Key Points
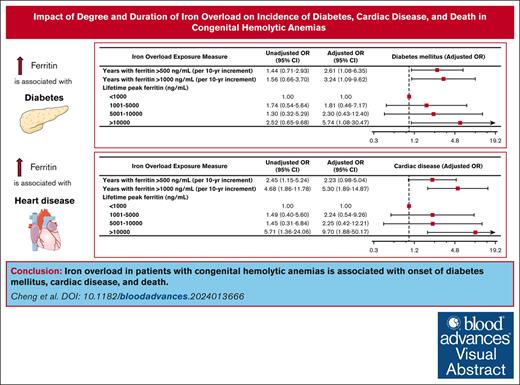
Iron overload in adults with congenital hemolytic anemias is associated with incidence of diabetes mellitus, cardiac disease, and death.
Peak ferritin and length of time with elevated ferritin are useful proxies for iron overload and correlate with clinical outcomes.
Visual Abstract
Iron overload and its complications are recognized to be morbid and fatal in patients with congenital hemolytic anemias. In patients with iron overload caused by congenital hemolytic anemias, there has been no study evaluating the dose-response relationship between serum markers of iron overload and long-term health complications. Filling this critical gap was the aim of this study. We evaluated outcomes in a 5-hospital observational cohort study of adults with congenital hemolytic anemias diagnosed with iron overload over a 40-year period and assessed associations between depth and duration of iron overload, as well as clinical complications including diabetes, heart disease, malignancy, bone density disorders, and death. One hundred seventy patients with congenital hemolytic anemias developing iron overload were included. More years experienced of ferritin >500 ng/mL and >1000 ng/mL were associated with the development of diabetes mellitus, with adjusted odds ratios (ORs) of 2.61 per 10-year increment (P = .034) and 3.24 per 10-year increment (P = .035), respectively. More years experienced of ferritin >1000 ng/mL were associated with the development of heart disease (adjusted OR, 5.30 per 10-year increment; P = .002). Peak lifetime ferritin of >10 000 ng/mL was associated with sixfold odds of developing diabetes (P = .04) and 10-fold odds of developing heart disease (P = .007). A peak ferritin >10 000 ng/mL was associated with an increase in mortality (adjusted OR, 6.77; P = .033). In conclusion, iron overload in patients with congenital hemolytic anemias is associated with diabetes mellitus, cardiac disease, and death. Prolonged exposure to relatively modest iron overload was associated with nearly threefold increased odds of diabetes.
Introduction
Humans are unable to actively excrete iron. Therefore, iron homeostasis requires modulation of intestinal iron uptake to reflect the body’s iron stores.1 In patients with iron overload disorders such as hereditary hemochromatosis, regulation of iron absorption and metabolism is disrupted, resulting in accumulation of iron in body tissues such as the liver and spleen.1 In other disease states such as beta-thalassemia, known derangements in the hepcidin axis secondary to ineffective erythropoiesis similarly lead to dysregulated iron absorption; iron overload in severe thalassemia is further exacerbated by dependence on blood transfusions.2 Improved control of iron overload has been shown to strongly correlate with a lower probability of heart disease and death in patients susceptible to hemosiderosis.3
Although the exact prevalence of hereditary hemochromatosis is unclear, it is estimated to be as high as 1 in 227 in people of Northern European descent.4 Conditions causing iron overload, however, extend far beyond classical hemochromatosis. For example, an estimated 26 000 patients with transfusion-dependent thalassemia are born annually.5 When combined with the population of patients with other hereditary or acquired anemias requiring chronic transfusions, iron overload and its associated complications contribute to a substantial global burden of adverse health outcomes.
Over the past several decades, life expectancies for patients with chronic hemolytic anemias such as thalassemia and sickle cell disease have improved substantially, in part due to the advent of iron chelators, among other innovations such as advances in blood safety and development of tailored therapies. As patients with these lifelong conditions are living longer into adulthood, there is a great need to better understand the long-term goals of iron chelation and the clinical risks of varying degrees of iron overload over long periods of time. These considerations need to be carefully balanced with the toxicity profiles of currently available iron chelators, which include adverse effects such as hepatotoxicity, nephrotoxicity, oculotoxicity, and ototoxicity. To date, there is significant practice variation among individuals and institutions in the use and titration of iron chelators.6-8 Emerging evidence suggests that even mild iron overload, when present over decades, can confer a substantial risk of malignancy, diabetes, and other adverse health outcomes, although these claims have not been studied in varied patient populations.9-15 To date, most studies related to iron overload and the associated long-term risks are limited to hereditary hemochromatosis. In patients with iron overload caused by congenital hemolytic anemias, there has been no study evaluating the temporal dose-response relationship between serum markers of iron overload and long-term health complications. Filling this critical gap was the aim of this study.
