Key Points
AA is common in middle-aged patients with SCA and is associated with an increased risk for stroke.
Age and LA volume are associated with an increased risk for AA and may be parameters for future risk stratification.
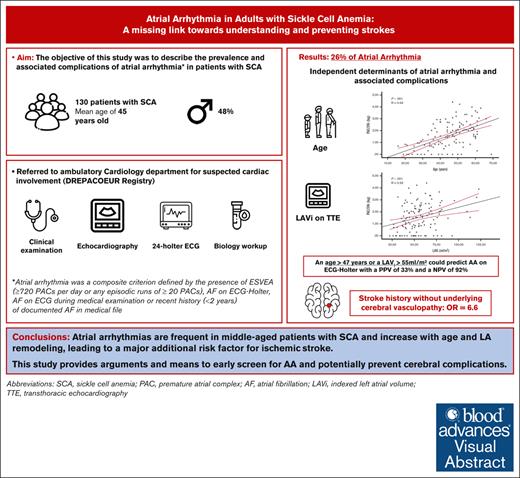
Visual Abstract
Although patients with homozygous sickle cell anemia (SCA) carry both significant left atrial (LA) remodeling and an increased risk of stroke, the prevalence of atrial arrhythmia (AA) has never been prospectively evaluated. The aim of this study was to identify the prevalence and predictors of atrial arrhythmia in SCA. From 2018 to 2022, consecutive adult patients with SCA were included in the DREPACOEUR prospective registry and referred to the physiology department for cardiac evaluation, including a 24-hour electrocardiogram monitoring (ECG-Holter). The primary endpoint was the occurrence of AA, defined by the presence of excessive supraventricular ectopic activity (ESVEA) on ECG-Holter (ie >720 premature atrial contractions [PACs] or any run ≥ 20 PACs) or any recent history of atrial fibrillation. Overall, 130 patients with SCA (mean age: 45±12 years, 48% of male) were included. AA was found in 34 (26%) patients. Age (52±9 vs. 42±12 years, P=0,002), LA dilation (LAVi, 71±24 vs. 52±14 mL/m², P<0.001) and history of stroke without underlying cerebral vasculopathy (26% vs. 5%, P=0.009, OR=6.6 (95%CI 1.4-30.3]) were independently associated with AA. Age and LAVi correlated with PAC load per 24 hours on ECG-Holter. An age over 47 years or a LAVi >55mL/m² could predict AA with a PPV of 33% and a NPV of 92%. AAs are frequent in middle-aged patients with SCA and increase with age and LA remodeling, leading to a major additional risk factor for ischemic stroke. This study provides arguments and means to early screen for AA and potentially prevent cerebral complications.
Introduction
Sickle cell disease (SCD) is the most common inherited blood disorder in the world.1 Major medical advances such as antibiotic prophylaxis, vaccination, hydroxyurea use, and red blood cell exchange have considerably increased life expectancy in patients with homozygous SCD, also called sickle cell anemia (SCA), but unmasked chronic disease complications, among which cardio- and cerebrovascular impairment have a major impact on morbidity and mortality.2-9
The pathophysiology of cardiac involvement in SCA is complex and still under investigation.5,10,11 The role of chronic anemia, hemolysis, and volume overload is acknowledged in the progression of cardiac chamber dilation, which starts early in childhood and increases with age.5,12-14 Furthermore, the atrial remodeling, associated with some evidence of myocardial fibrosis, and the existence of chronic low-grade inflammation provide a propitious environment for atrial rhythm disorders (ARDs), even in younger patients with SCA.15-22 In line with this, retrospective studies and health care database analyses reported an increased risk for atrial arrhythmia (AA) among patients with SCD and its association with ischemic stroke and mortality.23-28 Ischemic stroke is a major complication among patients with SCD and has multiple etiologies, but it is mainly related to the underlying cerebral vasculopathy.6,29 Interestingly, our group has also identified cardioembolism as a prevalent etiology of stroke in a small sample of adults with SCA, thus raising the possibility that cardioembolic risk is underestimated.7 Based on the early and major atrial remodeling observed in patients with SCA,5,13 we hypothesized that a significant proportion of strokes that occur in middle-aged adults might be attributable to ARDs.28,30-32 Such a rhythmic disorder might furthermore contribute to the development of postcapillary pulmonary hypertension and acute heart failure, both of which also have a major impact on the quality of life and the prognosis of patients with SCA.3,23,33,34 To our knowledge, there is currently no study that prospectively assessed AA prevalence, determinants, and its relation with ischemic stroke in patients with SCA.
