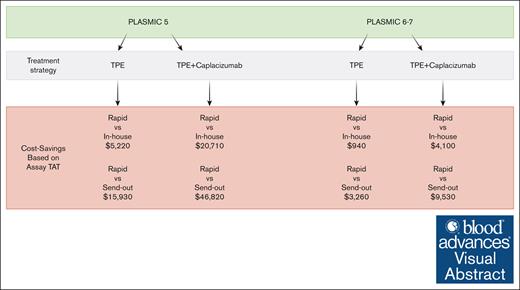
Rapid ADAMTS13 assay utilization is a cost-saving strategy in the care of patients with intermediate and high PLASMIC scores.
Cost savings with the rapid ADAMTS13 assay are greatest in the context of empiric treatment with caplacizumab.
Visual Abstract
While awaiting confirmatory results, empiric therapy for patients suspected to have immune thrombotic thrombocytopenic purpura (iTTP) provides benefits and also accrues risks and costs. Rapid assays for ADAMTS13 may be able to avoid the cost and risk exposure associated with empiric treatment. We conducted, to our knowledge, the first cost-effectiveness evaluation of testing strategies with rapid vs traditional ADAMTS13 assays in patients with intermediate- to high-risk PLASMIC scores, with and without caplacizumab use. We built a Markov cohort simulation with 4 clinical base-case analyses: (1) intermediate-risk PLASMIC score with caplacizumab; (2) intermediate-risk PLASMIC score without caplacizumab; (3) high-risk PLASMIC score with caplacizumab; and (4) high-risk PLASMIC score without caplacizumab. Each of these evaluated 3 testing strategies: (1) rapid assay (<1-hour turnaround); (2) in-house fluorescence resonance energy transfer (FRET)–based assay (24-hour turnaround); and (3) send-out FRET-based assay (72-hour turnaround). The primary outcome was the incremental net monetary benefit reported over a 3-day time horizon and across accepted willingness-to-pay thresholds in US dollars per quality-adjusted life-year (QALY). While accruing the same amount of QALYs, the rapid assay strategy saved up to $46 820 (95% CI, $41 961-$52 486) per patient tested. No parameter variation changed the outcome. In probabilistic sensitivity analyses, the rapid assay strategy was favored in 100% (3 base cases and scenario analyses) and 99% (1 base-case and scenario analysis) across 100 000 Monte Carlo iterations within each. Rapid ADAMTS13 testing for patients with intermediate- or high-risk PLASMIC scores yields significant per patient cost savings, achieved by reducing the costs associated with unnecessary therapeutic plasma exchange and caplacizumab therapy in patients without iTTP.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is a life-threatening thrombotic microangiopathy requiring immediate treatment to prevent progressive end organ damage and death.1 The annual incidence is 3 per million patients, with an approximate median age at presentation of 40 years.2 Acute iTTP is the result of severe deficiency in the metalloproteinase that cleaves von Willebrand factor, ADAMTS13, which results in the persistence of large von Willebrand factor multimers and formation of platelet-rich microthrombi. The diagnosis depends on clinical expertise to adjudicate a prior probability, the diagnostic assay result, and the ability to interpret these results in the context of prior and known test characteristics. The diagnosis is confirmed by ADAMTS13 activity <10%. However, the turnaround time (TAT) for ADAMTS13 activity testing is several days. Therefore, patients with an intermediate or high pretest probability of iTTP are often started on empiric treatment while awaiting diagnostic confirmation.3,4 Various clinical risk scores were developed to identify patients most likely to have iTTP. Two clinical tools used in practice include the PLASMIC and French scores.5,6
Current testing for ADAMTS13 activity includes the fluorescence resonance energy transfer (FRET)–based assay and chromogenic enzyme-linked immunosorbent assay.7 Concerningly, results can take days due to assay complexity, need for in-house trained technicians, and test batching.7,8 Additionally, many hospitals send out their testing to a reference laboratory, which further increases the duration of time until results are available. Two prior studies examined the use of various assays in iTTP and their cost-effectiveness. One found that using PLASMIC score in addition to in-house testing was cost-effective, whereas the other noted a shorter TAT of 1 day to be cost-effective.9,10 However, despite these cost-effectiveness analyses showing that a TAT of 1 day would be optimal, the assays remain the same, thus diagnostic delays are common. To help overcome these delays, newer assays focused on rapid TAT have been developed. For example, the rapid HemosIL AcuStar ADAMTS13 activity assay is a fully automated, chemiluminescent assay with an analytic TAT of <1 hour, which has demonstrated high concordance with chromogenic enzyme-linked immunosorbent assays and FRET-based assays.11-15 The rapid assay requires less resources in person-time in contrast to other assays (ie, Immucor, recently acquired by Werfen) that require multiple hour assay run times with technician requirement for monitoring and adjustment.
To our knowledge, no cost-effectiveness analysis has evaluated the utility of the new rapid assay with TAT <1 hour, in addition to varied PLASMIC scores at intermediate and high risks, to see which assay would constitute the cost-effective diagnostic strategy. Additionally, the cost-effectiveness of a rapid diagnostic strategy in the context of caplacizumab use for both intermediate- and high-probability PLASMIC scores, compared with that of without caplacizumab, is not known. We sought to fill these gaps by determining the cost-effectiveness of a rapid assay compared with an in-house test with a 24-hour TAT and a send-out test with a 72-hour TAT for these strategies: (1) intermediate- (PLASMIC 5) vs high-probability (PLASMIC 6-7) PLASMIC scores; and (2) patients treated empirically with therapeutic plasma exchange (TPE) with or without caplacizumab. We sought to determine whether the rapid assay would be worth investing in, should it present cost savings due to the rapid TAT. Our hypothesis was that the use of a rapid assay as part of a diagnostic and management strategy would be cost-effective, compared with traditional TAT test strategies.