We hypothesize that in adults with chronic hemolytic anemias and iron overload, there is a measurable dose-response relationship between readily obtainable serum markers of iron overload and long-term clinical outcomes. This multicenter observational study evaluated the clinical outcomes of this patient population and innovated metrics of long-term iron overload that demonstrated a statistically significant association with medical problems such as diabetes and heart disease, as well as all-cause mortality.
Methods
Study design and data collection
We performed a multicenter, observational cohort study of adult patients with iron overload and a congenital hemolytic anemia between 1 January 1988 and 2 June 2022. This study was approved by the institutional review board of Mass General Brigham Healthcare (approval PHS/2021P003058).
Patients were identified via a query of the Mass General Brigham Research Patient Data Registry, which contains detailed patient-level clinical chart data on >7 million patients.16,17 Patients were cared for at 1 of 5 hospitals affiliated with Mass General Brigham: Massachusetts General Hospital, Brigham and Women’s Hospital, Newton-Wellesley Hospital, Faulkner Hospital, and North Shore Medical Center. Retrospective data collection using manual chart review was conducted by the authors. Study data were collected and managed using a secure offline database. The date of database lock was 13 June 2022.
The database was queried for patients with chart diagnoses of sickle cell disorder, thalassemia, other hereditary hemolytic anemias, or anemia due to enzyme disorders, who also had chart documentation of iron chelators (including the terms “deferasirox,” “deferiprone,” “deferoxamine,” “desferrioxamine,” “Desferal,” “Jadenu,” and “Exjade”) in the notes available on the electronic medical record. Data collected included demographics, underlying blood disorder classification, age, sex, all available instances of ferritin, all available instances of hemoglobin A1c, iron chelation history, and medical problem list.
Each patient’s chart was manually examined. Patients with iron overload were identified initially via the presence of iron overload in their medical problem list per International Classification of Diseases, ninth revision (ICD-9) and ICD-10 codes. Each patient’s chart was then manually reviewed by the study authors, and the diagnosis was corroborated by notes from the patient’s treating hematologist. Ferritin at the time of diagnosis was not always available, because in some patients, their diagnosis of iron overload predated their arrival to the Boston area. There were no cases of discrepancies between the diagnosis code for iron overload and the clinical history of iron history based upon manual chart review. Patients with a diagnosis of iron overload were included in the study regardless of history of iron chelation. Patients whose clinical histories were not consistent with a diagnosis of iron overload were excluded. The age at diagnosis of iron overload was obtained via ambulatory notes. Additional information gathered via manual chart curation included the number of years during which the patient’s ferritin exceeded 500 and 1000 ng/mL. If the patient was diagnosed with diabetes mellitus, heart disease, malignancy, or bone mineral disease, the age at diagnosis of the condition was obtained. Patients were defined as diabetic if they had a documented diagnosis of diabetes mellitus on manual review of provider notes, a hemoglobin A1c >6.5%, or a diagnostic glucose tolerance test. Patients were considered to have cardiac disease if they had a known diagnosis of congestive heart failure, cardiomyopathy, or cardiac arrest. Patients were considered to have bone mineral disease if they had a documented diagnosis of osteoporosis or osteopenia or had a dual-energy X-ray absorptiometry result consistent with osteoporosis or osteopenia. Deceased patients were identified via documentation in the medical record; in our medical system, patients were only marked as deceased if this was confirmed by additional sources such as a death certificate or other reports. Because the goal of this study was to assess the diagnosis of clinical outcomes subsequent to iron overload, instances in which the diagnosis of diabetes mellitus, heart disease, malignancy, or bone mineral disease predated the diagnosis of iron overload were not included in the analysis.