In this study, we propose to shed new light on AA in patients with SCA through a comprehensive evaluation that integrated electrocardiographic monitoring with a patient’s phenotype, advanced cardiac imaging, and circulating biomarkers.
Methods
Study population
From November 2018 to November 2022, adult patients with SCD that were followed in the Sickle Cell Coordinating Referral Center of the Henri Mondor teaching hospital (Créteil, France) were included in DREPACOEUR, a multicenter, prospective registry, and were referred to the ambulatory cardiology department for documented or suspected cardiac involvement (see criteria below). The inclusion criteria involved patients with SCD, aged >15 years and 3 months (with parental consent obtained for those aged between 15 and 18 years), at steady state (at least 1 month following acute complication) with 1 of the following features:
Chest pain suggestive of angina
Worsening dyspnea without argument for a hematologic etiology, therefore justifying a cardiopulmonary assessment
Recent hospitalization for an episode of decompensated heart failure
Abnormalities documented on echocardiography including left ventricular ejection fraction (LVEF) <50%, tricuspid regurgitation velocity (TRV) ≥2.5 m/s,12,35 or E/A ratio <14
Abnormalities documented on cardiac magnetic resonance imaging including LVEF <50% or the presence of iron overload (T2∗ <20 milliseconds)
Elevation of cardiac biomarkers (N-terminal probrain natriuretic peptide [NT pro-BNP]/BNP/troponin) according to laboratory standards
Frequent or consistent palpitations or electrocardiographic abnormalities such as ST-segment changes, rhythm, or conduction disorders
Recent history of unexplained stroke
Surgery or hematopoietic stem cell transplant that justified a previous cardiothoracic assessment
During a 1-day outpatient clinic evaluation, these patients underwent a physical examination, an electrocardiogram (ECG), transthoracic echocardiography, a 6-minute walking test, and biology tests. Ambulatory pressure monitoring, 24-hour ECG monitoring (ECG-Holter), a computerized tomography (CT) coronary angiogram, or noninvasive testing for myocardial ischemia was also performed depending on the clinical features and the decision of the cardiologist and SCD experts. Cardiac CT and noninvasive testing for myocardial ischemia were the only investigations that were not necessarily performed on the day of the visit.
For this study, only patients with SCA (SS or Sβ0) who underwent a 24-hour ECG-Holter were included. Indeed, assessments for some patients with SCA were partly canceled or postponed because of the COVID-19 pandemic, and thus they could not undergo the ECG-Hotler. Written informed consent was collected from all participating patients; the database was declared to the Commission Nationale de l'Informatique et des Libertés (n°7830264) and was approved by the ethics committee (protocol 2013/NICB).
Data collection and analysis
All patients were interviewed, had their medical record examined, and underwent a physical examination by a sickle cell expert and a cardiologist. Height (cm) and weight (kg) were measured to calculate the body mass index. Blood pressure was measured as recommended.36 A resting ECG was performed to calculate the duration of corrected QT. A multidisciplinary meeting was held to discuss the results for each patient. This included sickle cell experts, cardiologists, pulmonologists, and radiologists. In addition, all patients with a history of stroke or transient ischemic attack were also included in the PCDREP prospective registry37 and a consensus reunion was set with a neurologist, a sickle cell expert, and a neuroradiologist to assess the likely cause according to the following predefined classification: cerebral vasculopathy, vascular thrombosis, possible cardioembolism related to ARD (such as atrial fibrillation [AF]) or a different cardioembolic mechanism (eg, patent foramen ovale), and other defined causes as previously described.6 A possible ARD-related stroke was defined as a stroke unrelated to cerebrovascular disease that occurred in patients with at least 1 of the following criteria: a history of AF, a new diagnosis of atrial arrythmia (defined hereafter), or undetermined etiology despite in-depth investigations that excluded other cardioembolic causes and included at least a contract echocardiography.38 Some patients with an unexplained history of stroke underwent long-term ECG monitoring (Reveal, Medtronic, Dublin) based on expert consensus decision. A history of cardiovascular risk factors was documented, including systemic hypertension, dyslipidemia, diabetes mellitus, active smoking, and sleep apnea obstructive syndrome. Routine laboratory tests (complete blood count, serum chemistry profile, lactate dehydrogenase, and NT pro-BNP) were performed during the same visit. In case of a history of stroke or transient ischemic attack, antiphospholipid syndrome was excluded by a biologic screening (anti-β2GP1 and anti-cardiolipin antibodies and lupus anticoagulant).39 These patients were closely followed at our center and some early rhythmological information, obtained during follow-up (<2 years), has been used to elaborate on the underlying mechanism of unexplained history of stroke in selected patients.