Methods
Model overview
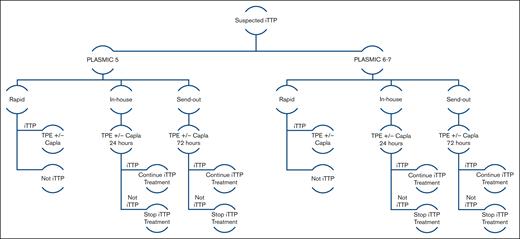
We built a Markov cohort model to determine the cost-effectiveness of 3 different ADAMTS13 assay testing strategies used in the care of adult patients with intermediate (PLASMIC score = 5) and high (PLASMIC score = 6-7) pretest probability for iTTP. Our model evaluated inpatient costs associated with suspected iTTP. Our model examined 3 testing strategies: (1) rapid turnaround (<1 hour); (2) 24-hour in-house, FRET-based evaluation; and (3) 72-hour send-out, FRET-based evaluation. For in-house and send-out diagnostic strategies, the patient proceeds with empiric treatment with TPE with or without caplacizumab until assay results return, at 24 and 72 hours, respectively (Figure 1). The study population were those patients presenting to a hospital with thrombotic microangiopathy and concern for iTTP. For the 24-hour in-house and the 72-hour send-out strategies, the shortest TAT was used to have each assay option accrue the least amount of therapy-related cost while waiting for results. Using the lowest bound of TAT allowed for us to estimate the most conservative potential benefit of a rapid testing strategy, favoring the null hypothesis. For the rapid assay testing strategy, a patient is started on treatment only if the diagnosis is confirmed and not started on treatment if iTTP diagnosis is ruled out. For the in-house and send-out assay testing strategies, the patient is empirically started on treatment while awaiting assay results. For patients in whom iTTP is ruled out upon receipt of ADAMTS13 activity level, empiric therapy is discontinued. Age- and sex-adjusted background mortality were used. Rituximab was not used in this model because it is typically initiated once confirmatory results of severe ADAMTS13 deficiency are available. This model characteristic additionally also favors the null hypothesis (ie, if rituximab were to be used empirically, then further costs and risks would accrue with in-house and send-out testing, compared with the rapid testing strategy). We constructed our model using TreeAge Pro Healthcare 2023 (TreeAge Software). The Consolidated Health Economic Evaluation Reporting Standards reporting guideline was implemented where applicable.
Markov model schematic of the 3 assay TATs (rapid, in-house, and send-out). All patients (PLASMIC = 5 or PLASMIC = 6-7) undergo treatment with TPE with or without caplacizumab. Unlike the rapid strategy, this treatment is empiric with in-house and send-out testing until 24 and 72 hours, respectively, after which only treatment for patients with confirmed iTTP is continued. Capla, caplacizumab.
Markov model schematic of the 3 assay TATs (rapid, in-house, and send-out). All patients (PLASMIC = 5 or PLASMIC = 6-7) undergo treatment with TPE with or without caplacizumab. Unlike the rapid strategy, this treatment is empiric with in-house and send-out testing until 24 and 72 hours, respectively, after which only treatment for patients with confirmed iTTP is continued. Capla, caplacizumab.
Model assumptions
The analytic time horizon was set at 3 days to analyze the impact of waiting up to 72 hours for assay results (ie, in the send-out testing strategy). We assumed all patients with an appropriately applied and calculated PLASMIC score of 5 for intermediate-risk (or 6-7 for high-risk) were hospitalized in the intensive care unit (ICU),16 rather than the general medicine floor, for the first 3 days of empiric treatment for an incident diagnosis. We assumed that the rapid assays, given concordance in prior studies, had similar diagnostic accuracy to current FRET-based assays.11-15 As a conservative assumption to favor the null hypothesis, we also assumed that earlier adjudication of the diagnosis (ie, with rapid testing) does not improve iTTP-related mortality or morbidity for patients over the 3-day time horizon.
Model input parameters
For transition probabilities, our model was informed by extensive literature published on clinical risk score characteristics, informed by the derivation and validation cohorts for the PLASMIC score (Table 1). All probabilities were converted to daily rates before converting back to probabilities, using the recommended transformation formula: p = 1-expˆ(-r∗t), in which p = probability, r = rate, and t = time.17 During each daily cycle, patients may experience adverse events from TPE, including transfusion-related lung injury, anaphylaxis, transfusion-associated circulatory overload, central line thrombosis, and central line infection; whereas for caplacizumab, these additionally include bleeding risk. The daily bleed rate was determined by tabulating the number of major bleed events across all real-world studies reporting major bleeding by the International Society on Thrombosis and Haemostasis criteria and dividing by a full per-person treatment-exposure period of caplacizumab use of 35 days, corresponding to the median duration of caplacizumab use in clinical trials. Although this approach may underestimate the daily bleed rate (ie, any bleed event that happened is assumed to have accrued 35 risk-exposure days), it was a necessity due to the lack of reporting of person-risk exposure time. In this context, we preferred to underestimate (rather than overestimate) these risks to again favor the null hypothesis. In addition, because these daily bleed probabilities were expected to be very small, we did not expect them to affect model results (ie, which of the 3 strategies are the cost-effective strategy). Nevertheless, in addition to testing all parameters (ie, bleeding risk and beyond) with ±50% extensive ranges in sensitivity analyses, we also conducted a set of scenario analyses in which we nullified all bleeding risk for all 4 base cases to see whether bleeding related to caplacizumab had any effect in changing model results (ie, which strategy was the cost-effective strategy).
