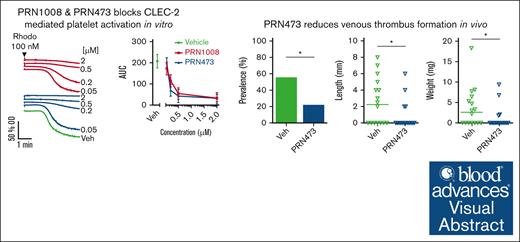
Key Points
PRN473 blocks CLEC-2–mediated platelet function in vitro and ex vivo and reduces thrombosis in 2 mouse models of venous thrombosis.
The data and lack of bleeding in PRN1008/rilzabrutinib clinical trials suggest a future use in immune-mediated thrombosis.
Visual Abstract
Platelet C-type lectin-like receptor 2 (CLEC-2) is a hem-immunoreceptor tyrosine–based activation motif-containing receptor that has a critical role in venous thrombosis but minimal involvement in hemostasis. CLEC-2 can be blocked by Btk inhibitors. Treatment with ibrutinib is associated with increased bleeding due to off-target inhibition of Src family kinases (SFKs). Patients with X-linked agammaglobulinemia (XLA) who lack Btk, however, do not bleed, suggesting selective Btk inhibition as a viable antithrombotic strategy. We assessed the effects of selective Btk inhibitors PRN1008 (rilzabrutinib) and PRN473 on platelet signaling and function mediated by CLEC-2 and glycoprotein-VI. We used healthy donors and XLA platelets to determine off-target inhibitor effects. Inferior vena cava (IVC) stenosis and Salmonella infection mouse models were used to assess antithrombotic effects of PRN473 in vivo. PRN1008 and PRN473 potently inhibited CLEC-2–mediated platelet activation to rhodocytin. No off-target inhibition of SFKs was seen. PRN1008 treatment of Btk-deficient platelets resulted in minor additional inhibition of aggregation and tyrosine phosphorylation, likely reflecting inhibition of Tec. No effect on G protein-coupled receptor-mediated platelet function was observed. PRN473 significantly reduced the number of thrombi in podoplanin-positive vessels after Salmonella infection and the presence of IVC thrombosis after vein stenosis. The potent inhibition of human platelet CLEC-2 and reduced thrombosis in in vivo models, together with the lack of off-target SFK inhibition and absence of bleeding reported in rilzabrutinib-treated patients with immune thrombocytopenia, suggest Btk inhibition as a promising antithrombotic strategy.
Introduction
Deep vein thrombosis (DVT) is increasingly being recognized as an inflammatory disorder in which the development of thrombosis is preceded by local inflammation (a phenomenon termed immunothrombosis).1 Similarly, hepatic portal vein thrombosis is a frequent complication of the continuous hepatic inflammation that occurs in patients with cirrhosis.2
The platelet receptor C-type lectin-like receptor 2 (CLEC-2) has been shown to have a critical role in inflammation-driven venous thrombosis. Mice deficient in CLEC-2 exhibit protection from portal vein thrombosis after Salmonella typhimurium infection,3 as well as in the inferior vena cava (IVC) stenosis model of DVT.4 In both models, inflammation in the vessel wall drives the upregulation of CLEC-2’s ligand podoplanin, which triggers platelet activation. Upregulation of podoplanin has also been reported in patients with hepatic thrombosis and other inflammatory liver disorders,5 as well as on the venous valves of patients with DVT.6 Importantly, CLEC-2 has been shown to have only minor or no role in bleeding.7,8 This suggests CLEC-2 blockade is a potential antithrombotic strategy for venous thrombosis with reduced bleeding side effects.
CLEC-2 is a hem-immunoreceptor tyrosine–based activation motif (ITAM)–containing receptor, signaling through Src, Syk, and Tec family kinases in a similar pathway to the other platelet ITAM-containing receptors, glycoprotein-VI (GPVI) and FcγRIIA. Inhibition of both Syk and the Tec family kinase Btk has been shown to block or delay CLEC-2, GPVI, and FcγRIIA signaling.9-13 Indeed, a lower incidence of thrombotic events has been reported in patients treated with the early-generation Btk inhibitor ibrutinib.6,14 These patients, however, also exhibit bleeding side effects, including increased rates of major hemorrhage.15,16
Patients with X-linked agammaglobulinemia (XLA), who are deficient in Btk, do not exhibit a bleeding diathesis/phenotype,17 with bleeding side effects caused by ibrutinib treatment attributed to off-target inhibition of Src family kinases.10,18 More selective Btk inhibitors should, therefore, avoid these off-target bleeding side effects but still maintain their antithrombotic effect.
PRN1008 (rilzabrutinib) is a third-generation Btk inhibitor currently undergoing phase 3 trials for the bleeding disorder immune thrombocytopenia (ITP), with no increase in bleeding reported in these patients despite their low platelet counts.19 PRN1008 and related compound PRN473 have covalent and noncovalent binding regions, enabling binding to Btk with high potency and long residence time.20,21 To a lesser degree, both agents inhibit the related kinases Tec, Txk, and Bmx22 but only have very limited binding to Src family kinases.20
In this study, we assessed the effect of Btk inhibitors PRN1008 and PRN473 on platelet signaling and function in vitro. We additionally assessed the effect of PRN473 ex vivo and in in vivo models of venous thrombosis.
