on acalabrutinib, whereas 9 patients (7.6%) in the prior-HTN group were on acalabrutinib. In general, BTKi dose reductions and medication switches were uncommon across both groups; however, BTKis were permanently discontinued because of HTN in 26 (22.0%) patients in the prior-HTN group and 18 (23.1%) patients in the de novo–HTN group. Additional baseline characteristics are described in Table 1.
Patient characteristics
| . | Prior-HTN (n = 118) . | De novo HTN (n = 78) . | Entire cohort (N = 196) . |
|---|---|---|---|
| Diagnosis | |||
| CLL/SLL | 112 (94.9%) | 72 (92.3%) | 184 (93.9%) |
| MCL | 3 (2.5%) | 3 (3.8%) | 6 (3.1%) |
| Other | 3 (2.5%) | 3 (3.8%) | 6 (3.1%) |
| BTKi type | |||
| Ibrutinib | 101 (85.6%) | 75 (96.2%) | 176 (89.8%) |
| Acalabrutinib | 9 (7.6%) | 1 (1.3%) | 10 (5.1%) |
| Other | 8 (6.8%) | 2 (2.6%) | 10 (5.1%) |
| Current treatment line | |||
| 1 | 57 (48.3%) | 39 (50.0%) | 96 (49.0%) |
| 2 | 32 (27.1%) | 18 (23.1%) | 50 (25.5%) |
| ≥3 | 29 (24.6%) | 21 (26.9%) | 50 (25.5%) |
| Age, median (range), y | 67.5 (42.0-88.0) | 65.5 (37.0-87.0) | 67 (37.0-88.0) |
| Race | |||
| Caucasian | 100 (92.6%) | 73 (97.3%) | 173 (94.5%) |
| Other | 8 (7.4%) | 2 (2.7%) | 10 (5.5%) |
| Missing | 10 | 3 | 13 |
| Sex | |||
| Female | 34 (29.3%) | 20 (25.6%) | 54 (27.8%) |
| Male | 82 (70.7%) | 58 (74.4%) | 140 (72.2%) |
| History of DM | 25 (21.2%) | 9 (11.5%) | 34 (17.3%) |
| History of CAD | 5 (4.2%) | 0 (0.0%) | 5 (2.6%) |
| History of CKD | 2 | 0 | 2 |
| BTKi on a clinical trial | 29 (24.6%) | 17 (21.8%) | 46 (23.5%) |
| BTKi dose reduction | |||
| No | 84 (71.2%) | 59 (75.6%) | 143 (73.0%) |
| Yes, for HTN | 7 (5.9%) | 2 (2.6%) | 9 (4.6%) |
| Yes, for other reasons | 27 (22.9%) | 17 (21.8%) | 44 (22.4%) |
| Time to BTKi dose reduction; median days (range) | 382.5 (0.0-1651.0) | 365.0 (0.0-1574.0) | 366.0 (0.0-1651.0) |
| Switch to other BTKi | |||
| No | 111 (94.1%) | 73 (93.6%) | 184 (93.9%) |
| Yes, for HTN | 3 (2.5%) | 2 (2.6%) | 5 (2.6%) |
| Yes, for other reasons | 4 (3.4%) | 3 (3.8%) | 7 (3.6%) |
| Time to switching to other BTKi; median days (range) | 308.0 (85.0-1631.0) | 1445.0 (386.0-3775.0) | 777.0 (85.0-3775.0) |
| BTKi stopped | |||
| No | 92 (78.0%) | 59 (75.6%) | 151 (77.0%) |
| Yes, for HTN | 26 (22.0%) | 18 (23.1%) | 44 (22.4%) |
| Yes, for other reasons | 0 (0.0%) | 1 (1.3%) | 1 (0.5%) |
| . | Prior-HTN (n = 118) . | De novo HTN (n = 78) . | Entire cohort (N = 196) . |
|---|---|---|---|
| Diagnosis | |||
| CLL/SLL | 112 (94.9%) | 72 (92.3%) | 184 (93.9%) |