Base-case input parameters and their probability distributions for PSA
| Result or transition . | Input parameter . | Probability distribution . | Study or data source . |
|---|---|---|---|
| Probability of iTTP based on PLASMIC (6-7) | 0.81 | β-PERT (0.41, 1) | Bendapudi et al5 |
| Probability of iTTP based on PLASMIC (5) | 0.045 | β-PERT (0.023, 0.068) | Bendapudi et al5 |
| Daily mortality in patients with iTTP with caplacizumab and TPE (converted to daily using a conservative median per-person treatment period of 35 d) | 0.000266 | β-PERT (0.00013, 0.00040) | Peyvandi et al18 |
| Daily mortality in patients without iTTP patients with TPE | 0.00411 | β-PERT (0.0021,0.0.0062) | Li et al19 |
| Daily probability of ISTH-defined major bleeding with caplacizumab (converted to daily using a conservative median per-person treatment period of 35 d across available real-world reports) | .000592 | β-PERT (0.000296, 0.000888) | Coppo et al, Dutt et al, and Knobl et al20-22 |
| Daily probability of ICH with caplacizumab (converted to daily using a conservative median per-person treatment period of 35 d across available real-world reports) | .000423 | β-PERT (0.000212, 0.000635) | Dutt et al, Knobl et al, and Izquierdo et al21-23 |
| Mortality of (ISTH-defined) non-ICH major bleeding | 0.00440 | β-PERT (0.0022, 0.0066) | Franco et al24 |
| Mortality of ICH | 0.00758 | β-PERT (0.0038, 0.011) | Franco et al24 |
| Probability of TRALI | 0.0000833 | β-PERT (0.000042, 0.00013) | Pandey et al25 |
| Probability of TACO | 0.000639 | β-PERT (0.00032, 0.00096) | Narick et al26 |
| Daily probability of severe anaphylaxis | 0.000000606 | β-PERT (0.0000003, 0.0000009) | Som et al27 |
| Daily probability of catheter thrombosis | 0.0000106 | β-PERT (0.0000053, 0.000016) | Som et al27 |
| Daily probability of central line infection | 0.0000218 | β-PERT (0.000011, 0.000033) | Som et al27 |
| Daily mortality of TRALI | 0.00107 | β-PERT (0.00054, 0.0016) | Li et al28 |
| Daily mortality of TACO | 0.000410 | β-PERT (0.0002, 0.006) | Li et al28 |
| Daily mortality of central line infection | 0.0568 | β-PERT (0.028, 0.085) | Ziegler et al29 |
| Costs | |||
| Caplacizumab dose (11 mg) | 8 112.62 | Fixed | CMS 202330,31 |
| TPE, per session (professional and technical cost) | 5 573.47 | Gamma (36, 154.82) | Average of all 3 US reports: White et al, Connell et al, Goshua et al, and Kim et al10,31-33 |
| ICU bed per day | 1 291.3 | Gamma (36, 35.87) | Goshua et al31 |
| Rapid testing (HemosIL Acustar ADAMTS13 activity) | 546.02 | Gamma (36, 15.17) | White et al32 |
| In-house testing | 442.51 | Gamma (36, 12.29) | Kim et al9 |
| Send-out testing | 582.85 | Gamma (36, 16.19) | Kim et al9 |
| iTTP hospitalization without complications | 6 667.84 | Gamma (36, 185.22) | MS-DRG 81034 |
| iTTP hospitalization with a complication (nonmajor) | 8 482.30 | Gamma (36, 235.62) | MS-DRG 80934 |
| iTTP hospitalization with a major complication (ie, ICH) | 15 155.00 | Gamma (36, 420.97) | MS-DRG 80834 |
| Result or transition . | Input parameter . | Probability distribution . | Study or data source . |
|---|---|---|---|
| Probability of iTTP based on PLASMIC (6-7) | 0.81 | β-PERT (0.41, 1) | Bendapudi et al5 |
| Probability of iTTP based on PLASMIC (5) | 0.045 | β-PERT (0.023, 0.068) | Bendapudi et al5 |
| Daily mortality in patients with iTTP with caplacizumab and TPE (converted to daily using a conservative median per-person treatment period of 35 d) | 0.000266 | β-PERT (0.00013, 0.00040) | Peyvandi et al18 |
| Daily mortality in patients without iTTP patients with TPE | 0.00411 | β-PERT (0.0021,0.0.0062) | Li et al19 |
| Daily probability of ISTH-defined major bleeding with caplacizumab (converted to daily using a conservative median per-person treatment period of 35 d across available real-world reports) | .000592 | β-PERT (0.000296, 0.000888) | Coppo et al, Dutt et al, and Knobl et al20-22 |
| Daily probability of ICH with caplacizumab (converted to daily using a conservative median per-person treatment period of 35 d across available real-world reports) | .000423 | β-PERT (0.000212, 0.000635) | Dutt et al, Knobl et al, and Izquierdo et al21-23 |
| Mortality of (ISTH-defined) non-ICH major bleeding | 0.00440 | β-PERT (0.0022, 0.0066) | Franco et al24 |
| Mortality of ICH | 0.00758 | β-PERT (0.0038, 0.011) | Franco et al24 |
| Probability of TRALI | 0.0000833 | β-PERT (0.000042, 0.00013) | Pandey et al25 |
| Probability of TACO | 0.000639 | β-PERT (0.00032, 0.00096) | Narick et al26 |
| Daily probability of severe anaphylaxis | 0.000000606 | β-PERT (0.0000003, 0.0000009) | Som et al27 |
| Daily probability of catheter thrombosis | 0.0000106 | β-PERT (0.0000053, 0.000016) | Som et al27 |
| Daily probability of central line infection | 0.0000218 | β-PERT (0.000011, 0.000033) | Som et al27 |
| Daily mortality of TRALI | 0.00107 | β-PERT (0.00054, 0.0016) | Li et al28 |
| Daily mortality of TACO | 0.000410 | β-PERT (0.0002, 0.006) | Li et al28 |
| Daily mortality of central line infection | 0.0568 | β-PERT (0.028, 0.085) | Ziegler et al29 |
| Costs | |||
| Caplacizumab dose (11 mg) | 8 112.62 | Fixed | CMS 202330,31 |
| TPE, per session (professional and technical cost) | 5 573.47 | Gamma (36, 154.82) | Average of all 3 US reports: White et al, Connell et al, Goshua et al, and Kim et al10,31-33 |