Methods
Antibodies and reagents
The anti-phospho-tyrosine (4G10) monoclonal antibody (mAb) was from Millipore (Abingdon, United Kingdom). The horseradish peroxidase (HRP)-conjugated goat anti-rat immunoglobulin G (IgG; SC-2032) and anti-Syk polyclonal antibody (pAb) (SC-1077) were from Santa Cruz Biotechnology (Dallas, TX). Phospho-specific pAb against pY1217 was from Cell Signaling Technology (Hitchin, United Kingdom), against linker for activation of T cells (LAT) pY200 and Btk pY223 were from Abcam (Cambridge, United Kingdom), against Btk pY551 was from BD Biosciences (San Jose, CA), and against Src pY418 was from Life Technologies (Carlsbad, CA). Eptifibatide was from GlaxoSmithKline (Brentford, United Kingdom). The fluorescein (FITC)-conjugated rat anti-mouse P-selectin mAb and phycoerythrin (PE)-conjugated rat anti-mouse integrin αIIbβ3 mAb were from Emfret Analytics (Eibelstadt, Germany). The FITC-conjugated rat anti-mouse, HRP-conjugated anti-mouse and anti-rabbit secondary pAbs, and Hyperfilm enhanced chemiluminescence (ECL) autoradiography film were from Amersham Biosciences (GE Healthcare, Bucks, United Kingdom). The anti-hamster podoplanin primary mAb was from Invitrogen (Paisley, United Kingdom). The rat anti-mouse CD31 primary mAb was from BD Pharmigen (Wokingham, United Kingdom). The PE-conjugated rat anti-mouse CD41 mAb was from Biolegend (London, United Kingdom). The AF647-conjugated goat anti-hamster IgG secondary pAb was from Invitrogen. The FITC-conjugated goat anti-rat IgG secondary pAb was from DAKO (Hamburg, Germany). The AF568-conjugated rabbit anti-mouse annexin A5 pAb was from ThermoFisher (Waltham, MA). The AF647-conjugated rat anti-mouse CD62P mAb was from BD Pharmigen. The FITC-conjugated rabbit anti-mouse fibrinogen pAb was from DAKO. The glucose, pH 7 and pH 10 buffer solutions, Bolt running buffer, Bolt antioxidant, ECL reagent were from ThermoFisher. Rhodocytin was a gift from Johannes Eble (Münster, Germany). Collagen-related peptide (CRP) was from CambCol (Cambridge, United Kingdom). Collagen (Horm, equine tendon, 95% type I, and 5% type IV) from Takeda (Linz, Austria). Thrombin receptor activating peptide was from Severn Biotech (Kidderminster, United Kingdom). PAR4 peptide was from Alta Biosciences (Birmingham, United Kingdom). Podoplanin-Fc was made in-house in HEK 293T cells; details can be found in the supplemental Material. PRN1008 and PRN473 were from Principia Biopharma (Palo Alto, CA). Non–fatty acid–free bovine serum albumin was from First Link (UK) ltd (Birmingham, United Kingdom). ChronoLume and adenosine triphosphate (ATP) standard were from ChronoLog Corporation (Havertown, PA). Methanol and ethanol were from VWR Chemicals (Lutterworth, United Kingdom). Transblot Turbo western blotting buffer was from Bio-Rad (Kidlington, United Kingdom). All other reagents were purchased from Sigma-Aldrich (Poole, United Kingdom).
Approvals and ethics
Ethical approval for collecting blood from healthy volunteers was granted by Birmingham University Internal Ethical Review (ERN_11-0175), in accordance with the Declaration of Helsinki. Ethical approval for collection of blood from patients with XLA was granted by the North West–Haydock National Health Service (NHS) Research Ethics Committee (15/NW/0079, amendment 3).
Blood collection
Blood was taken by venepuncture into 4% sodium citrate from consenting patients or healthy, drug-free volunteers.
Human platelet preparation
Warmed acid citrate dextrose (ACD) 1:10 (volume-to-volume ratio [v/v]) was added to citrated whole blood and then centrifuged (200g for 20 minutes at room temperature), and platelet-rich plasma (PRP) was collected. PRP was then centrifuged (1000g for 10 minutes at room temperature) in the presence of 0.2 μg/mL prostacyclin, and the supernatant was discarded. The platelet pellet was resuspended in 24-mL modified Tyrode HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (134-mM NaCl, 0.34-mM Na2HPO4, 2.9-mM KCl, 12-mM NaHCO3, 20-mM HEPES, 5-mM glucose, and 1-mM MgCl2; pH 7.3) and 3-mL ACD, and then centrifuged (1000g for 10 minutes at room temperature) in the presence of 0.2 μg/mL prostacyclin. Supernatant was discarded, and the platelet pellet was resuspended in modified Tyrode HEPES buffer to the required concentration. Platelets were rested for 30 minutes before use in any experiments.
Light transmission aggregometry and granule secretion
Aggregation and ATP secretion in washed platelets (2 × 108/mL) or PRP under stirring conditions (1200 rpm) at 37°C were measured in a lumi-aggregometer (Model 700; ChronoLog, Havertown, PA) for 5 minutes. Aggregation was monitored by measuring changes in light transmission, with ChronoLume (D-luciferin–luciferase mixture) added to enable simultaneous measurement of ATP release by luminescence. Inhibitors (50 nM to 2 μM) or vehicle (dimethyl sulfoxide [DMSO]) were incubated with washed platelets or PRP for 1 hour before stimulation.
Protein phosphorylation
Washed platelets at 4 × 108/mL were pretreated with 9-μM eptifibatide to block integrin αIIbβ3 activation. Agonists were added while stirring at 1200 revolutions per minute (rpm) in an aggregometer at 37°C for 180 seconds, unless stated otherwise. Inhibitors (50 nM to 2 μM) or vehicle (DMSO) were incubated with washed platelets for 1 hour before stimulation. Activation was terminated with 5× sodium dodecyl sulfate (SDS)–reducing sample buffer. Lysates were separated by SDS–polyacrylamide gel electrophoresis, electro-transferred, and western blotted. Western blots were probed with the stated antibodies and imaged using ECL autoradiography film. For analysis of levels of phosphorylation, western blot films were scanned, and band intensities were measured using ImageJ 1.5 (National Institutes of Health), with values normalized to those seen in vehicle-treated platelets.
Podoplanin-Fc generation
Human podoplanin–Fc plasmid was made by inserting a human podoplanin extracellular domain sequence into the IgFc mammalian vector pFuse-rIgG-Fc (Invitrogen) to yield a construct encoding podoplanin fused at the COOH terminus to the Fc region of rabbit IgG1. For expression and purification of recombinant podoplanin–Fc fusion protein, 293T cells were transfected with the construct DNA by polyethylenimine method. Three days after transfection, the cell culture supernatant was harvested, filtered with a 0.22-μM filter, and then purified through a protein A agarose affinity column. The recombinant protein was then eluted by 1 M glycine solution at pH 3.0, followed by neutralization and dialysis in phosphate-buffered saline. The protein solution was aliquoted and stored at –20°C before use. The molecular weight and purity of protein were verified by SDS–polyacrylamide gel electrophoresis.
Flow adhesion
Citrated whole blood, stained with 4-μM 3,3'-dihexyloxacarbocyanine iodide (DiOC6), was perfused over podoplanin- (10 μg/mL) or collagen-coated (200 μg/mL) channels (Ibidi μ-slides VI 0.1) at venous (150 s–1) or arterial (1000 s–1) shear, respectively, for 10 minutes. Accumulation of labeled platelets was measured by taking z-stacks (41 images; step size, 0.5 μm) every 30 seconds for 2 separate locations using an Evos FL Auto imaging system (Life Technologies) using a 20× objective. Z-stacks for each condition were then analyzed using ImageJ (version 1.52; National Institutes of Health) and an in-house macro that generates platelet intensity and surface coverage of thrombi for each time point.