| MCL | 3 (2.5%) | 3 (3.8%) | 6 (3.1%) |
| Other | 3 (2.5%) | 3 (3.8%) | 6 (3.1%) |
| BTKi type | |||
| Ibrutinib | 101 (85.6%) | 75 (96.2%) | 176 (89.8%) |
| Acalabrutinib | 9 (7.6%) | 1 (1.3%) | 10 (5.1%) |
| Other | 8 (6.8%) | 2 (2.6%) | 10 (5.1%) |
| Current treatment line | |||
| 1 | 57 (48.3%) | 39 (50.0%) | 96 (49.0%) |
| 2 | 32 (27.1%) | 18 (23.1%) | 50 (25.5%) |
| ≥3 | 29 (24.6%) | 21 (26.9%) | 50 (25.5%) |
| Age, median (range), y | 67.5 (42.0-88.0) | 65.5 (37.0-87.0) | 67 (37.0-88.0) |
| Race | |||
| Caucasian | 100 (92.6%) | 73 (97.3%) | 173 (94.5%) |
| Other | 8 (7.4%) | 2 (2.7%) | 10 (5.5%) |
| Missing | 10 | 3 | 13 |
| Sex | |||
| Female | 34 (29.3%) | 20 (25.6%) | 54 (27.8%) |
| Male | 82 (70.7%) | 58 (74.4%) | 140 (72.2%) |
| History of DM | 25 (21.2%) | 9 (11.5%) | 34 (17.3%) |
| History of CAD | 5 (4.2%) | 0 (0.0%) | 5 (2.6%) |
| History of CKD | 2 | 0 | 2 |
| BTKi on a clinical trial | 29 (24.6%) | 17 (21.8%) | 46 (23.5%) |
| BTKi dose reduction | |||
| No | 84 (71.2%) | 59 (75.6%) | 143 (73.0%) |
| Yes, for HTN | 7 (5.9%) | 2 (2.6%) | 9 (4.6%) |
| Yes, for other reasons | 27 (22.9%) | 17 (21.8%) | 44 (22.4%) |
| Time to BTKi dose reduction; median days (range) | 382.5 (0.0-1651.0) | 365.0 (0.0-1574.0) | 366.0 (0.0-1651.0) |
| Switch to other BTKi | |||
| No | 111 (94.1%) | 73 (93.6%) | 184 (93.9%) |
| Yes, for HTN | 3 (2.5%) | 2 (2.6%) | 5 (2.6%) |
| Yes, for other reasons | 4 (3.4%) | 3 (3.8%) | 7 (3.6%) |
| Time to switching to other BTKi; median days (range) | 308.0 (85.0-1631.0) | 1445.0 (386.0-3775.0) | 777.0 (85.0-3775.0) |
| BTKi stopped | |||
| No | 92 (78.0%) | 59 (75.6%) | 151 (77.0%) |
| Yes, for HTN | 26 (22.0%) | 18 (23.1%) | 44 (22.4%) |
| Yes, for other reasons | 0 (0.0%) | 1 (1.3%) | 1 (0.5%) |
CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; SLL, small lymphocytic lymphoma.
Among patients with de novo HTN and prior-HTN, ACEis/ARBs constituted the most common anti-HTN drug class (66% of all patients), although CCBs (51%), BBs (36%), and HCTZ (29%) were also prescribed frequently (see Table 2). Common drug combinations included ACEi/ARBs with CCBs (27%), ACEi/ARBs with HCTZ (20%), CCBs with HCTZ (11%), and BBs with HCTZ (8%). The median number of MAP measurements per patient was 12 (interquartile range, 7-19), and the median time from baseline to the end of follow-up was 3 years (interquartile range, 1.4-4.8 years). For patients with de novo HTN, the median time from BTKi initiation to anti-HTN therapy initiation was 394 days (interquartile range, 192-961 days).