| ICU bed per day | 1 291.3 | Gamma (36, 35.87) | Goshua et al31 |
| Rapid testing (HemosIL Acustar ADAMTS13 activity) | 546.02 | Gamma (36, 15.17) | White et al32 |
| In-house testing | 442.51 | Gamma (36, 12.29) | Kim et al9 |
| Send-out testing | 582.85 | Gamma (36, 16.19) | Kim et al9 |
| iTTP hospitalization without complications | 6 667.84 | Gamma (36, 185.22) | MS-DRG 81034 |
| iTTP hospitalization with a complication (nonmajor) | 8 482.30 | Gamma (36, 235.62) | MS-DRG 80934 |
| iTTP hospitalization with a major complication (ie, ICH) | 15 155.00 | Gamma (36, 420.97) | MS-DRG 80834 |
CMS, Centers for Medicare and Medicaid Services; ICH, intracranial hemorrhage; ISTH, International Society on Thrombosis and Haemostasis; TRALI, transfusion-related acute lung injury; TACO, transfusion-associated cirulatory overload.
Health utilities were informed by literature on critically ill adults hospitalized in the medical ICU, originally derived with validated EQ-5D methodology.35,36 Costs were estimated in 2023 US dollars, with inflation to 2023 costs using the medical component of the consumer price index.37 Medication cost (ie, caplacizumab) was obtained from the Centers for Medicare and Medicaid Services.30 For the cost of TPE, we used an average of the cost of 1 TPE session reported across all 3 studies reporting in the US context, which included both technical and professional costs.10,32,33 Baseline cost of hospitalization and treatment-related complication management were obtained from Centers for Medicare and Medicaid Services Medicare Severity Diagnosis-Related Groups (MS-DRGs) as follows in all treatment strategies: applying MS-DRG 810 for iTTP managed without complications; MS-DRG 809 for complications that include any of transfusion-associated circulatory overload, transfusion-related lung injury, anaphylaxis, nonintracranial hemorrhage major bleed, and central line complication (ie, infection or thrombosis); and MS-DRG 808 for the complication of intracranial hemorrhage (Table 1).34 The MS-DRG codes for a major hematologic diagnosis were also specified to favor the null hypothesis, with only intracranial hemorrhage qualifying for the most expensive hospitalization category that accounts for a major complication. The cost of an ICU bed was sourced from our institution’s cost reporting.31 For assay costs, the rapid HemosIL assay was sourced from the Instrumentation Laboratory,32 whereas in-house and send-out assays were sourced from prior literature using the cost from institutional reported and projected costs.9 All model parameters were subjected to extensive sensitivity analyses to elucidate whether any model parameters changed the final result in all base cases (ie, which strategy is the cost-effective strategy).
Cost-effectiveness analysis
Four base-case analyses were examined, all with TPE anchoring treatment as follows: (1) PLASMIC 5 with caplacizumab; (2) PLASMIC 5 without caplacizumab; (3) PLASMIC 6 to 7 with caplacizumab; and (4) PLASMIC 6 to 7 without caplacizumab. For completeness, the model was also applied to a PLASMIC score of 0 to 4, in which patients were not started on empiric TPE or caplacizumab. The primary outcome was the incremental cost-effectiveness ratio (ICER) for each base case, unless the intervention was cost-saving, in which case the incremental net monetary benefits (iNMBs) were reported because ICERs should not be reported in the cost-saving (ie, “dominated”) context. The iNMB is a reformulation of the ICER to present the same concept of value as an ICER and is calculated as the difference between 2 individual NMBs (hence, it is termed “incremental”), with each strategy having an NMB calculated. The individual strategy NMB is calculated for each strategy as a product of effectiveness (quality-adjusted life years [QALYs]) and the willingness-to-pay ($ per QALY), whose product is cost in $ units, and is then subtracted by the total cost of each testing strategy also in $ units, to result in a monetary value that captures a health strategy’s value in $ units. The iNMB is then calculated as the difference (ie, “incremental”) between individual strategy NMBs. To minimize emphasis on any 1 willingness-to-pay (WTP) point estimate, most recently derived to be $104 000 per QALY (95% uncertainty interval, $51 000-$209 000 per QALY) in the US context,38 we used the full range of accepted willingness-to-pay thresholds in the United States, reporting model outputs across WTPs of $50 000 per QALY, $100 000 per QALY, and $150 000 per QALY.
Sensitivity and scenario analyses
To determine how sensitive our results were to all the individual and collective parameter uncertainties, we performed extensive, deterministic, and probabilistic sensitivity analyses (PSAs), varying all input parameter estimates ±50% (Table 1). We performed PSA across >100 000 Monte Carlo simulations using (β)-PERT distributions for transition probabilities and utilities, as well as (γ) distributions for costs. In addition, in scenario analyses for each base case, we nullified the possibility of any bleeding events with caplacizumab to prove that the small (ie, daily) probability of bleeding does not affect the model’s result (ie, which strategy is the cost-effective strategy).