Twenty-four hour ECG-Holter
A simultaneous 3-channel ECG-Holter (Novacor, Rueil-Malmaison, France) was performed on the day before the outpatient visit. The data were analyzed by a cardiologist blinded to the medical record. Premature atrial contractions (PACs) and premature ventricular contractions were quantified. Excessive supraventricular ectopic activity (ESVEA) was defined as ≥720 PACs per day or any episodic runs of ≥20 PACs, as previously defined by the European Heart Rhythm Association consensus.40 AF was defined as an irregular heart rhythm without P waves that lasted for at least 30 seconds and it was further characterized as paroxysmal if it stopped spontaneously or with intervention within 7 days of onset.41 Persistent and permanent AF was identified based on the current guidelines.41 AA was a composite criterion defined by the presence of ESVEA, AF on ECG-Holter, AF on ECG during the medical examination, or a recent history (<2 years) of documented AF in the medical file. Ventricular arrhythmia was defined according to European guidelines as the occurrence of sustained (>30 seconds) or nonsustained ventricular tachycardia, >500 premature ventricular contractions on ECG-Holter, or history of ventricular tachycardia ablation.10,42
Echocardiography evaluation
All patients underwent comprehensive 2-dimensional, M-mode Doppler and tissue Doppler echocardiography that was performed on the same ultrasound system (Vivid E95, General Electric, Boston, MA) according to contemporary guidelines by experienced sonographers blinded to the medical records.43,44 All studies were interpreted offline and in a blinded fashion by a single cardiologist. Cardiac output left ventricular (LV) end-diastolic volume and mass and left atrial (LA) volume were indexed to body surface area (cardiac index, left ventricular end-diastolic volume indexed to body surface area, LV mass indexed to body surface area [LVMi], and LA volume indexed to body surface area [LAVi], respectively). LVEF was calculated using Simpson biplane method. The LV global longitudinal strain was acquired using automated functional imaging (using Speckle Tracking Echocardiography) of 3 clips to perfectly track all myocardial segments in the apical 4-chamber, 2-chamber, and 3-chamber views. Mitral inflow velocity pattern, peak velocities of early (E) and late (A) waves and E wave deceleration time were recorded. Tissue Doppler echocardiography was performed to measure early (e′) diastolic tissue velocities at the lateral mitral annulus. The lateral E/e′ ratio was used as a surrogate of LV filling pressure.45 The right ventricular function was assessed using tricuspid annular plane systolic excursion and S' wave. TRV was recorded to assess pulmonary arterial pressure.46
Study objective
The objective of this study was to describe the prevalence and associated complications of AA in patients with SCA included in the DREPACOEUR registry.
Statistical analysis
All analyzes were performed using SPSS 26 (IBM, Armonk, NY). The data are presented as percentages for categorical data and as mean ± standard deviation or median (interquartile range) for continuous variables depending on the normality of their distribution. Comparisons between groups for continuous variables were performed using 1-way analysis of variance. The χ2 test was used to compare dichotomous variables. A multivariate analysis was then performed using forward stepwise logistic regression on the clinical, biological and echocardiography parameters significantly associated with AA occurrence (P < .05). In addition to the statistical significance, variables selection for the logistic regression took into account clinical considerations and the absence of multicollinearity. In the case of missing values in the multivariate analysis, mean imputation was performed. Overall, computation was only used for the 6 patients with missing systolic pulmonary artery pressure (sPAP) data. Two-tailed Pearson correlation analyzes were conducted on independent parameters associated with the end point. Odds ratios (ORs) are presented with a 95% confidence interval. Area under the receiver operating characteristic curve (AUC) was used to determine the accuracy of age and LAVi for identifying AA. A P value <.05 indicated statistical significance.