Statistical analyses
Multivariable logistic modeling was used to assess the associations between depth of iron overload (as measured by lifetime peak ferritin measurement) or duration of iron overload (as measured by years of ferritin >500 ng/mL and >1000 ng/mL) and complications of iron overload, including diabetes mellitus, heart disease, malignancy, and bone density disorders (osteopenia and osteoporosis), as well as death. Categorical variables used in the analysis included living or dead, sex, underlying blood disorder (sickle cell disease, beta-thalassemia major, or other condition), diagnosis of malignancy, diagnosis of heart disease, diagnosis of diabetes mellitus, and diagnosis of bone mineral disease (osteopenia or osteoporosis). Continuous variables included age of iron overload diagnosis, current age, peak ferritin in ng/mL, years spent with ferritin >500 ng/mL, years spent with ferritin >1000 ng/mL, age at diagnosis of malignancy, age at diagnosis of heart disease, age at diagnosis of diabetes mellitus, and age at diagnosis of bone mineral disease. All multivariate logistical models were controlled for sex, years of life lived since diagnosis of iron overload, and underlying congenital hemolytic anemia (sickle cell disease, beta-thalassemia major, or other congenital hemolytic anemia).
Results
Study population
A total of 296 patients with congenital hemolytic anemias diagnosed with iron overload were identified via the database query. Of these, 170 adult patients were included in statistical analysis. A total of 126 patients were excluded from this study because of missing essential data in their medical records or because they did not have a congenital hemolytic anemia. Baseline characteristics of the included patient population are presented in Table 1. The median age at diagnosis of iron overload was 31.5 years (range, 2-70). Ninety-seven patients (57%) were female. At the time of data collection, 18% of patients were deceased, with an average age of death of 46 years (range, 24-76). The cohort included 105 patients with sickle cell disease (61%), 30 patients with beta-thalassemia major (18%), and 35 patients with another congenital hemolytic anemia (including beta-thalassemia intermedia, hemoglobin H disease, and others). The prevalence of clinical outcomes of interest (diabetes mellitus, cardiac disease, malignancy, and bone mineral disease) are detailed in Table 1. The cohort included a total of 2003 patient-years of iron overload exposure (mean, 11.8 years per patient).
Demographics and baseline characteristics of study population
| Parameter . | Result . | % . |
|---|---|---|
| Median age at diagnosis of iron overload (range), y | 31.5 (2-70) | |
| Sickle cell disease | 30 (11-65) | |
| Beta-thalassemia major | 24.5 (2-61) | |
| Other congenital hemolytic anemias∗ | 42 (18-70) | |
| Female, n (%) | 97 | 57 |
| Ethnicities | ||
| Black | 79 | 46 |
| White | 47 | 28 |
| Hispanic or Latino | 12 | 7 |
| Asian | 19 | 11 |
| Other | 8 | 5 |
| Unknown | 5 | 3 |
| Deceased, n (%) | 30 | 18 |
| Sickle cell disease, n (%) | 105 | 62 |
| Beta-thalassemia major | 30 | 18 |
| Other congenital hemolytic anemias∗ | 35 | 21 |
| Mean peak ferritin | 6659 | |
| Diabetes, n (%) | 44 | 26 |
| Heart disease, n (%) | 37 | 22 |
| Malignancy, n (%) | 14 | 8 |
| Bone mineral disease, n (%) | 46 | 27 |
| Chelator history | ||
| Deferasirox | 61 | 36 |
| Deferoxamine | 15 | 9 |
| Deferiprone | 8 | 5 |
| Dual chelation | 4 | 2 |
| Unknown | 103 | 48 |
| Parameter . | Result . | % . |
|---|---|---|
| Median age at diagnosis of iron overload (range), y | 31.5 (2-70) | |
| Sickle cell disease | 30 (11-65) | |
| Beta-thalassemia major | 24.5 (2-61) | |
| Other congenital hemolytic anemias∗ | 42 (18-70) | |
| Female, n (%) | 97 | 57 |
| Ethnicities | ||
| Black | 79 | 46 |
| White | 47 | 28 |
| Hispanic or Latino | 12 | 7 |
| Asian | 19 | 11 |
| Other | 8 | 5 |
| Unknown | 5 | 3 |
| Deceased, n (%) | 30 | 18 |
| Sickle cell disease, n (%) | 105 | 62 |
| Beta-thalassemia major | 30 | 18 |
| Other congenital hemolytic anemias∗ | 35 | 21 |
| Mean peak ferritin | 6659 | |
| Diabetes, n (%) | 44 | 26 |
| Heart disease, n (%) | 37 | 22 |
| Malignancy, n (%) | 14 | 8 |
| Bone mineral disease, n (%) | 46 | 27 |
| Chelator history | ||
| Deferasirox | 61 | 36 |
| Deferoxamine | 15 | 9 |
| Deferiprone | 8 | 5 |
| Dual chelation | 4 | 2 |
| Unknown | 103 | 48 |
Represents individuals with beta-thalassemia intermedia, hereditary spherocytosis, hemoglobin E disease, congenital dyserythropoietic anemia, hemoglobin H disease, pyruvate kinase deficiency, and glucose-6-phosphate dehydrogenase deficiency.