Results
Base case
The estimated cost, QALYs, and iNMBs with 95% credible intervals (CIs) across all 3 testing strategies are reported in Table 2. Across all 4 base-case analyses, the rapid testing strategy with rapid assay was cost-saving (ie, better than cost-effective) while accruing the same amount of QALYs (Table 2). The savings with rapid assay use vs send-out testing with a 72-hour TAT per-patient-tested ranged from $3260 (95% CI, $739-$8190; PLASMIC 6-7, without caplacizumab) to $46 820 (95% CI, $41 960-$52 490; PLASMIC 5, with caplacizumab); and from $940 (95% CI, $90-$2630) to $20 710 (95% CI, $19 050-$22 630) vs in-house testing with a 24-hour TAT. When looking specifically at a PLASMIC score of 0 to 4, the costs of the strategies were directly related to assay cost variation with results demonstrating: rapid, $8510; in-house, $8400; and send-out, $8540. Notably, even when looking at the most modest per-person benefit base case (ie, rapid vs in-house testing, without caplacizumab use), proportionally weighted across the population of patients in PLASMIC derivation and validation cohorts, the per-person savings for rapid testing in a cohort of patients with thrombotic microangiopathy would be ∼$1300 savings per patient tested (regardless of PLASMIC score).
iNMB per patient tested and empirically treated for iTTP for rapid (less than 1 hour) vs send-out (72 hours) and rapid (less than 1 hour) vs in-house (24 hours) testing over a 3-day time horizon
| Testing Strategy Comparison . | Cost (USD) . | Effectiveness (QALYs) . | Base-case iNMB (USD) (95% credible interval) . | Scenario iNMB (95% credible interval) . |
|---|---|---|---|---|
| PLASMIC = 5 (intermediate), TPE with caplacizumab | ||||
| Rapid vs send-out | 13 310 vs 60 130 | 0.0051 | 46 820 (41 960-52 490) | 46 810 (41 950-52 480) |
| Rapid vs in-house | 13 310 vs 34 020 | 0.0051 | 20 710 (19 050-22 630) | 20 700 (19 040-22 620) |
| PLASMIC = 5 (intermediate), TPE without caplacizumab | ||||
| Rapid vs send-out | 11 830 vs 27 770 | 0.0051 | 15 930 (11 130-21 530) | 15 930 (11 160-21 570) |
| Rapid vs in-house | 11 830 vs 17 050 | 0.0051 | 5 220 (3 590-7 100) | 5 220 (3 600-7 110) |
| PLASMIC = 6 or 7 (high), TPE with caplacizumab | ||||
| Rapid vs send-out | 50 960 vs 60 220 | 0.0051 | 9 530 (2 210-22 540) | 9 530 (2 210-22 540) |
| Rapid vs in-house | 50 960 vs 54 790 | 0.0051 | 4 100 (830-9 870) | 4 100 (830-9 970) |
| PLASMIC = 6 or 7 (high), TPE without caplacizumab | ||||
| Rapid vs send-out | 24 550 vs 21 810 | 0.0051 | 3 260 (730-8 190) | 3 260 (730-8 190) |
| Rapid vs in-house | 24 550 vs 25 510 | 0.0051 | 940 (90-2 630) | 940 (90-2 630) |
| Testing Strategy Comparison . | Cost (USD) . | Effectiveness (QALYs) . | Base-case iNMB (USD) (95% credible interval) . | Scenario iNMB (95% credible interval) . |
|---|---|---|---|---|
| PLASMIC = 5 (intermediate), TPE with caplacizumab | ||||
| Rapid vs send-out | 13 310 vs 60 130 | 0.0051 | 46 820 (41 960-52 490) | 46 810 (41 950-52 480) |
| Rapid vs in-house | 13 310 vs 34 020 | 0.0051 | 20 710 (19 050-22 630) | 20 700 (19 040-22 620) |
| PLASMIC = 5 (intermediate), TPE without caplacizumab | ||||
| Rapid vs send-out | 11 830 vs 27 770 | 0.0051 | 15 930 (11 130-21 530) | 15 930 (11 160-21 570) |
| Rapid vs in-house | 11 830 vs 17 050 | 0.0051 | 5 220 (3 590-7 100) | 5 220 (3 600-7 110) |
| PLASMIC = 6 or 7 (high), TPE with caplacizumab | ||||
| Rapid vs send-out | 50 960 vs 60 220 | 0.0051 | 9 530 (2 210-22 540) | 9 530 (2 210-22 540) |
| Rapid vs in-house | 50 960 vs 54 790 | 0.0051 | 4 100 (830-9 870) | 4 100 (830-9 970) |
| PLASMIC = 6 or 7 (high), TPE without caplacizumab | ||||
| Rapid vs send-out | 24 550 vs 21 810 | 0.0051 | 3 260 (730-8 190) | 3 260 (730-8 190) |
| Rapid vs in-house | 24 550 vs 25 510 | 0.0051 | 940 (90-2 630) | 940 (90-2 630) |
iNMB calculated for all scenario analyses (last column). All point estimates rounded to maximum 4 significant digits.
USD, US dollar.