Frequency of BP medications/combination use among all patients
| . | Patients on the drug class N = 118, % . | Days on treatment (median) . |
|---|---|---|
| Anti-HTN class∗ | ||
| ACEi/ARB | 66 | 722 |
| CCB | 51 | 482 |
| BB | 36 | 1036 |
| HCTZ | 29 | 690 |
| BP drug combinations∗ | ||
| ACEi/ARB + HCTZ | 20 | |
| CCB + HCTZ | 11 | |
| BB + HCTZ | 8 | |
| ACEi/ARB + CCB | 27 | |
| Number of BP drugs prescribed at a time | ||
| 1 | 39 | |
| 2 | 40 | |
| 3 | 16 | |
| ≥4 | 5 |
| . | Patients on the drug class N = 118, % . | Days on treatment (median) . |
|---|---|---|
| Anti-HTN class∗ | ||
| ACEi/ARB | 66 | 722 |
| CCB | 51 | 482 |
| BB | 36 | 1036 |
| HCTZ | 29 | 690 |
| BP drug combinations∗ | ||
| ACEi/ARB + HCTZ | 20 | |
| CCB + HCTZ | 11 | |
| BB + HCTZ | 8 | |
| ACEi/ARB + CCB | 27 | |
| Number of BP drugs prescribed at a time | ||
| 1 | 39 | |
| 2 | 40 | |
| 3 | 16 | |
| ≥4 | 5 |
Indicates the percentage of patients taking this class or combination of alone or with other categories.
MAP reductions with specific anti-HTN drug types and combinations
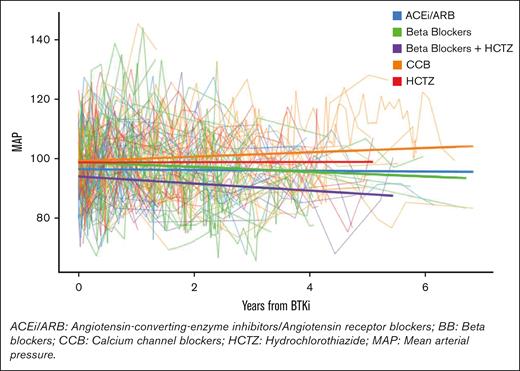
Of patients with prior-HTN, no single anti-HTN class provided a statistically significant reduction in mean MAP, although all classes except CCBs showed nonsignificant reductions in mean MAPs (negative coefficients). In terms of 2-drug combination therapies, only the combination of BBs and HCTZ provided a statistically significant mean reduction in MAP of −5.05 mmHg (95% CI, 10.0 to −0.0596; P value = .047; see Figure 1). The population that benefited from this combination included patients who were on either a BB or HCTZ before starting a BTKi and the second anti-HTN agent, as well as patients who were previously on an alternative anti-HTN regimen and began receiving both a BB and HCTZ after their BTKi start date.
MAP trend in patients with preexisting HTN taking anti-HTN medications and a BTKi concurrently.
MAP trend in patients with preexisting HTN taking anti-HTN medications and a BTKi concurrently.
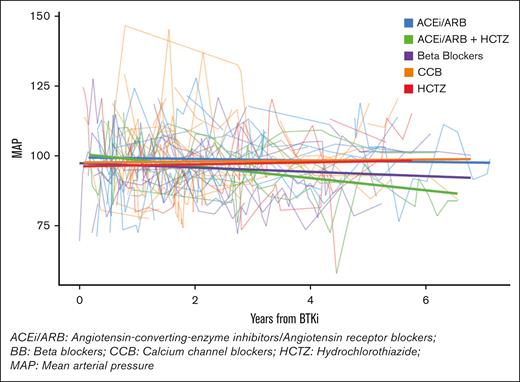
As with patients with prior-HTN, patients with de novo HTN did not exhibit a statistically significant reduction in mean MAP after receiving any single anti-HTN class. Patients with de novo HTN also responded best to combination therapy, exhibiting a statistically significant mean MAP reduction on an ACEi/ARB combined with HCTZ (−5.47 mmHg; 95% CI, 10.9 to −0.001; P value = .05; see Figure 2). When all patients were combined, ACEi/ARBs plus HCTZ, and BBs plus HCTZ, provided statistically significant mean MAP reductions. Additional findings are outlined in Table 3.
MAP trend in patients with de novo HTN taking anti-HTN medications and a BTKi concurrently.
MAP trend in patients with de novo HTN taking anti-HTN medications and a BTKi concurrently.