Association of degree and duration of iron overload with development of complications
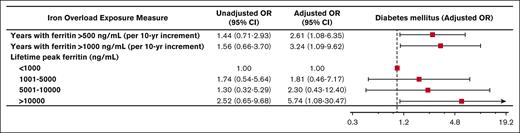
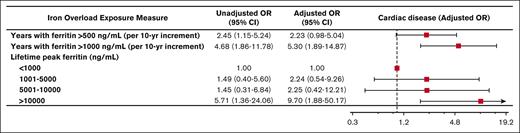
During the observation period, 44 patients (26%) developed diabetes mellitus, 37 patients (22%) developed cardiac disease, 46 patients (27%) developed a bone density disorder, and 14 patients (8.2%) developed malignancy. Of patients who developed diabetes, the median age of diagnosis was 35 years. The median age of diagnosis of cardiac disease was 42.5 years. More years experienced of ferritin >500 ng/mL and >1000 ng/mL were associated with the development of diabetes mellitus (adjusted odds ratio [OR], 2.61; 95% confidence interval [CI], 1.08-6.35 per 10-year increment; P = .034 and adjusted OR, 3.24; 95% CI, 1.09-9.62 per 10-year increment; P = .035, respectively; Figure 1). Similarly, more years experienced of ferritin >500 ng/mL and >1000 ng/mL were associated with the development of cardiac disease (adjusted OR, 2.23; 95% CI, 0.98-5.04 per 10-year increment; P = .055 and adjusted OR, 5.30; 95% CI, 1.89-14.87 per 10-year increment; P = .002, respectively; Figure 2). Peak lifetime ferritin of >10 000 ng/mL was associated with approximately sixfold odds of development of diabetes and 10-fold odds of development of cardiac disease (P = .040 and P = .007, respectively; Figures 1 and 2).
Iron overload and diabetes mellitus. Forest plot illustrating the results of univariable and multivariable logistic models assessing associations between various measures of iron overload duration and degree and development of diabetes mellitus. Multivariable logistic models were controlled for sex, years of life lived since diagnosis of iron overload, and underlying congenital hemolytic anemia (sickle cell disease, beta-thalassemia major, or other congenital hemolytic anemias).
Iron overload and diabetes mellitus. Forest plot illustrating the results of univariable and multivariable logistic models assessing associations between various measures of iron overload duration and degree and development of diabetes mellitus. Multivariable logistic models were controlled for sex, years of life lived since diagnosis of iron overload, and underlying congenital hemolytic anemia (sickle cell disease, beta-thalassemia major, or other congenital hemolytic anemias).
Iron overload and cardiac disease. Forest plot illustrating the results of univariable and multivariable logistic models assessing associations between various measures of iron overload duration and degree and development of cardiac disease. Multivariable logistic models were controlled for sex, years of life lived since diagnosis of iron overload, and underlying congenital hemolytic anemia (sickle cell disease, beta-thalassemia major, or other congenital hemolytic anemias).
Iron overload and cardiac disease. Forest plot illustrating the results of univariable and multivariable logistic models assessing associations between various measures of iron overload duration and degree and development of cardiac disease. Multivariable logistic models were controlled for sex, years of life lived since diagnosis of iron overload, and underlying congenital hemolytic anemia (sickle cell disease, beta-thalassemia major, or other congenital hemolytic anemias).