Sensitivity analyses
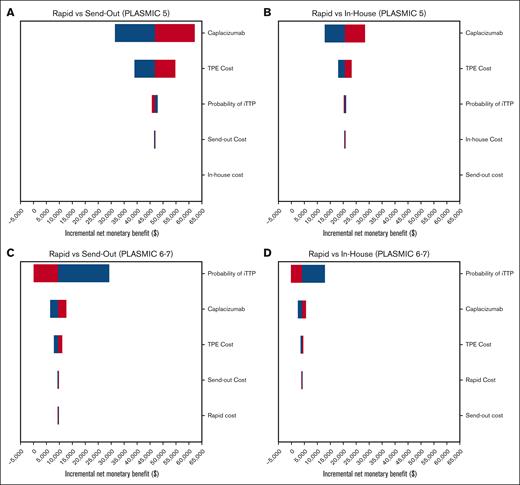
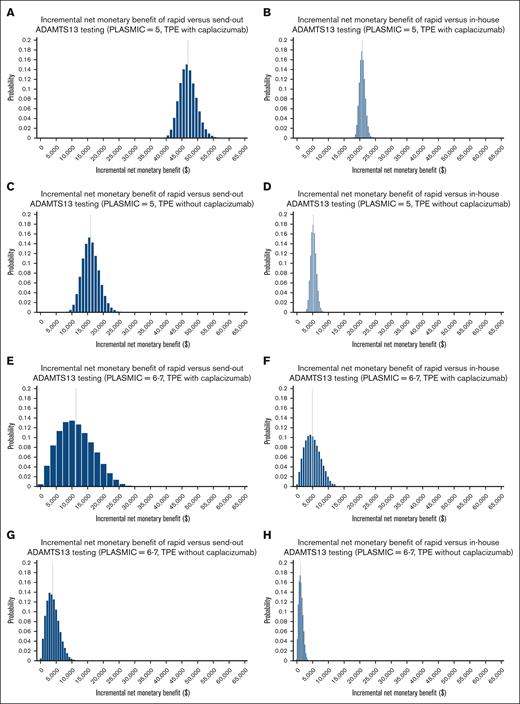
The top 5 parameters that all models were most sensitive to were: caplacizumab cost, TPE cost, probability of having iTTP, and cost of ADAMTS13 assays (2 assays were parameters per sensitivity analysis; Figure 2). The only parameter variation in broad sensitivity analyses that could steer the cost-effective strategy away from the rapid testing strategy occurred when the probability of iTTP with a PLASMIC 6 to 7 was at least 99.7% (for send-out vs rapid) and at least 97.1% (for in-house vs rapid). These probabilities are implausibly high compared with published cohorts and are not relevant to clinical practice.5 Nevertheless, despite including these broad ranges in PSAs and across all willingness-to-pay thresholds, the rapid assay was favored in 100% of iterations in the context of an intermediate-risk PLASMIC score (with and without caplacizumab) and 99.94% and 98.81% in the context of a high-risk PLASMIC score with and without caplacizumab use, respectively (Table 3). We additionally report PSA outputs as iNMB distributions for each strategy compared with rapid testing for all intermediate- and high-risk PLASMIC scores empirically treated with TPE, with and without caplacizumab use, generating all 8 distributions (Figure 3). An iNMB greater than $0 denotes cost-effectiveness of rapid testing vs the comparator strategy, which is visualized on the x-axis in all instances (Figure 3).
Tornado diagrams of incremental net monetary benefit. Tornado diagrams of incremental net monetary benefit for rapid vs send-out and rapid vs in-house diagnostic strategies in the PLASMIC = 5 (A and B, respectively) and PLASMIC = 6-7 (C and D, respectively) clinical contexts, with the use of caplacizumab and TPE. The x-axis is the iNMB for each pairwise strategy comparison. Positive iNMB (ie, >$0, moving right on the x-axis) favors the rapid strategy.
Tornado diagrams of incremental net monetary benefit. Tornado diagrams of incremental net monetary benefit for rapid vs send-out and rapid vs in-house diagnostic strategies in the PLASMIC = 5 (A and B, respectively) and PLASMIC = 6-7 (C and D, respectively) clinical contexts, with the use of caplacizumab and TPE. The x-axis is the iNMB for each pairwise strategy comparison. Positive iNMB (ie, >$0, moving right on the x-axis) favors the rapid strategy.
PSAs of all base-case and scenario analyses across all accepted willingness-to-pay thresholds in the United States
| Willingness-to-pay threshold (USD/QALY) . | Rapid Base-case | ∗Scenario . | In-house Base-case | ∗Scenario . | Send-out Base-case | ∗Scenario . |
|---|---|---|---|
| PLASMIC = 5 (intermediate), TPE with caplacizumab | |||
| 50 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 100 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 150 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| PLASMIC = 5 (intermediate), TPE without caplacizumab | |||
| 50 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 100 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 150 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| PLASMIC = 6 or 7 (high), TPE with caplacizumab | |||
| 50 000 | 99.94% | 99.94% | 0.06% | 0.06% | 0% | 0% |
| 100 000 | 99.94% | 99.94% | 0.06% | 0.06% | 0% | 0% |
| 150 000 | 99.94% | 99.94% | 0.06% | 0.06% | 0% | 0% |
| PLASMIC = 6 or 7 (high), TPE without caplacizumab | |||
| 50 000 | 98.81% | 98.81% | 1.19% | 1.19% | 0% | 0% |
| 100 000 | 98.81% | 98.81% | 1.19% | 1.19% | 0% | 0% |
| 150 000 | 98.81% | 98.81% | 1.19% | 1.19% | 0% | 0% |
| Willingness-to-pay threshold (USD/QALY) . | Rapid Base-case | ∗Scenario . | In-house Base-case | ∗Scenario . | Send-out Base-case | ∗Scenario . |
|---|---|---|---|
| PLASMIC = 5 (intermediate), TPE with caplacizumab | |||
| 50 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 100 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 150 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| PLASMIC = 5 (intermediate), TPE without caplacizumab | |||
| 50 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 100 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| 150 000 | 100% | 100% | 0% | 0% | 0% | 0% |
| PLASMIC = 6 or 7 (high), TPE with caplacizumab | |||
| 50 000 | 99.94% | 99.94% | 0.06% | 0.06% | 0% | 0% |
| 100 000 | 99.94% | 99.94% | 0.06% | 0.06% | 0% | 0% |
| 150 000 | 99.94% | 99.94% | 0.06% | 0.06% | 0% | 0% |
| PLASMIC = 6 or 7 (high), TPE without caplacizumab | |||
| 50 000 | 98.81% | 98.81% | 1.19% | 1.19% | 0% | 0% |
| 100 000 | 98.81% | 98.81% | 1.19% | 1.19% | 0% | 0% |
| 150 000 | 98.81% | 98.81% | 1.19% | 1.19% | 0% | 0% |
All parameters varied simultaneously over 100 000 Monte Carlo iterations in each sensitivity analysis.