Mean MAP reductions with specific BP medications/combinations
| . | Estimate . | 95% CI . | P value . | |
|---|---|---|---|---|
| Lower . | Higher . | |||
| Prior-HTN | ||||
| ACEi/ARB | −1.30 | −4.74 | 2.13 | .45 |
| BBs | −2.06 | −5.10 | 0.97 | .18 |
| CCBs | 2.33 | −0.74 | 5.41 | .13 |
| HCTZ | −1.17 | −4.10 | 1.76 | .43 |
| BBs and HCTZ∗ | −5.05 | −10.0 | −0.0596 | .047 |
| de novo HTN | ||||
| ACEi/ARB | −0.13 | −3.57 | 3.31 | .94 |
| BBs | −2.71 | −6.88 | 1.45 | .20 |
| CCBs | 0.11 | −5.66 | 5.90 | .96 |
| HCTZ | −4.26 | −9.46 | 0.93 | .10 |
| ACEi/ARB and HCTZ∗ | −5.47 | −10.9 | −0.001 | .05 |
| All patients combined | ||||
| ACEi/ARB and HCTZ∗ | −3.16 | −6.22 | −0.10 | .04 |
| BBs and HCTZ∗ | −5.05 | −9.61 | −0.48 | .03 |
| . | Estimate . | 95% CI . | P value . | |
|---|---|---|---|---|
| Lower . | Higher . | |||
| Prior-HTN | ||||
| ACEi/ARB | −1.30 | −4.74 | 2.13 | .45 |
| BBs | −2.06 | −5.10 | 0.97 | .18 |
| CCBs | 2.33 | −0.74 | 5.41 | .13 |
| HCTZ | −1.17 | −4.10 | 1.76 | .43 |
| BBs and HCTZ∗ | −5.05 | −10.0 | −0.0596 | .047 |
| de novo HTN | ||||
| ACEi/ARB | −0.13 | −3.57 | 3.31 | .94 |
| BBs | −2.71 | −6.88 | 1.45 | .20 |
| CCBs | 0.11 | −5.66 | 5.90 | .96 |
| HCTZ | −4.26 | −9.46 | 0.93 | .10 |
| ACEi/ARB and HCTZ∗ | −5.47 | −10.9 | −0.001 | .05 |
| All patients combined | ||||
| ACEi/ARB and HCTZ∗ | −3.16 | −6.22 | −0.10 | .04 |
| BBs and HCTZ∗ | −5.05 | −9.61 | −0.48 | .03 |
Bold values indicate statistically significant findings.
BP combination was statistically significant after controlling for the possible effects of a single drug class.
MAP reductions seen with an increased number of concurrent anti-HTN drugs
As shown in Table 2, 61% of patients were on >1 anti-HTN medication at any given time during follow-up. Across both pre-HTN and de novo–HTN groups, 79 patients (40%) were on a maximum of 2 anti-HTN medications, 31 patients (16%) were on 3 anti-HTN medications, and 10 patients (5%) were on ≥4 agents concurrently. Patients in the de novo group exhibited a trend of MAP reduction with an increasing number of medications and a statistically significant mean MAP reduction (−5.70 mmHg) on ≥3 anti-HTN drugs (95% CI, 9.94 to −1.46; P value = .008) when compared with MAPs during periods of no anti-HTN drug use. Patients in the pre-HTN group had a suggestive mean MAP reduction on ≥4 anti-HTN drugs (reduction of −4.81 mmHg; 95% CI, 9.93 to 0.30; P = .07) when compared with just 1 anti-HTN drug. When pre-HTN and de novo HTN groups were combined, a statistically significant mean reduction in BP was seen in patients on ≥4 agents (reduction of −6.27 mm Hg; 95% CI, 11.8 to −0.69; P = .02). Additional data pertaining to MAP reductions are available in Table 4.