Citrated whole blood was thrombin inhibited (40-μM D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone [PPACK]) and recalcified (3.75-mM MgCl2 and 7.5-mM CaCl2), then perfused over fibrinogen and collagen microspots in a Maastricht flow chamber at 1000 per second. Platelets were labeled for activation markers using anti–annexin A5, anti-CD62P, and anti-fibrinogen antibodies described above. End point images were obtained on an EVOS AMF4300 microscope (Life Technologies), as described previously.23
Flow cytometry
Whole blood was diluted 1:9 (v/v) in staining solution (anti–P-selectin and anti–activated integrin αIIbβ3 antibodies in phosphate-buffered saline) and then stimulated by indicated agonists 1:10 (v/v) for 20 minutes in the dark at room temperature. Samples were then fixed with ice-cold 1% paraformaldehyde and analyzed on an Accuri C6 flow cytometer (BD Biosciences, Oxford, United Kingdom). Platelets were gated using forward and side scatter. Inhibitors (200 nM to 5 μM) or vehicle (DMSO) were incubated in whole blood for 1 hour before antibody addition and stimulation.
Animal models
All animal procedures were undertaken with UK Home Office approval, in accordance with the Animals (Scientific Procedures) Act 1986 (license numbers PP9677279, P06779746, and PC427E5DD). All experiments were performed on wild-type C57Bl6 mice.
For ex vivo assessment of platelet function, blood was taken from the IVC of mice into ACD (1:10 v/v).
Salmonella infection thrombosis model was performed as previously described.3 PRN473 pretreatment of mice was by PRN473 formulated (6.66 g/kg) diet for 7 days. Control diet was used in nontreated control animals.
PRN473- and control diet–treated mice were infected intraperitoneally with 5 × 105 attenuated S typhimurium. Mice continued on assigned diet for 7 days after infection. Tissues were then removed and immediately snap frozen. Frozen tissues were then sectioned and stained by immunohistochemistry to detect vasculature (CD31), thrombi (CD41), and podoplanin. Sections were imaged and quantified as previously described.3
IVC stenosis DVT model was performed as previously described.4 Briefly, mice were treated with 80 mg/kg PRN473 or vehicle by oral gavage once daily for 4 days before being anesthetized by isoflurane and a laparotomy performed. IVC side branches were then identified and tied off. A ligature was then placed around the IVC itself to induce stenosis, with a 30-gauge spacer used to maintain a small degree of vessel patency. The incision was closed, and mice were allowed to recover. Buprenorphine analgesia was used postoperatively. Mice were euthanized after 6 hours, and the IVC was examined for thrombus presence and size.
Btk occupancy assessment
Frozen spleen samples were thawed on ice and homogenized in Omni tubes preloaded with ceramic beads and prepared CelLytic-M plus protease/phosphatase lysis buffer using the Omni Bead Ruptor homogenizer. The homogenate was incubated on ice for complete lysis and then clarified by centrifugation. Protein concentration of the cell lysates was determined by bicinchoninic acid (BCA) assay (Thermo Fisher-Pierce) according to the manufacturer’s instructions.
Aliquots of vehicle lysate samples were treated with 1-μM PRN473 for 1 hour at room temperature for background reading. Aliquots of all vehicle plus dosed spleen lysates and background-reading vehicle lysates were then treated with biotinylated Btk probe PRN299 for 1 hour at room temperature.
The probe-treated lysates were then transferred to streptavidin-coated 96-well plates (Life Technologies) and incubated at room temperature for 1 hour for biotin capture. The captured protein in all assay wells was treated with mouse anti-human Btk Clone 53 primary antibody (BD Biosciences) overnight at 4°C. After overnight primary antibody incubation, all assay wells were washed and treated with anti-mouse HRP secondary antibody (Jackson Immuno). Peroxidase is detected by prepared SuperSignal enzyme-linked immunosorbent assay (ELSA) Femto chemiluminescent substrate (Thermo Fisher-Pierce) on the EnVision 2105 multilabel reader (Perkin Elmer).
The average of all background reading wells was calculated, then subtracted from all sample readings in the same plate. The background-subtracted vehicle and dosed readings were then used to calculate percentage occupancy for each dosed sample by dividing the dosed samples from the vehicle sample, then multiplying by 100, and subtracting from 100. The resulting percentage occupancy values were then used as a measure of the amount of PRN473 bound to target Btk enzyme in the treated samples at each time point.
Plasma PRN473 level measurement
Frozen citrated plasma samples were thawed and diluted (1:5) with 10 ng/mL acetonitrile. After centrifugation at 4000 rpm for 5 minutes, the supernatant was diluted (1:1) in 1% formic acid before being analyzed by liquid chromatography tandem mass spectrometry.
Statistical analysis
All data are presented as mean ± standard error of the mean, with statistical significance taken as P value <.05 unless otherwise stated. Graphs were plotted using GraphPad Prism 9 (GraphPad Software Inc, La Jolla, CA). Statistical analysis was performed using 1- or 2-way analysis of variance with corrections for multiple comparisons, unless otherwise stated. All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software Inc).
Results
PRN1008/473 block CLEC-2– and GPVI–mediated platelet activation
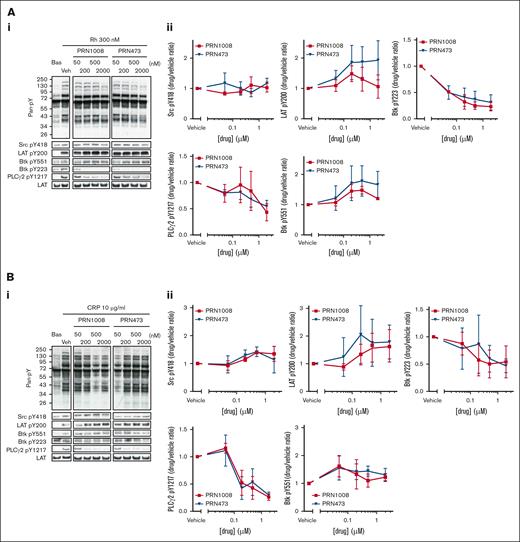
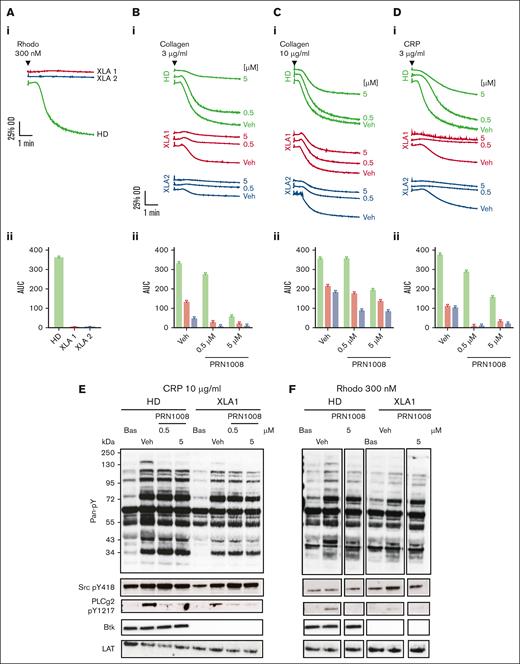
Previous studies have shown CLEC-2 downstream signaling can be blocked by low-concentration Btk inhibition with ibrutinib and acalabrutinib.11 Therefore, we assessed the effect of PRN1008 and PRN473 inhibition on tyrosine phosphorylation after activation of washed platelets with 300-nM rhodocytin. Both inhibitors blocked Btk Y223 phosphorylation and markedly reduced downstream PLCg2 Y1217 phosphorylation at concentrations >200 nM (Figure 1A). Phosphorylation of LAT Y200 and Src Y418, as well as Btk’s transphosphorylation site Y551, all of which are upstream of Btk Y223, were unaffected.