No associations were observed between duration or degree of iron overload and development of malignancy or bone density disease. More years experienced of ferritin >500 ng/mL and >1000 ng/mL were not significantly associated with the development of bone density disease (adjusted OR, 1.28; 95% CI, 0.53-3.13 per 10-year increment; P = .581 and adjusted OR, 1.13; 95% CI, 0.36-3.60 per 10-year increment; P = .830, respectively). Similarly, more years experienced of ferritin >500 ng/mL and >1000 ng/mL were not significantly associated with the development of malignancy (adjusted OR, 0.35; 95% CI, 0.077-1.59 per 10-year increment; P = .175 and adjusted OR, 0.20; 95% CI, 0.028-1.47 per 10-year increment; P = .114, respectively).
Association of degree and duration of iron overload with death
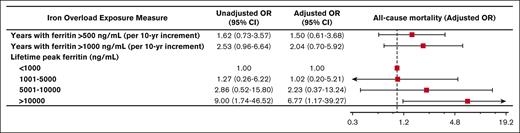
During the observation period, 30 patients (18%) died. Peak lifetime ferritin of >10 000 ng/mL was associated with approximately sevenfold odds of death (adjusted OR, 6.77; 95% CI, 1.17-39.27; P = .033). Associations between duration of iron overload and death did not reach statistical significance (Figure 3).
Iron overload and mortality. Forest plot illustrating the results of univariable and multivariable logistic models assessing associations between various measures of iron overload duration and incident death. Multivariable logistic models were controlled for sex, years of life lived since diagnosis of iron overload, and underlying congenital hemolytic anemia (sickle cell disease, beta-thalassemia major, or other congenital hemolytic anemias).
Iron overload and mortality. Forest plot illustrating the results of univariable and multivariable logistic models assessing associations between various measures of iron overload duration and incident death. Multivariable logistic models were controlled for sex, years of life lived since diagnosis of iron overload, and underlying congenital hemolytic anemia (sickle cell disease, beta-thalassemia major, or other congenital hemolytic anemias).
Discussion
In our cohort of adult patients with congenital hemolytic anemias, we observed a statistically significant correlation between iron overload and important clinical outcomes including diabetes, cardiac disease, and death. Our study reveals that easily calculable metrics such as peak lifetime ferritin and duration of elevations in ferritin >500 ng/mL or >1000 ng/mL are clinically meaningful predictors of adverse clinical outcomes. Specifically, this study’s results demonstrate that prolonged modest elevations in ferritin (as measured by the number of years with ferritin measured >500 ng/mL) is associated with an incidence of diabetes mellitus. Furthermore, a higher degree of iron overload, as measured by the number of years with ferritin >1000 ng/mL, carries even higher odds of developing diabetes mellitus, as well as a statistically significant association with heart disease. Peak ferritin also meaningfully correlates with diabetes, heart disease, and death. To our knowledge, this is the first study of adults with congenital hemolytic anemias in which a cumulative exposure-based dose-response association of ferritin with important long-term clinical outcomes was performed.
Previous studies have demonstrated that iron overload in patients with hereditary hemochromatosis are at elevated risk of malignancies, cirrhosis, cardiac disease, and diabetes.18,19 The role of iron overload and cardiac disease has also been demonstrated in transfusion-dependent thalassemia.20,21 However, there remains a paucity of studies to investigate the risk of developing other long-term health outcomes in patients with congenital hemolytic anemias. This area of investigation is becoming more urgent because the adult patient population continues to grow as a result of improved therapeutics and diagnostic technology. Our study provides evidence that iron overload in the adult population is a strong risk factor for the development of multiple disease processes and overall mortality and, furthermore, provides an accessible clinical metric to monitor over time and identify patients at highest risk of developing cardiac and endocrine complications.
Our study has several strengths, including a large cohort of adult patients obtained from multiple centers, as well as high-quality manual data extraction with meticulous review of diagnoses and laboratory data by physician investigators. However, it also has important limitations. For example, this was an observational study reliant on retrospective data collection and, as such, is vulnerable to selection bias and confounding. Although this multi-institutional study included patients from 5 hospitals in the greater Boston area, the results from this study may not be generalizable to the broader population of people with congenital hemolytic anemias given the local practice patterns and epidemiologic characteristics. Furthermore, this analysis includes several referral centers for rare and severe hematologic conditions, which may introduce a source of selection bias that is not representative of the general population of patients with similar diagnoses. Although our study was performed with meticulous manual chart review by the study authors who synthesized the available patient notes and laboratory results to enhance confidence in diagnosis and clinical outcomes, only data from the 10 included hospitals were available for manual review. Because of this, there is an inherent risk of being unable to completely capture important elements of a patient’s clinical outcomes, especially if the patient is simultaneously managed by a hospital system whose medical records are not available for our review.