Mean MAP reduction per number of BP medications
| . | Estimate . | 95% CI . | P value . | |
|---|---|---|---|---|
| Lower . | Higher . | |||
| Prior HTN | ||||
| 2 BP medications | −1.44 | −4.72 | 1.84 | .39 |
| 3 BP medications | −0.23 | −4.70 | 4.23 | .91 |
| ≥4 BP medications | −4.81 | −9.93 | 0.30 | .07 |
| de novo HTN | ||||
| 1 BP medication | −2.08 | −5.65 | 1.49 | .25 |
| 2 BP medications | −2.41 | −6.43 | 1.61 | .24 |
| ≥3 BP medications | −5.70 | −9.94 | −1.46 | .008 |
| All patients combined | ||||
| 1 BP medication | −1.06 | −4.50 | 2.38 | .54 |
| 2 BP medications | −2.28 | −5.93 | 1.36 | .22 |
| 3 BP medications | −1.60 | −6.11 | 2.90 | .48 |
| ≥4 BP medications | −6.27 | −11.8 | −0.69 | .02 |
| . | Estimate . | 95% CI . | P value . | |
|---|---|---|---|---|
| Lower . | Higher . | |||
| Prior HTN | ||||
| 2 BP medications | −1.44 | −4.72 | 1.84 | .39 |
| 3 BP medications | −0.23 | −4.70 | 4.23 | .91 |
| ≥4 BP medications | −4.81 | −9.93 | 0.30 | .07 |
| de novo HTN | ||||
| 1 BP medication | −2.08 | −5.65 | 1.49 | .25 |
| 2 BP medications | −2.41 | −6.43 | 1.61 | .24 |
| ≥3 BP medications | −5.70 | −9.94 | −1.46 | .008 |
| All patients combined | ||||
| 1 BP medication | −1.06 | −4.50 | 2.38 | .54 |
| 2 BP medications | −2.28 | −5.93 | 1.36 | .22 |
| 3 BP medications | −1.60 | −6.11 | 2.90 | .48 |
| ≥4 BP medications | −6.27 | −11.8 | −0.69 | .02 |
Percentage of normotensive MAPs with specific anti-HTN drugs and combinations
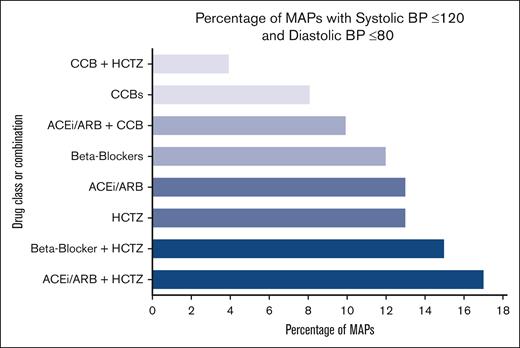
Both anti-HTN combinations that provided statistically significant mean MAP reductions in all patients also led to the greatest percentage of normotensive MAPs, as defined by a systolic BP of ≤120 and a diastolic BP of ≤80. The definition of normotension was pulled from standard guidelines written for the American College of Cardiology/American Heart Association.21 As shown in Figure 3, 17% of the MAPs collected for patients on an ACEi/ARB and HCTZ were in the normotensive range, the most of any single drug or combination of drugs used alone or with other agents. Second only to the combination of ACEis/ARBs with HCTZ, BBs plus HCTZ led to MAPS that were 15% normotensive.
Percentage of normotensive MAPs with specific BP medications/combinations in all patients.
Percentage of normotensive MAPs with specific BP medications/combinations in all patients.