PRN1008 and PRN473 inhibit CLEC-2– and GPVI-mediated signaling. (A-B) Healthy donor–washed platelets (4 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (50, 200, 500, or 2000 nM) of Btk inhibitors PRN1008 and PRN473 for 1 hour, then stimulated with snake venom toxin rhodocytin 300 nM (A) or CRP 10 μg/mL (B) for 180 seconds in the presence of eptifibatide (9 μM) and lysed with reducing sample buffer. Whole cell lysates were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and western blotted for tyrosine phosphorylation of indicated proteins. Total LAT was used as a loading control. Representative western blots (i) and normalized densitometry quantification (ii). Mean ± standard error of the mean (SEM) of 4 identical experiments. Bas, basal; Veh, vehicle; pan-pY, panphosphotyrosine.
PRN1008 and PRN473 inhibit CLEC-2– and GPVI-mediated signaling. (A-B) Healthy donor–washed platelets (4 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (50, 200, 500, or 2000 nM) of Btk inhibitors PRN1008 and PRN473 for 1 hour, then stimulated with snake venom toxin rhodocytin 300 nM (A) or CRP 10 μg/mL (B) for 180 seconds in the presence of eptifibatide (9 μM) and lysed with reducing sample buffer. Whole cell lysates were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and western blotted for tyrosine phosphorylation of indicated proteins. Total LAT was used as a loading control. Representative western blots (i) and normalized densitometry quantification (ii). Mean ± standard error of the mean (SEM) of 4 identical experiments. Bas, basal; Veh, vehicle; pan-pY, panphosphotyrosine.
Blockade of GPVI downstream signaling has also been shown with Btk inhibition; however, loss of GPVI–mediated platelet function only occurred at higher concentrations in which off-target inhibition of Src phosphorylation was observed.10 The effect of the inhibitors on GPVI downstream signaling after activation by 10 μg/mL CRP was thus assessed (Figure 1B). Phosphorylation of Btk Y223 and PLCg2 Y1217 were again lost at concentrations >200 nM, with Btk Y551 unaffected. No off-target inhibition of Src phosphorylation was observed with either inhibitor, even with high concentrations (2 μM).
These findings show platelet CLEC-2 and GPVI signaling can be effectively blocked by Btk inhibition.
PRN1008/473 block (hem)ITAM- but not G protein-coupled receptor-mediated platelet aggregation and secretion
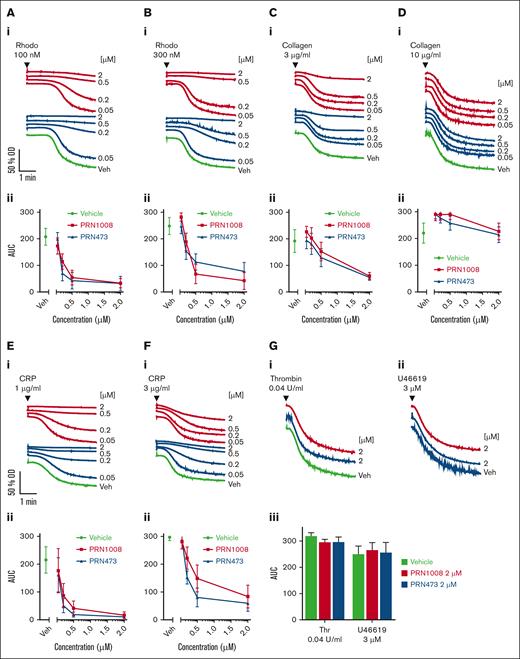
The effects of PRN1008 and PRN473 on aggregation and secretion were assessed in washed platelets. Both PRN1008 and PRN473 blocked CLEC-2–mediated platelet aggregation induced by rhodocytin (Figure 2A-B). GPVI–mediated platelet aggregation to low concentrations of CRP was also blocked; however, only minor inhibition to high concentrations of CRP and to collagen-induced aggregation was observed, with no blockade at all to high concentrations (Figure 2C-F). No effect on G protein–coupled receptor–mediated aggregation to thrombin or the thromboxane mimetic U46619 was observed with either inhibitor at high concentrations (2 μM; Figure 2G). Blockade of dense granule secretion was consistently observed at lower concentrations (>50 nM) than aggregation to (hem)ITAM receptor agonists (supplemental Figure 1). No inhibition of adenosine diphosphate (ADP)-mediated aggregation in PRP was observed with either Btk inhibitor, even at 20 μM (data not shown).
PRN1008 and PRN473 inhibit CLEC-2– and GPVI–mediated platelet aggregation. Healthy donor–washed platelets (2 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (50, 200, 500, or 2000 nM) of Btk inhibitors PRN1008 and PRN473 for 1 hour before platelet aggregation to snake venom toxin rhodocytin 100 (A) and 300 nM collagen (B); 3 (C) and 10 μg/mL CRP (D); 1 (E) and 3 μg/mL (F) or thrombin (0.04 U/mL) and thromboxane A2 mimetic U46619 (3 μM) (G) were measured by lumi-aggregometry. Representative traces (i) and quantification (ii). Mean ± SEM (n = 6). OD, optical density; Rhodo, rhodocytin; Veh, vehicle.
PRN1008 and PRN473 inhibit CLEC-2– and GPVI–mediated platelet aggregation. Healthy donor–washed platelets (2 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (50, 200, 500, or 2000 nM) of Btk inhibitors PRN1008 and PRN473 for 1 hour before platelet aggregation to snake venom toxin rhodocytin 100 (A) and 300 nM collagen (B); 3 (C) and 10 μg/mL CRP (D); 1 (E) and 3 μg/mL (F) or thrombin (0.04 U/mL) and thromboxane A2 mimetic U46619 (3 μM) (G) were measured by lumi-aggregometry. Representative traces (i) and quantification (ii). Mean ± SEM (n = 6). OD, optical density; Rhodo, rhodocytin; Veh, vehicle.
High concentrations of PRN1008/473 inhibit CLEC-2– and GPVI–mediated platelet activation in whole blood, including under flow conditions.