Results
Population characteristics
Among the 215 patients with SCD who were prospectively included in the DREPACOEUR registry during this period, 181 (84%) were SS or Sβ0-thalassemia (SCA), 7 (3%) were Sβ+ thalassemia, and 27 (13%) had an SC genotype. The current study focused on the 130 patients with SCA who underwent a 24-hour ECG-Holter the day before their visit in the outpatient clinic. Patients without rhythm monitoring at the time of the analysis were not included in this study. This mainly concerned patients who postponed because of the COVID-19 pandemic or patients who had their ECG-Holter canceled to avoid 2 consecutive day visits in a risk-prone environment. A flowchart of the study and additional information regarding patients who had their ECG-Holter canceled are available in the supplemental Figure 1 and Table 1. The baseline clinical and laboratory characteristics of the study population are summarized in Table 1. Notably, it was comparable with the subgroup affected by canceled appointment (supplemental Table 1). Briefly, the mean age was 45 ± 12 years, 48% were males, and they were mostly referred for dyspnea (41%) and chest pain (18%), whereas palpitation or ECG changes occurred in only 12%. Patients had frequent disease complications (systemic hypertension, thromboembolic disease, and chronic kidney disease), including a history of stroke in 25 patients (19%). Interestingly, 56% of these strokes (14 patients) were compatible with an ARD mechanism without underlying cerebral vasculopathy or other identified cause (supplemental Table 2). No positive anti-phospholipid antibody was found in patients with a history of stroke. Most of the patients were treated with hydroxyurea (70%) and/or were included in an erythrocytapheresis program (25%). Despite an overall preserved LVEF in most of the patients (LVEF = 58% ± 6%), 8 (6%) had an LV dysfunction (LVEF <50%) and global longitudinal strain was slightly reduced (−17.9% ± 2.7%; Table 2). The LA chamber was dilated (LAVi = 57 ± 19 mL/m2) when compared with the general population with a normal value of <34 mL.m2.43 The right ventricular function was preserved (tricuspid annular plane systolic excursion = 25 ± 5 mm). TRV was 2.6 ± 0.3 m/s with 23 patients (19%) above 3 m/s. No severe or torrential tricuspid regurgitation was observed. A total of 58 patients (46%) underwent a coronary CT scan without any coronary artery disease reported.
Clinical and biological characteristics of the studied population
| Characteristics . | Patients (n = 130) . |
|---|---|
| Demographic and clinical | |
| Age, y | 45 ± 12 |
| Male, n (%) | 62 (48) |
| Body mass index (kg/m2) | 24 ± 4 |
| Systolic blood pressure (mm Hg) | 129 ± 17 |
| Diastolic blood pressure (mm Hg) | 75 ± 11 |
| Heart rate (bpm) | 72 ± 13 |
| Oxygen saturation (%) (n = 105) | 95 ± 3 |
| Referred for, n (%) | |
| Worsening of dyspnea | 56 (41) |
| Chest pain | 23 (18) |
| Palpitations or ECG changes | 15 (12) |
| Elevation of a cardiac biomarker | 7 (5) |
| Cardiac imaging abnormalities | 9 (7) |
| Episode of decompensated heart failure | 7 (5) |
| Recent stroke | 7 (5) |
| Pretransplant assessment (kidney/HSCs) | 6 (5) |
| Cardiovascular risk factors, n (%) | |
| History of stroke | 25 (19) |
| Classification of stroke∗, n = 25 | |
| Cerebral vasculopathy | 8 (32) |
| Possible ARD-related stroke or undetermined cause | 14 (56) |
| Other defined cause | 3 (12) |
| History | |
| Arterial hypertension | 74 (57) |
| Diabetes | 4 (3) |
| Dyslipidemia | 1 (1) |