Additionally, it is well-known that ferritin is an imperfect measure of end-organ deposition of iron, especially in patients with transfusional iron overload.22 Given the high missingness of gold standard diagnostic data for iron deposition such as hepatic and cardiac magnetic resonance imaging, our study does not incorporate the findings of these advanced imaging methods into its analysis. Ferritin is also a known acute phase reactant and may increase in response to numerous stressors independent of an iron overloaded state. Although our covariates (years with ferritin >500 ng/mL or >1000 ng/mL) are intended to capture a more accurate representation of chronic iron overload, it may be confounded by ferritin elevations explained by unrelated clinical stressors. Accordingly, this retrospective study is vulnerable to confounding variables and cannot comment upon the causative relationship between elevated ferritin and the clinical outcomes that were measured in this study.
Separately, although intensive manual review of each patient chart was used to acquire accurate diagnoses, as an observational study, it is possible that certain diagnoses of diabetes mellitus, heart disease, or bone mineral disease may not have been appropriately coded in the available data, because protocolized screening for these diagnoses was not done. In particular, diabetes mellitus is likely to be an underrecognized entity in patients with congenital hemolytic anemias, because the usual hemoglobin A1c assay used for screening in the general population may be falsely low due to increased red cell turnover. Despite this shortcoming, our study demonstrated a statistically significant association between even mild persistent elevations in ferritin and diabetes mellitus and supports a need for more aggressive and accurate screening of diabetes mellitus in this patient population.
Finally, our study did not demonstrate a statistically meaningful correlation between ferritin elevation and the diagnosis of malignancy, despite a known increased risk of hepatocellular carcinoma in patients with hereditary hemochromatosis, and prior literature suggesting a higher prevalence of solid and hematologic malignancies in patients with transfusion-dependent thalassemia.23,24 This may reflect (1) inadequate power of our study population to detect a significant correlation, especially given the overall low prevalence of malignancy in the studied population; (2) a primarily young adult population with underrepresentation of older patients who are at highest risk of malignancies; (3) difference in the average degree of iron deposition in the liver in our study samples compared with those previously studied patients with hereditary hemochromatosis; or (4) a difference in biology and risk factor profiles between different disease processes predisposing patients to iron overload.
In conclusion, we observed that prolonged elevations in ferritin as well as the degree of ferritin elevation are strongly associated with the onset of diabetes and heart disease, as well as overall mortality, in adult patients with congenital hemolytic anemia and iron overload. This finding supports the need for adequate surveillance of long-term clinical complications and proposes an accessible and easy-to-measure method of risk stratifying patients. Further studies are needed to refine metrics of prolonged iron overload and correlate these findings with gold standard diagnostics of end-organ iron deposition, such as magnetic resonance imaging, as well as to perform prospective investigations of ferritin elevations as defined in this study to demonstrate its ability to predict clinical complications and risk stratify patients.
Acknowledgments
A.N.C. is funded by the American Society of Hematology's Hematology Opportunities for the Next Generation of Research Scientists (HONORS) Award. H.A.-S. is funded by the National Heart, Lung, and Blood Institute (grant K23HL159313).
Authorship
Contribution: A.N.C. contributed to concept and design, data collection, creation of tables and figures, writing of the first draft of the manuscript, and final approval; and H.A.-S. contributed to concept and design, data collection, data analysis, critical revisions of the intellectual content, and final approval.
Conflict-of-interest disclosure: H.A.-S. reports universal disclosures regarding research funding to institution from Amgen, Sobi, Vaderis, Novartis, and Agios; and consultancy fees from Alnylam, Amgen, Alpine, argenx, Agios, Sobi, Novartis, and Pharmacosmos. A.N.C. declares no competing financial interests.
Correspondence: Hanny Al-Samkari, Division of Hematology Oncology, Massachusetts General Hospital, Bartlett Hall, Office 133, 55 Fruit St, Boston, MA 02114; email: hal-samkari@mgh.harvard.edu.
References
Author notes
Original data are available on request from the author, Aaron N. Cheng (aaron.cheng@pennmedicine.upenn.edu).