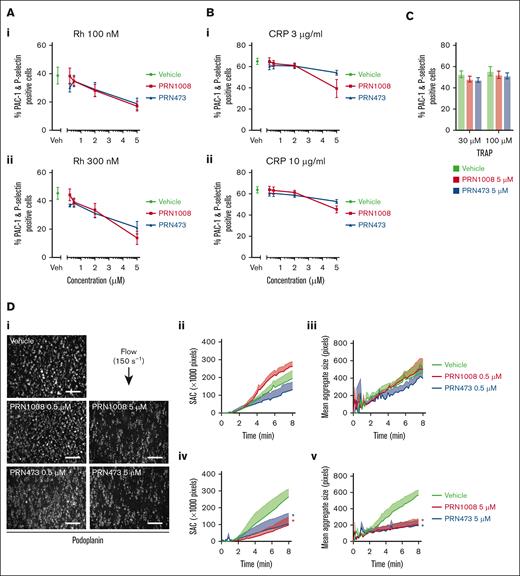
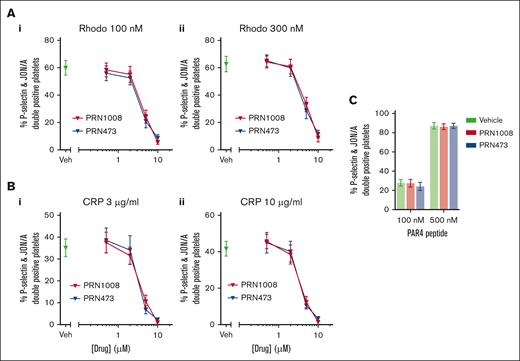
Platelet activation in response to CLEC-2 stimulation with rhodocytin was measured using flow cytometry (P-selectin and active integrin αIIbβ3 [PAC-1]) in whole blood after Btk inhibitor incubation. Strong inhibition of platelet activation was observed with 5 μM of both inhibitors (Figure 3A). CRP-induced platelet activation was also inhibited at similar concentrations; however, not as strongly as CLEC-2 (Figure 3B). No inhibition of platelet activation to thrombin receptor activating peptide was observed (Figure 3C).
PRN1008 inhibits CLEC-2– and GPVI–mediated platelet activation and CLEC-2–induced thrombus formation in whole blood. Citrated healthy donor whole blood was incubated with vehicle (0.02% DMSO) or indicated concentration (0.2, 0.5, 2, or 5 μM) of Btk inhibitors PRN1008 and PRN473 for 1 hour. Platelet activation, indicated by activated integrin αIIbβ3 (PAC-1) and P-selectin surface expression, was then assessed by flow cytometry in response to stimulation with snake venom toxin rhodocytin (100 and 300 nM) (A) or CRP (3 and 10 μg/mL) (B) or thrombin receptor activating peptide (TRAP; 30 μM and 100 uM) (C). Mean ± SEM (n = 4-8). (D) Citrated healthy donor whole blood was incubated with vehicle (0.02% DMSO) or indicated concentration (0.5 or 5 μM) of Btk inhibitors PRN1008 or PRN473 for 1 hour. Blood was then labeled with DiOC6 dye (10 minutes) and perfused over recombinant podoplanin-coated (10 μg/mL) channels at 150 per second for 8 minutes. Representative images (i); quantification of platelet surface area coverage (ii, iv); and mean aggregate size in images captured every 10 seconds (iii, v) are shown. Mean ± SEM (n = 3-4 per condition). Scale bar, 100 μm. Statistical analysis by 2-way analysis of variance (ANOVA) with Tukey correction for multiple comparisons. ∗P < .05; comparison with vehicle indicated by color.
PRN1008 inhibits CLEC-2– and GPVI–mediated platelet activation and CLEC-2–induced thrombus formation in whole blood. Citrated healthy donor whole blood was incubated with vehicle (0.02% DMSO) or indicated concentration (0.2, 0.5, 2, or 5 μM) of Btk inhibitors PRN1008 and PRN473 for 1 hour. Platelet activation, indicated by activated integrin αIIbβ3 (PAC-1) and P-selectin surface expression, was then assessed by flow cytometry in response to stimulation with snake venom toxin rhodocytin (100 and 300 nM) (A) or CRP (3 and 10 μg/mL) (B) or thrombin receptor activating peptide (TRAP; 30 μM and 100 uM) (C). Mean ± SEM (n = 4-8). (D) Citrated healthy donor whole blood was incubated with vehicle (0.02% DMSO) or indicated concentration (0.5 or 5 μM) of Btk inhibitors PRN1008 or PRN473 for 1 hour. Blood was then labeled with DiOC6 dye (10 minutes) and perfused over recombinant podoplanin-coated (10 μg/mL) channels at 150 per second for 8 minutes. Representative images (i); quantification of platelet surface area coverage (ii, iv); and mean aggregate size in images captured every 10 seconds (iii, v) are shown. Mean ± SEM (n = 3-4 per condition). Scale bar, 100 μm. Statistical analysis by 2-way analysis of variance (ANOVA) with Tukey correction for multiple comparisons. ∗P < .05; comparison with vehicle indicated by color.
We further assessed the effect of PRN1008 and PRN473 in whole blood using flow adhesion assays over recombinant podoplanin at venous shear (150 s–1) and collagen at arterial shear (1000 s–1). Significant reductions in platelet adhesion and aggregate size on podoplanin were observed at high (5 μM) but not low concentrations (500 nM) of both inhibitors, whereas high (5 μM) but not low concentrations (500 nM) of both inhibitors reduced thrombus size but not platelet adhesion on collagen (Figure 3D; supplemental Figure 2).
Platelets from patients with XLA have reduced responses to GPVI stimulation, which are further inhibited by PRN1008.
To assess whether the observed effects of the inhibitors were due solely to Btk inhibition or mediated by off-target effects, we studied washed platelets from patients with XLA using aggregometry. Platelets from these patients had no response to CLEC-2 stimulation with rhodocytin and had reduced responses to GPVI stimulation with CRP and collagen (Figure 4A-D). Addition of low (500 nM) and high (5 μM) concentrations of PRN1008 to XLA platelets had no additional inhibition with high concentrations (10 μg/mL) of collagen but did reduce the aggregation seen with low concentrations (3 μg/mL) of collagen and CRP (Figure 4B-D). We also measured PLCɣ2 phosphorylation in healthy donor and XLA platelets. Low level PLCɣ2 Y1217 phosphorylation was present in XLA platelets after GPVI stimulation with CRP, which was blocked by PRN1008. CLEC-2 stimulation with rhodocytin in the XLA platelets resulted in only very low level PLCɣ2 phosphorylation (Figure 4E-F).