| Obesity | 8 (6) |
| Obstructive sleep apnea syndrome | 13 (10) |
| Chronic kidney disease† | 44 (34) |
| Active smoking | 16 (12) |
| Thromboembolic history (PE/DVT) | 35 (27) |
| Treatment of SCD, n (%) | |
| None | 15 (12) |
| Hydroxyurea | 81 (62) |
| Erythrocytapheresis | 10 (8) |
| Hydroxyurea + erythrocytapheresis | 22 (17) |
| Bloodletting | 2 (2) |
| Anticoagulation therapy and indication, n (%) | |
| Stroke complicating patient foramen ovale | 1 (1) |
| Cryptogenic stroke | 2 (2) |
| History of AF (disregarding complications) | 15 (12) |
| Venous thromboembolism | 14 (11) |
| Biology | |
| Hemoglobin (g/dL) | 8.7 ± 1.5 |
| Leukocytes (×109/L) | 7.7 ± 2.6 |
| Platelets (×109/L) | 313 ± 132 |
| Potassium (mmol/L) | 4.3 ± 0.5 |
| Creatinine (μmol/L) | 63 (52-91) |
| GFR (based on CKD-EPI formula in mL/min per 1.73m2) | 105 (69-121) |
| ASAT (IU/L) | 42 (33-60) |
| ALAT (IU/L) | 24 (18-36) |
| LDH (IU/L) | 439 (253-494) |
| Total bilirubin (μmol/L) | 31 (21-47) |
| Free bilirubin (μmol/L) | 21 (13-33) |
| Nt Pro-BNP (ng/L) | 145 (57-400) |
| Nt Pro-BNP ≥160n g/L | 61 (47) |
| Troponin T (Hs) (ng/L), n = 101 | 5 (3-11) |
| Characteristics . | Patients (n = 130) . |
|---|---|
| Demographic and clinical | |
| Age, y | 45 ± 12 |
| Male, n (%) | 62 (48) |
| Body mass index (kg/m2) | 24 ± 4 |
| Systolic blood pressure (mm Hg) | 129 ± 17 |
| Diastolic blood pressure (mm Hg) | 75 ± 11 |
| Heart rate (bpm) | 72 ± 13 |
| Oxygen saturation (%) (n = 105) | 95 ± 3 |
| Referred for, n (%) | |
| Worsening of dyspnea | 56 (41) |
| Chest pain | 23 (18) |
| Palpitations or ECG changes | 15 (12) |
| Elevation of a cardiac biomarker | 7 (5) |
| Cardiac imaging abnormalities | 9 (7) |
| Episode of decompensated heart failure | 7 (5) |
| Recent stroke | 7 (5) |
| Pretransplant assessment (kidney/HSCs) | 6 (5) |
| Cardiovascular risk factors, n (%) | |
| History of stroke | 25 (19) |
| Classification of stroke∗, n = 25 | |
| Cerebral vasculopathy | 8 (32) |
| Possible ARD-related stroke or undetermined cause | 14 (56) |
| Other defined cause | 3 (12) |
| History | |
| Arterial hypertension | 74 (57) |
| Diabetes | 4 (3) |
| Dyslipidemia | 1 (1) |
| Obesity | 8 (6) |
| Obstructive sleep apnea syndrome | 13 (10) |
| Chronic kidney disease† | 44 (34) |
| Active smoking | 16 (12) |
| Thromboembolic history (PE/DVT) | 35 (27) |
| Treatment of SCD, n (%) | |
| None | 15 (12) |
| Hydroxyurea | 81 (62) |
| Erythrocytapheresis | 10 (8) |
| Hydroxyurea + erythrocytapheresis | 22 (17) |
| Bloodletting | 2 (2) |
| Anticoagulation therapy and indication, n (%) | |
| Stroke complicating patient foramen ovale | 1 (1) |
| Cryptogenic stroke | 2 (2) |
| History of AF (disregarding complications) | 15 (12) |
| Venous thromboembolism | 14 (11) |
| Biology | |
| Hemoglobin (g/dL) | 8.7 ± 1.5 |
| Leukocytes (×109/L) | 7.7 ± 2.6 |
| Platelets (×109/L) | 313 ± 132 |
| Potassium (mmol/L) | 4.3 ± 0.5 |
| Creatinine (μmol/L) | 63 (52-91) |
| GFR (based on CKD-EPI formula in mL/min per 1.73m2) | 105 (69-121) |
| ASAT (IU/L) | 42 (33-60) |
| ALAT (IU/L) | 24 (18-36) |
| LDH (IU/L) | 439 (253-494) |
| Total bilirubin (μmol/L) | 31 (21-47) |
| Free bilirubin (μmol/L) | 21 (13-33) |
| Nt Pro-BNP (ng/L) | 145 (57-400) |
| Nt Pro-BNP ≥160n g/L | 61 (47) |
| Troponin T (Hs) (ng/L), n = 101 | 5 (3-11) |
The ordinal data are reported as mean ± standard deviation when they have a normal distribution, as median (Q1-Q3) when they do not have a normal distribution, and nominal data as absolute value and percentage.
ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; bpm, beats per minute; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DVT, deep veinous thrombosis; Hs, high sensitivity; HSCs, hematopoietic stem cells; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PE, pulmonary embolism; Q; quartile.
Additional information in supplemental Table 2.
GFR <90 mL/min per 1.73m2 according to CKD-EPI
Echocardiographic variables in the study population
| Echocardiography data . | Patients (n = 130) . |
|---|---|
| LV function and remodeling | |
| LVEF in Simpson biplane (%) | 58 ± 6 |
| LVEF <50%, n (%) | 8 (6) |
| GLS (%) | −17.9 ± 2.7 |
| Cardiac output (L/min) | 6.6 ± 1.8 |
| Indexed LV volume (mL/m2) | 82 ± 22 |
| Indexed LV mass (g/m2) | 108 ± 36 |
| Diastolic function | |
| E (cm/s) | 87 ± 24 |
| EDT (ms) | 190 ± 48 |
| A (cm/s) | 64 ± 22 |
| E/A | 1.5 ± 0.6 |
| E' lateral (cm/s) | 13 ± 4 |
| E/E'lateral | 8 ± 3 |
| Indexed LA volume (mL/m2) | 57 ± 19 |
| Right ventricular function and right pressures | |
| TAPSE (mm) | 25 ± 5 |
| S-wave (cm/s) | 14 ± 3 |
| TRV (m/s), n = 124 | 2.6 ± 0.3 |
| TRV ≥3 m/s, n (%) | 23 (19) |
| Pulmonary acceleration time (ms) | 132 ± 31 |
| Indexed tricuspid ring (mm/m2) | 19 ± 3 |
| Telesystolic area of the right atrium (cm2) | 20 ± 6 |
| Systolic pulmonary arterial pressure (mm Hg) | 33 ± 9 |
| Right atrium pressure (mm Hg) | 5 ± 3 |
| Valve disease | |
| Severe valve disease (grade 4), n (%) | 1 (1) |
| Mild to moderate valve disease (grades 2 and 3), n (%) | 15 (12) |
| Echocardiography data . | Patients (n = 130) . |
|---|---|
| LV function and remodeling | |
| LVEF in Simpson biplane (%) | 58 ± 6 |
| LVEF <50%, n (%) | 8 (6) |
| GLS (%) | −17.9 ± 2.7 |
| Cardiac output (L/min) | 6.6 ± 1.8 |
| Indexed LV volume (mL/m2) | 82 ± 22 |
| Indexed LV mass (g/m2) | 108 ± 36 |
| Diastolic function | |
| E (cm/s) | 87 ± 24 |
| EDT (ms) | 190 ± 48 |
| A (cm/s) | 64 ± 22 |
| E/A | 1.5 ± 0.6 |
| E' lateral (cm/s) | 13 ± 4 |
| E/E'lateral | 8 ± 3 |
| Indexed LA volume (mL/m2) | 57 ± 19 |
| Right ventricular function and right pressures | |
| TAPSE (mm) | 25 ± 5 |
| S-wave (cm/s) | 14 ± 3 |
| TRV (m/s), n = 124 | 2.6 ± 0.3 |
| TRV ≥3 m/s, n (%) | 23 (19) |
| Pulmonary acceleration time (ms) | 132 ± 31 |
| Indexed tricuspid ring (mm/m2) | 19 ± 3 |
| Telesystolic area of the right atrium (cm2) | 20 ± 6 |
| Systolic pulmonary arterial pressure (mm Hg) | 33 ± 9 |
| Right atrium pressure (mm Hg) | 5 ± 3 |
| Valve disease | |
| Severe valve disease (grade 4), n (%) | 1 (1) |
| Mild to moderate valve disease (grades 2 and 3), n (%) | 15 (12) |
The ordinal data are reported as mean ± standard deviation and the nominal data are reported as absolute value and percentage.