USD, US dollar.
Scenario analyses: all bleeding events (and thus cost of managing them) nullified.
Probablistic histograms. Probabilistic distribution (1st to 100th percentile) of iNMB for rapid testing compared with (1) in-house and (2) send-out testing for each of 4 base-case scenarios: (i) PLASMIC = 5, TPE with caplacizumab (A and B); (ii) PLASMIC = 5, TPE without caplacizumab (C and D); (iii) PLASMIC = 6-7, TPE with caplacizumab (E and F); and (iv) PLASMIC = 6-7, TPE without caplacizumab (G and H). The x- and y-axes are aligned to be identical across all 8 distributions. Positive iNMB (ie, >$0, moving right on the x-axis) favors the rapid strategy.
Probablistic histograms. Probabilistic distribution (1st to 100th percentile) of iNMB for rapid testing compared with (1) in-house and (2) send-out testing for each of 4 base-case scenarios: (i) PLASMIC = 5, TPE with caplacizumab (A and B); (ii) PLASMIC = 5, TPE without caplacizumab (C and D); (iii) PLASMIC = 6-7, TPE with caplacizumab (E and F); and (iv) PLASMIC = 6-7, TPE without caplacizumab (G and H). The x- and y-axes are aligned to be identical across all 8 distributions. Positive iNMB (ie, >$0, moving right on the x-axis) favors the rapid strategy.
Scenario analyses
For the scenario analysis, the risk of caplacizumab-related bleeding (and its associated cost) was nullified to examine the impact on each strategy. There was no significant difference in the analysis output when bleeding costs were removed; the cost savings from the rapid assay testing strategy remained nearly identical to base-case scenarios including the cost of bleeding. For the intermediate-risk PLASMIC score with caplacizumab scenario, savings were $46 810 (95% CI, $41 950-$52 480) for rapid vs send-out and $20 700 (95% CI, $19 040-$22 620) for rapid vs in-house. For those with a high-risk PLASMIC score with caplacizumab, savings associated with rapid vs send-out and rapid vs in-house were $9530 (95% CI, $2210-$22 540) and $4100 (95% CI, $830-$9970), respectively. The conclusion in all scenario analyses remained the same; the rapid assay testing strategy is a cost-saving or cost-effective strategy in the same proportion of iterations as in the base-case analyses (Table 3).
Discussion
This study extends the literature in several ways and is, to our knowledge, the first to evaluate the potential cost savings associated with TATs of rapid (<1 hour), in-house (24 hours), and send-out (72 hours) ADAMTS13 testing in patients with intermediate and high PLASMIC scores, with and without caplacizumab use. First, regardless of the PLASMIC score, a rapid TAT diagnostic strategy was the most cost-effective strategy. In fact, it is cost-saving. Second, regardless of whether caplacizumab is used, the rapid assay remains a cost-saving option compared with traditional TATs, with more savings when caplacizumab is used. These results are achieved because the rapid testing strategy reduces the costs of unnecessary TPE and caplacizumab therapy in patients without iTTP. The highest cost savings occur in those individuals with intermediate-risk PLASMIC scores who are initiated on TPE and caplacizumab while awaiting traditional confirmatory results. When comparing the costs of the send-out vs the rapid testing strategies with intermediate- and high-risk PLASMIC scores, the cost savings are $46 820 and $9529 for every patient tested and empirically treated, respectively. These data highlight that the rapid assay testing strategy is a cost-saving strategy, irrespective of PLASMIC score (ie, intermediate or high-risk) and is greatest with caplacizumab use. The per-person cost savings are greater when caplacizumab is used empirically vs when it is not, due to medication cost averted with the rapid testing strategy. Prior studies did not examine rapid testing, intermediate- vs high-risk PLASMIC scores, nor use of caplacizumab.9,10
These results have several broader applications. The Oklahoma registry noted that there is a large gap between those with suspected TTP and those confirmed to have severe ADAMTS13 deficiency (11.29 vs 1.74 per 1 million people annually).39 Given that patients present across a variety of institutions (ie, academic, urban, and rural) with variability in available resources, rapid ADAMTS13 testing would help expedite diagnosis in all cases. The small portion of suspected thrombotic microangiopathies (TMAs) that are iTTP, as well as diagnostic uncertainties present in diagnosis, allows for the rapid, and technically simpler, assay to be crucial in expediting the care for individuals with suspected iTTP. At American centers with extensive clinical iTTP experience, there is an inability to consistently perform same-day testing, leading to diagnostic delays and cost expenditures with empiric treatment initiation.7,20,40 The rapid HemosIL assay lowers the barrier to rapid test availability, given that there is not an intensive technical component and easier technical methodology, a practical limitation of FRET-based assays at both urban and rural centers.15 Rapid results allow for a more rapid diagnostic adjudication of iTTP (ie, vs not iTTP in the thrombotic microangiopathy context), supporting the potential for an improved yield in the rapid transfer of individuals with confirmed iTTP to centers capable of TPE and further reducing time required for simple plasma infusion, which is inferior.41 The rapid TAT would also help exclude iTTP and consider alternative thrombotic microangiopathies with alternative therapies sooner. One additional benefit of rapid testing may lie in the care of older patients, who often present atypically and for whom diagnostic uncertainty can lead to treatment delays.42 Thus, institutions should consider investing in a rapid assay option to help with cost-saving and rapid triage of patients with suspected iTTP.