PRN1008 blocks GPVI-mediated aggregation and PLCɣ2 phosphorylation in XLA platelets at low agonist concentrations of agonist. (A-D) Healthy donor and XLA washed platelets (2 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (0.5 and 5 μM) of PRN1008 for 1 hour before platelet aggregation to snake venom toxin rhodocytin 300 nM (A), collagen 3 (B) and 10 μg/mL (C), and CRP (3 μg/mL) (D) was measured by lumi-aggregometry. Representative traces (i) and quantification (ii); n = 3 for healthy donor; n = 2 for XLA. (E-F) Healthy donor and XLA washed platelets (4 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (0.5 or 5 μM) of PRN1008 for 1 hour, then stimulated with snake venom toxin rhodocytin 300 nM (E) or CRP 10 μg/mL (F) for 180 seconds in the presence of eptifibatide (9 μM) and lysed with reducing sample buffer. Whole cell lysates were then separated by SDS-PAGE and western blotted for tyrosine phosphorylation of indicated proteins. Total LAT was used as a loading control. Representative western blots (n = 1). OD, optical density; Veh, vehicle.
PRN1008 blocks GPVI-mediated aggregation and PLCɣ2 phosphorylation in XLA platelets at low agonist concentrations of agonist. (A-D) Healthy donor and XLA washed platelets (2 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (0.5 and 5 μM) of PRN1008 for 1 hour before platelet aggregation to snake venom toxin rhodocytin 300 nM (A), collagen 3 (B) and 10 μg/mL (C), and CRP (3 μg/mL) (D) was measured by lumi-aggregometry. Representative traces (i) and quantification (ii); n = 3 for healthy donor; n = 2 for XLA. (E-F) Healthy donor and XLA washed platelets (4 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (0.5 or 5 μM) of PRN1008 for 1 hour, then stimulated with snake venom toxin rhodocytin 300 nM (E) or CRP 10 μg/mL (F) for 180 seconds in the presence of eptifibatide (9 μM) and lysed with reducing sample buffer. Whole cell lysates were then separated by SDS-PAGE and western blotted for tyrosine phosphorylation of indicated proteins. Total LAT was used as a loading control. Representative western blots (n = 1). OD, optical density; Veh, vehicle.
Dietary administration of PRN473 to mice blocks CLEC-2–mediated platelet function ex vivo and venous hepatic thrombosis formation in vivo.
We have previously shown that CLEC-2 plays a critical role in mouse models of thromboinflammation3,4; therefore, we tested the effect of PRN473 in these in vivo models.
First, we confirmed inhibition of mouse platelet activation by PRN473 and PRN1008 in vitro. Strong inhibition of mouse platelet activation (P-selectin and active integrin αIIbβ3 [JON/A]) was observed at high concentrations (>5 μM) of PRN1008 and PRN473 in whole blood after CLEC-2 and GPVI stimulation (Figure 5A-B). No effect on PAR4-mediated platelet activation was seen at high concentration (5 μM; Figure 5C).
PRN1008 and PRN473 block platelet activation in mouse whole blood. (A) Mouse washed platelets (2 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (0.5, 2, 5, or 10 μM) of Btk inhibitors PRN1008 for 1 hour. Platelet activation, indicated by activated integrin αIIbβ3 (JON/A) and P-selectin surface expression, was then assessed by flow cytometry in response to stimulation with snake venom toxin rhodocytin 100 (Ai) and 300 nM (Aii) or CRP 3 (Bi) and 10 μg/mL (Bii) or PAR4 receptor activating peptide (100 or 500 nM) (C). Mean ± SEM (n = 3-6). Rhodo, rhodocytin.
PRN1008 and PRN473 block platelet activation in mouse whole blood. (A) Mouse washed platelets (2 × 108/mL) were incubated with vehicle (0.02% DMSO) or indicated concentration (0.5, 2, 5, or 10 μM) of Btk inhibitors PRN1008 for 1 hour. Platelet activation, indicated by activated integrin αIIbβ3 (JON/A) and P-selectin surface expression, was then assessed by flow cytometry in response to stimulation with snake venom toxin rhodocytin 100 (Ai) and 300 nM (Aii) or CRP 3 (Bi) and 10 μg/mL (Bii) or PAR4 receptor activating peptide (100 or 500 nM) (C). Mean ± SEM (n = 3-6). Rhodo, rhodocytin.
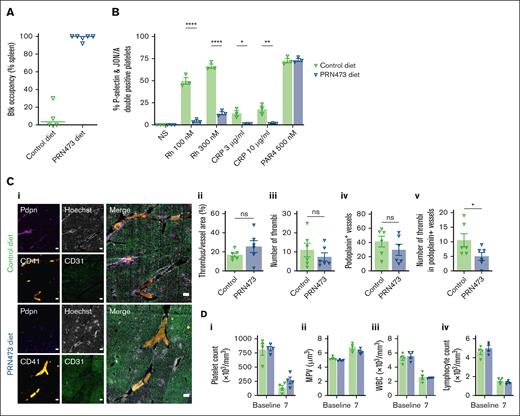
Next, we assessed the effect of dietary administration of PRN473. Mice fed diet formulated with 6.66 g/kg PRN473 for 7 days, had full or near complete Btk occupancy in blood (Figure 6A) and exhibited abrogated CLEC-2– and GPVI-mediated but normal protease-activated receptor (PAR)4-mediated responses to ex vivo platelet activation (Figure 6B). Reduced platelet adhesion, thrombus size, P-selectin, and phosphatidyl serine exposure were also observed when blood from PRN473-treated mice was flowed over Horm collagen at arterial shear (1000 s–1; supplemental Figure 3A). Reduced platelet adhesion to fibrinogen at arterial shear was also seen (supplemental Figure 3B).
PRN473 reduces Salmonella-induced liver thrombosis in mice. (A-B) WT mice were fed control or PRN473 formulated diet for 7 days, then had spleens harvested for Btk occupancy analysis (A) or citrated whole blood taken for platelet activation analysis by flow cytometry (B), indicated by activated integrin αIIbβ3 (JON/A) and P-selectin surface expression, after stimulation with snake venom toxin rhodocytin (100 and 300 nM), CRP (3 and 10 μg/mL), or PAR4 receptor activating peptide (500 nM). Mean ± SEM (n = 3). Statistical analysis by 2-way ANOVA with Sídák correction for multiple comparisons. (C) WT mice fed control or PRN473 formulated diet were infected with 5 × 105 CFU S typhimurium on day 7, with thrombi in portal vein assessed at day 14 (7 days after infection). Representative immunohistochemistry staining of frozen liver sections (scale bar, 200 μm) (i) and quantification of thrombus area per unit vessel area (ii), quantification of number of thrombi (iii), quantification of number of podoplanin-expressing vessels (iv), number of thrombi in podoplanin-positive levels (v) (n = 6). (D) Peripheral blood counts of S typhimurium–infected mice at baseline and 7 days after infection. Mean ± SEM (n = 4). (A-B) ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. CFU, colony forming units; WT, wild type.