Discussion
In this retrospective study evaluating the efficacy of different anti-HTN drug classes in patients with HTN diagnosed before and after ibrutinib initiation, the greatest PB reductions were seen in patients on combination therapies. Combination regimens with ≥2 drugs intuitively seemed to provide the best control, but 2 specific combination regimens stood out once we controlled for the effects of all agents: BBs plus HCTZ provided a statistically significant mean MAP reduction in patients with prior-HTN, and ACEi/ARBs plus HCTZ provided a statistically significant mean MAP reduction in patients with de novo HTN. In all patients, these 2 combination regimens provided the greatest percentages of normotensive MAPs. Although prospective data will be needed to confirm whether these findings should inform formal guidelines on BTKi-related HTN, these data are hypothesis generating in identifying effective classes and combination regimens that appear to have real value in patients with BTKi-associated HTN.10,15,20,22
Multiple studies have shown a relationship between HTN and an increased risk of MACEs such as myocardial infarctions, stroke, heart failure, arrhythmias, and cardiac death, highlighting critical motivation for identifying optimal therapies to control HTN in the setting of BTKi use. Dickerson et al used multivariate regression to show that new or worsened HTN was associated with an increased risk of MACEs (hazard ratio, 2.17); however, initiation of an anti-HTN medication mitigated this risk (hazard ratio, 0.40).14 More generally, a large body of literature unequivocally substantiates the claim that a person’s life-time risk of developing cardiovascular disease is significantly higher if they have longstanding, severe HTN. In a study from the INTERHEART group, HTN was responsible for 18% of the population-attributable risk of a first myocardial infarction.23
Nearly 90% of the patients in our study were treated with ibrutinib, in large part because the US Food and Drug Administration (FDA) had not yet approved acalabrutinib or zanubrutinib for patients with CLL before our data cut-off in 2018. Acalabrutinib received FDA approval for MCL and CLL in 2017 and 2019, respectively. Zanubrutinib received FDA approval for MCL in 2019 and for CLL in 2023. Although second-generation BTKis like zanubrutinib and acalabrutinib are considered more targeted and better tolerated than ibrutinib, data suggest that patients taking second-generation BTKis still face an increased risk of MACE.24-26 Chen et al found a 27% increase in the risk of MACE for each 5-mmHg increase in systolic BP (P < .001) in patients on acalabrutinib.11 As in our study, Chen et al also found that individual anti-HTN drug classes (include ACEis, ARBs, BBs, CCBs, and diuretics) provided equivalent BP control, whereas combination therapy with >1 drug class seemed to provide a superior −3.32 mmHg-reduction in systolic BP.11 We hope that future studies replicate our methods with second generation and noncovalent BTKis so as to identify the combination regimens that might provide the most benefit.
The 2017 multisociety HTN guidelines do not have specific recommendations for patients on BTKis but do note that it would not be unusual for progressive HTN to prompt the initiation or intensification of antihypertensive therapy in those on tyrosine kinase inhibitors such as sunitinib or sorafenib.21 For the general population with HTN, treatment with thiazide diuretics, an ACEi/ARB, and/or CCB is recommended. Many patients will require ≥2 drugs from different pharmacologic classes to reach BP goals, which is consistent with our current study. Agents from different classes may even have a synergistic effect like the effect between thiazides, which inadvertently stimulate the renin-angiotensin-aldosterone system, and ACEi/ARBs, which counteract this compensatory mechanism. Perhaps this is why the combination of ACEi/ARBs with HCTZ was a particularly effective combination for patients with de novo HTN in our study. When patients require ≥2 medications to manage HTN, consultation with a cardiologist should be considered for increased monitoring and to discuss the next best anti-HTN agent should the patient need 3 drugs.
The European Society of Cardiology 2022 cardio-oncology guidelines recognize the risk of arterial HTN in patients treated with ibrutinib, along with other factors that may affect patients with lymphoid malignancies, such as stress, pain, steroid use, renal impairment, and reduced exercise.27 Recommendations for antihypertensive therapy broadly encompass patients on any type of cancer treatment and suggest that an ACEi or ARB should be used as a first-line treatment with the addition of a dihydropyridine CCB for those with a systolic BP of ≥160 mmHg and diastolic BP of ≥100mm Hg. BBs may be especially effective in patients with cancer with evidence of high sympathetic tone, stress, and/or pain.
Ultimately, larger prospective studies are needed to devise formal guidelines that aid clinicians in choosing optimal anti-HTN medications for patients on ibrutinib or second-generation BTKis, especially because the median duration of ibrutinib treatment was 74 months during the 8-year follow-up from the RESONATE-2 study.28 Given expectations for prolonged therapy, it would be useful to know if and when HTN resolves after a BTKi is reduced or discontinued in patients with de novo HTN. Our study may have contributed to current knowledge of BTKi-associated HTN by identifying specific combination therapies that may exhibit efficacy in this setting; but, even here, we noticed a relatively low percentage of normotensive MAPs in patients on the best drug combinations, proving that there’s room for improvement in all facets of our approach to BTKi-associated HTN.