This study has several strengths. First, we analyzed the cost-effectiveness of diagnostic testing strategy with and without caplacizumab use, with our findings supporting the cost-saving nature regardless of caplacizumab use (and greater cost savings when caplacizumab is used vs not). Second, we performed scenario analyses to nullify the cost related to bleeding that may arise on caplacizumab to ensure that this was not significantly affecting the cost-saving results observed. With the scenario analyses, we observed no significant changes in cost savings, further supporting that cost savings observed with the rapid assay are not related to bleeding but to the cost of unnecessary caplacizumab and TPE. Third, our model structure was designed to favor the null hypothesis by analyzing the shortest TAT for the in-house and send-out test strategies, thus minimizing the accrual of additional cost (and risk) that would occur while awaiting results. Fourth, these findings are relevant to any rapid assay that is produced, with similar cost savings, unless that theoretical rapid assay is logarithmically more expensive. This can be directly calculated from our findings. Fifth, this cost-effectiveness analysis is independent of industry influence.43
This study also has limitations. First, rituximab is used as part of the initial therapy in iTTP, and it was not included as a cost in our study.40 We decided to not include it because rituximab is often not given in clinical practice unless and until severe ADAMTS13 deficiency is confirmed.44,45 It should also be noted that the additional cost (and risk) associated with empiric rituximab infusion would serve as additional cost (and risk) to be averted with the rapid testing strategy. This would in turn only strengthen our finding that the rapid turnaround testing strategy is the cost-effective strategy. Additionally, there will be initial cost in acquiring the assay to perform this rapid, fully automated testing. Although the upfront cost of purchasing assay equipment was not accounted for in our model, and we also did not include the initial cost of the equipment for in-house testing, the iNMB specific to each institution can be easily calculated by subtracting the quotient resulting from dividing the purchase price by the expected number of patients tested and empirically treated at the same institution over the course of the equipment’s warranty. In addition, we used a TAT of 24 and 72 hours for the in-house and send-out testing options, respectively. However, there are often delays beyond this timeline. Longer TATs with in-house and send-out testing would expose patients to more time facing risks of TPE and/or caplacizumab as well as their associated cost, which would in turn only strengthen our result. In fact, we see this effect in the iNMB comparisons of rapid vs in-house (1-day difference) and rapid vs send-out (3-day difference), with greater iNMB in the case of the latter than the former. Additionally, the PLASMIC score cannot be applied to pediatric patients or individuals who are pregnant, limiting the generalizability of our findings to these cohorts. However, should similar scores be developed for these populations, models could be structured to evaluate assay TAT based on the pretest probability of scoring systems for iTTP in those contexts. The impact of the pretest probability of iTTP on the negative predictive value of ADAMTS13 testing should also be considered, with a lower negative predictive value when prevalence is higher.
In summary, to our knowledge, we performed the first cost-effectiveness analysis evaluating the utilization of a rapid testing strategy compared with in-house and send-out testing strategies for the diagnostic adjudication of iTTP, in the context of both intermediate and high PLASMIC scores, and with and without empiric caplacizumab initiation. The rapid ADAMTS13 activity assay demonstrated cost savings in the care of both patients with intermediate- and high-risk PLASMIC scores, with or without the use of caplacizumab. These results are consistent across extensive sensitivity and scenario analyses. The implication of this study is that the use of rapid testing will result in overall per-person cost savings, rather than expenditures, for hospitals and health care systems. This result occurs because of speedier determination of who needs treatment for iTTP and who does not. Institutions should consider investing in the cost-saving option of a rapid turnaround assay.
Acknowledgments
G.G. is supported by The Frederick A. DeLuca Foundation and the Yale Bunker Endowment.
The funding sources had no role in the study design, collection, analysis or interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
Authorship
Contribution: C.A. and G.G. conceived the analysis, and all authors wrote and edited the manuscript.
Conflict-of-interest disclosure: H.M.K. has received expenses and/or personal fees from UnitedHealth Group, Element Science, Eyedentify, and F-Prime in the past 3 years; is a cofounder of Refactor Health and Hugo Health; and is associated with contracts through Yale New Haven Hospital from the Centers for Medicare & Medicaid Services and through Yale University from the Food and Drug Administration, Johnson & Johnson, Google, and Pfizer. A.C. has served as a consultant for MingSight, Sanofi, and Synergy, and has received authorship royalties from UpToDate. The remaining authors declare no competing financial interests.
Correspondence: George Goshua, Section of Hematology, Department of Internal Medicine, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06520; email: george.goshua@yale.edu.
References
Author notes
These data were presented as an oral presentation at the American Society of Hematology (ASH) Annual Meeting in San Diego, CA, in 2023 and were selected for presentation at the 2024 Highlights of ASH in January 2024.
Data used in this study are from publicly sourced research and outlined within the article. Data are available on request from the corresponding author, George Goshua (george.goshua@yale.edu).
The full-text version of this article contains a data supplement.