PRN473 reduces Salmonella-induced liver thrombosis in mice. (A-B) WT mice were fed control or PRN473 formulated diet for 7 days, then had spleens harvested for Btk occupancy analysis (A) or citrated whole blood taken for platelet activation analysis by flow cytometry (B), indicated by activated integrin αIIbβ3 (JON/A) and P-selectin surface expression, after stimulation with snake venom toxin rhodocytin (100 and 300 nM), CRP (3 and 10 μg/mL), or PAR4 receptor activating peptide (500 nM). Mean ± SEM (n = 3). Statistical analysis by 2-way ANOVA with Sídák correction for multiple comparisons. (C) WT mice fed control or PRN473 formulated diet were infected with 5 × 105 CFU S typhimurium on day 7, with thrombi in portal vein assessed at day 14 (7 days after infection). Representative immunohistochemistry staining of frozen liver sections (scale bar, 200 μm) (i) and quantification of thrombus area per unit vessel area (ii), quantification of number of thrombi (iii), quantification of number of podoplanin-expressing vessels (iv), number of thrombi in podoplanin-positive levels (v) (n = 6). (D) Peripheral blood counts of S typhimurium–infected mice at baseline and 7 days after infection. Mean ± SEM (n = 4). (A-B) ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. CFU, colony forming units; WT, wild type.
To assess the effect of Btk inhibition on Salmonella infection–driven thrombosis in the liver, mice were treated with PRN473 or control diet for 7 days before being intraperitoneally injected with attenuated S typhimurium. Livers were then removed at the peak of thrombus formation 7 days after infection, and thrombus size was assessed.3 No difference in relative thrombus area between control and PRN473-treated mice was found (Figure 6Ci-ii). However, there was a trend toward a reduction in the overall number of thrombi in the PRN473-treated group (Figure 6Ciii-iv), as well as a statistically significant reduction in thrombi forming in podoplanin-expressing vessels (Figure 6Cv). Consistent with this, a trend for reduced platelet consumption and reduced increase in mean platelet volume was found in PRN473-treated mice (Figure 6Di-ii). Because Btk inhibitors also block signaling downstream of the B-cell receptor, we assessed white blood cell counts, finding no difference in either total white blood cell count or lymphocyte count between the PRN473-treated and control mice (Figure 6Diii-iv).
Oral administration of PRN473 blocks DVT formation in vivo
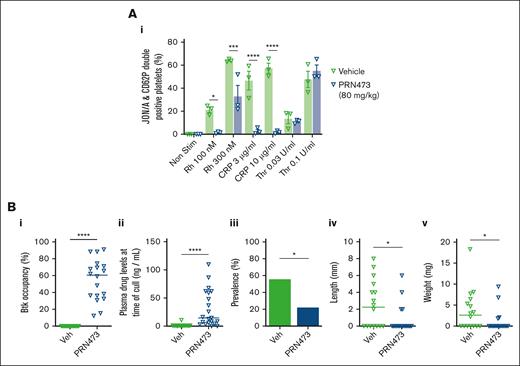
We also tested whether PRN473 or vehicle control administered via oral gavage (80 mg/kg) once daily for 4 days affected platelet activation. Similarly, blood from these mice had reduced CLEC-2– and GPVI-mediated but normal PAR4-mediated platelet activation (Figure 7Ai). After 4 days of dosing by oral gavage, mice underwent IVC stenosis surgery, followed by examination for presence of thrombosis 6 hours later. Plasma and spleens were harvested for drug level analysis. Median splenic occupancy and plasma levels for PRN473-treated mice were 60.5% and 14.5 ng/mL, respectively (Figure 7Bi-ii). Mice treated with PRN473 had fewer thrombi, and in those mice in which thrombi did form, these were smaller (Figure 7Biii-v). All thrombi formed in mice whose plasma concentrations of PRN473 were <8.5 ng/mL (supplemental Figure 4).
PRN473 inhibits IVC thrombosis in mice. (A) WT mice were gavaged daily with vehicle or PRN473 (80 mg/kg) for 4 days, then had platelet activation measured in citrated whole blood by flow cytometry, as indicated by activated integrin αIIbβ3 (JON/A) and P-selectin surface expression, after stimulation with snake venom toxin rhodocytin (100 and 300 nM), CRP (3 and 10 μg/mL), or thrombin (0.03 and 0.1 U/mL). GPRP was added to thrombin stimulation to prevent fibrin clot formation. Mean ± SEM (n = 3). Statistical analysis by 2-way ANOVA with Sídák correction for multiple comparisons. (B) IVC stenosis model of DVT was performed on WT mice gavaged with vehicle or PRN473 (80 mg/kg) for 4 days. Mice were euthanized 6 hours after IVC ligation and had spleens harvested for Btk occupancy analysis (i) and citrated plasma taken for drug concentration measurements (ii), and assessment for thrombus prevalence (iii), length (iv), and weight (v). Median: n = 16-18 mice per condition for splenic Btk occupancy and thrombus analysis; n = 17-21 mice per condition for plasma drug concentration measurements. Statistical analysis by Mann-Whitney test. ∗P < .01; ∗∗∗P = .0002; ∗∗∗∗P < .0001. GPRP, Gly-Pro-Arg-Pro; Veh, vehicle; WT, wild type.
PRN473 inhibits IVC thrombosis in mice. (A) WT mice were gavaged daily with vehicle or PRN473 (80 mg/kg) for 4 days, then had platelet activation measured in citrated whole blood by flow cytometry, as indicated by activated integrin αIIbβ3 (JON/A) and P-selectin surface expression, after stimulation with snake venom toxin rhodocytin (100 and 300 nM), CRP (3 and 10 μg/mL), or thrombin (0.03 and 0.1 U/mL). GPRP was added to thrombin stimulation to prevent fibrin clot formation. Mean ± SEM (n = 3). Statistical analysis by 2-way ANOVA with Sídák correction for multiple comparisons. (B) IVC stenosis model of DVT was performed on WT mice gavaged with vehicle or PRN473 (80 mg/kg) for 4 days. Mice were euthanized 6 hours after IVC ligation and had spleens harvested for Btk occupancy analysis (i) and citrated plasma taken for drug concentration measurements (ii), and assessment for thrombus prevalence (iii), length (iv), and weight (v). Median: n = 16-18 mice per condition for splenic Btk occupancy and thrombus analysis; n = 17-21 mice per condition for plasma drug concentration measurements. Statistical analysis by Mann-Whitney test. ∗P < .01; ∗∗∗P = .0002; ∗∗∗∗P < .0001. GPRP, Gly-Pro-Arg-Pro; Veh, vehicle; WT, wild type.