We also acknowledge that comorbidities may drive the selection of certain anti-HTN agents when supportive data are convincing. For example, an ACEi/ARB and a BB are likely to be chosen in patients with a recent myocardial infarction or a diagnosis of heart failure because these agents are known to reduce morbidity and mortality.21,29 Some physicians may reach for ACEis/ARBs in all patients with chronic kidney disease because research shows a reduced risk of progression to end-stage kidney disease in patients with severely increased albuminuria.30 An ACEi/ARB is also an appropriate first-line choice in a patient with diabetes mellitus and excessive albuminuria; whereas thiazide diuretics have an adverse effect on glucose metabolism.31 Diuretics are typically avoided in patients susceptible to orthostatic hypotension, and BBs or CCBs tend to double as a rate-control strategy for patients with atrial fibrillation.
Lastly, our data show that CCBs are not associated with a statistically significant MAP change either as monotherapy or when combined with other anti-HTN drugs. There is even a suggestion that CCBs may contribute to higher MAPs in patients on therapy to reduce BTKi-induced HTN. This phenomenon deserves further investigation, especially because CCBs are commonly used. Tang et al has gone as far as suggesting that ACEis and ARBs might be considered a first-line treatment option for BTKi-induced HTN, in part because they lack drug–drug interactions with BTKis.32 However, caution is still warranted when using ACEis, especially because Munir et al found that prior use of an ACEi was correlated with a risk of sudden or cardiac death in patients receiving ibrutinib and rituximab during the phase 3 FLAIR trial (relative risk, 50.2; 95% CI, 6.3-399; P < .0001).33
Limitations
Our study had several limitations beyond the constraints of a small sample size, retrospective data, and relatively few patients on second-generation BTKis. First, for the prior-HTN cohort, we did not evaluate the duration or severity of HTN before the index date. This means that we could not evaluate whether starting a BTKi made a patient’s known HTN significantly worse. Furthermore, any possible worsening of a patient’s HTN seen immediately after BTKi initiation might have been the result of stopping or reducing an anti-HTN medication immediately before the index date, and not actually attributable to a BTKi.
Although this study evaluates HTN management using a large-scale statistical model, it fails to provide details about decisions made between patients and providers to start specific anti-HTN medications or dose reduction of a BTKi. As such, we do not know how many patients started a BTKi at a reduced dose. We also do not know how often decisions to add or modify anti-HTN therapies hinged primarily on more aggressive treatment of cardiovascular risk factors or comorbidities like chronic kidney disease.
Additionally, patients were only included if they had at least 3 months of real-world MAP data, but there were no stipulations on how many BP measurements would be available during his period or any other interval. Large variations in BP data are easily evident in both Figures 1 and 2. Lastly, as with the available data from clinical trials, all the BP measurements used in this study came from clinic visits. Given our expanding knowledge of real-world BP variations and the limitations of clinic measurements, we hope that wearable technologies and other reliable methods of acquiring real-world BP data may inform future studies on BTKi-induced HTN.
Conclusion
Treatment of HTN in patients taking ibrutinib is challenging, but combination therapies appear to be necessary in patients diagnosed with HTN before and after BTKi initiation. Patients with prior-HTN appear to benefit from combination regimens with BBs and HCTZ, whereas patients with de novo HTN appear to benefit from ACEi/ARBs with HCTZ. Large prospective studies are needed to formulate formal guidelines on the best anti-HTN regimens to use in patients taking a BTKi.
Acknowledgments
This study was supported by research funding from AstraZeneca to the Fred Hutchinson Cancer Center where the collection, management, analysis, and interpretation of data were conducted. This work was also supported, in part, by the National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748 and the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number T32HL007093. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: L.S. completed this manuscript, which was edited by all other listed authors; M.S. led data collection and analysis efforts at the Fred Hutchinson Cancer Center; J.V. and T.G. performed the statistical analysis for this project; and all other authors not specifically listed were involved in study design, data collection, interpretation of data, and reviewing and editing the manuscript.
Conflict-of-interest disclosure: Although the collection, management, analysis, and interpretation of data included in this manuscript was conducted at the Fred Hutchinson Cancer Center, funding was provided externally by AstraZeneca.
Correspondence: Mazyar Shadman, Clinical Research Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109; email: mshadman@fredhutch.org.
References
Author notes
Data are available on request from the corresponding author, Mazyar Shadman (mshadman@fredhutch.org).