Discussion
In this study, we show that the third-generation Btk inhibitor PRN1008 and related compound PRN473 block Btk Y223 and downstream tyrosine kinase phosphorylation. They inhibit CLEC-2– and GPVI–mediated platelet function but leave G protein-coupled receptor-mediated function intact. Importantly, although it is not the first to show DVT reduction in Btk inhibitor–treated mice,11,24 it is the first to show that non–Src-targeting Btk inhibitors that lack bleeding side effects reduce thrombosis formation in in vivo mouse models of venous thromboinflammation.
These data are an important addition to the increasing evidence that suggests Btk inhibitors may be effective treatments of human thromboinflammatory disease. We and others have shown that Btk inhibitors reduce DVT in meta-analyses of trials of these B-cell malignancy drugs.6,14 Translational studies have not been forthcoming, however, because the first- and second-generation Btk inhibitors (ibrutinib and acalabrutinib) have bleeding side effects. The bleeding caused by these drugs has been shown by us and others to likely be due to off-target effects on other kinases, such as Src, that result in simultaneous blockade of multiple platelet signaling pathways.10,18,25 Our study does not find off-target Src inhibition by PRN473 or PRN1008 but does hint at other off-target effects (possibly on Tec)22 because of the increase in inhibition of GPVI–mediated platelet function by PRN1008 when added to platelets from patients with XLA.
The bleeding side effect of the first- and second-generation Btk inhibitors is not shared by the third-generation, more specific, Btk inhibitor PRN1008 (also known as rilzabrutinib). The phase 2 safety study of rilzabrutinib for the bleeding disorder ITP has been completed with no excess bleeding observed.19 Further evidence that specific Btk inhibition is unlikely to result in bleeding is demonstrated by the fact that patients with congenital Btk deficiency do not have any bleeding side effects.17,26
In the Salmonella infection model of liver thrombosis the effect of Btk inhibition was not as marked as in previous studies using CLEC-2–deficient mice in which near complete abrogation of thrombus formation was seen.3 This was not due to the dosing strategy because excellent Btk occupancy was achieved. The explanation for this may reside in our in vitro flow adhesion data, which show that it is platelet activation and thrombus propagation that are reduced by Btk inhibition and not platelet adhesion to podoplanin. Another explanation could be the lack of uniformity of podoplanin-expressing vessels; thrombosis was only reduced in those vessels that expressed podoplanin but not in the liver overall. There may also be other mechanisms contributing to liver thrombosis in the S typhimurium–infected mice, of which we are unaware.
The significant reduction in thrombosis in our DVT model is similar, however, to previous studies that have shown a reduction in DVT formation in ibrutinib-treated mice.11,24
We have also shown that PRN473 and PRN1008 are able to block GPVI–mediated platelet activation. Previous studies looking at PRN1008 in GPVI–mediated platelet function did not show any such blockade.20 In those studies, the agonist used was collagen. This is known to signal via integrin α2β1 as well as GPVI, and this may explain the lack of effect.27 The evidence our study provides opens the door for further studies looking at the effects on GPVI via atherosclerotic plaque–mediated platelet activation.13,28,29
Our study suffers from the same limitations that apply to many in vivo models, namely their unknown applicability to human disease. There are also some additional limitations; high concentrations (2-5 μM) of drug were required to block CLEC-2– and GPVI–mediated platelet function in whole blood, likely due to a high degree of plasma protein binding not found in washed platelets.30 We would have liked to study the drug’s effects on protein phosphorylation at these concentrations in whole blood, but this was not possible due to the drug being washed off the studied kinases during these experiments.10 It would have been ideal to study blood taken ex vivo from patients taking PRN1008 to look at the effect of pharmacologically achievable levels of drug. In addition, we would have liked to provide more conclusive data from the work with platelets from patients with XLA. However, due to the COVID-19 pandemic, these patients were heavily shielded and stopped attending hospital for appointments due to their lack mature B cells. This made the majority of our cohort of patients with XLA unavailable for study. In addition, our study did not investigate the effects of Btk inhibitors on monocyte and neutrophil function and the role Btk blockade in these cells plays in the reduction of thromboinflammation; this is the subject of a planned further study.
Overall, our study shows that PRN1008 and PRN473 are effective inhibitors of CLEC-2– and GPVI–mediated platelet function, and they have minimal off-target effects on other kinases and platelet signaling pathways. PRN473 reduces thrombosis in 2 different mouse models of immunothrombosis. Together with existing evidence that Btk inhibitors reduce venous thrombosis in patients and the lack of bleeding side effects reported thus far in clinical studies of PRN1008-treated patients with ITP,19 these data provide compelling evidence to start translational studies in patients with venous thromboinflammatory disease.
Acknowledgments
The authors thank Rachel Lamerton, Ruby Persaud, Joshua Bourne, and Julie Rayes for help and advice with histology, and Sarah Chadwick and the staff at the University of Birmingham Biomedical Services Unit for their help with the mouse models.
S.P.W. holds a British Heart Foundation (BHF) Chair (CH03/003). M.H. was supported by a postgraduate scholarship from Umm Al-Qura University and Ministry of Higher Education (Riyadh, Saudi Arabia). A.B. is supported by a BHF Senior Basic Science Research Fellowship (FS/19/30/34173).
This work was funded by Principia Biopharma/Sanofi.
The National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre (NIHR203326) and the British Heart Foundation Accelerator (AA/18/2/34218) have supported the University of Birmingham Institute of Cardiovascular Sciences where this research is based. The opinions expressed in this article are those of the authors and do not represent any of the listed organizations.
Authorship
Contribution: C.W.S. and P.L.R.N. designed and performed experiments, analyzed data, and wrote and revised the manuscript; J.C., H.C.B., N.J.J., V.-S.I., M.H., L.G.-Q., S.J., M.P.-T., K.R., L.N.T., T.O., J.L., M.C.F., S.M., and M.F. performed experiments, analyzed data, and revised the manuscript; M.S., A.H., C.L., and P.A.N. revised the manuscript; and S.P.W., M.R.T., A.F.C., A.C., and A.B. designed experiments and revised the manuscript.
Conflict-of-interest disclosure: P.L.R.N., M.R.T., and S.P.W. have received research grants from Novartis, Principia Biopharma, and Rigel Pharmaceuticals. P.L.R.N. has received research funding from AstraZeneca and honoraria from Bayer, Grifols, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Phillip L. R. Nicolson, University of Birmingham, Institute of Cardiovascular Sciences, Edgbaston, Birmingham B15 2TT, United Kingdom; email: p.nicolson@bham.ac.uk.
References
Author notes
Data are available on reasonable request from the corresponding author, Phillip L. R. Nicolson (p.nicolson@bham.ac.uk).
The full-text version of this article contains a data supplement.