A, mitral end-diastolic velocity; ASE, American Society of Echocardiography; E, mitral protodiastolic velocity; E', mitral protodiastolic annular velocity; EDT, E wave deceleration time; GLS, global longitudinal strain; LVEDVi, indexed LV end-diastolic volume; S, tricuspid annular mesosystolic velocity; TAPSE, tricuspid annular plane systolic excursion.
A total of 100 patients (77%) underwent the 6-minute walking test with a median distance of 475 ± 130 m (75% ± 17% of the predicted value) and frequently showed oxygen desaturation with 68 patients (60%) falling below 88% during the test (supplemental Data; Table 3).
Rhythmic data and 24-hour ECG- Holter results
| Characteristics . | Patients . |
|---|---|
| Corrected QT on surface ECG (ms) | 417 ± 29 |
| Corrected QT, >440 ms, n (%) | 24 (18) |
| Heart rate (bpm) | 72 ± 13 |
| Antiarrhythmic and anticoagulation medication, n (%) | 45 (35) |
| Beta-blockers | 43 (33) |
| Cordarone | 5 (4) |
| Flecaine | 4 (3) |
| Anticoagulation for AF history | 15 (12) |
| Indication for beta-blockers, n = 45, n (%) | |
| Antianginal | 5 (11) |
| Atrial hyperexcitability | 22 (49) |
| Ventricular hyperexcitability | 8 (18) |
| Dilated heart disease | 11 (24) |
| LV obstruction | 3 (7) |
| Arterial hypertension | 4 (9) |
| AA, n (%) | 34 (26) |
| Detail | |
| History of paroxysmal AF, n (%) | 11 (8) |
| Permanent or persistent AF, n (%) | 5 (4) |
| 24-Holter results | |
| Total PACs per 24 h, n = 125 | 34 (5-229), (min 0; max 25 204) |
| PAC of >720 per 24 h, n = 125 | 17 (14) |
| Episodic runs of ≥20 PACs | 6 (5) |
| ESVEA, n (%), n = 125 | 20 (16) |
| ESVEA without previous history of AF, n = 125 | 16 (13) |
| AF, n (%) | 7 (5) |
| New diagnosis of AF, n (%) | 2 (2) |
| Ventricular data | |
| Ventricular arrhythmia, n (%) | 26 (20) |
| Characteristics . | Patients . |
|---|---|
| Corrected QT on surface ECG (ms) | 417 ± 29 |
| Corrected QT, >440 ms, n (%) | 24 (18) |
| Heart rate (bpm) | 72 ± 13 |
| Antiarrhythmic and anticoagulation medication, n (%) | 45 (35) |
| Beta-blockers | 43 (33) |
| Cordarone | 5 (4) |
| Flecaine | 4 (3) |
| Anticoagulation for AF history | 15 (12) |
| Indication for beta-blockers, n = 45, n (%) | |
| Antianginal | 5 (11) |
| Atrial hyperexcitability | 22 (49) |
| Ventricular hyperexcitability | 8 (18) |
| Dilated heart disease | 11 (24) |
| LV obstruction | 3 (7) |
| Arterial hypertension | 4 (9) |
| AA, n (%) | 34 (26) |
| Detail | |
| History of paroxysmal AF, n (%) | 11 (8) |
| Permanent or persistent AF, n (%) | 5 (4) |
| 24-Holter results | |
| Total PACs per 24 h, n = 125 | 34 (5-229), (min 0; max 25 204) |
| PAC of >720 per 24 h, n = 125 | 17 (14) |
| Episodic runs of ≥20 PACs | 6 (5) |
| ESVEA, n (%), n = 125 | 20 (16) |
| ESVEA without previous history of AF, n = 125 | 16 (13) |
| AF, n (%) | 7 (5) |
| New diagnosis of AF, n (%) | 2 (2) |
| Ventricular data | |
| Ventricular arrhythmia, n (%) | 26 (20) |
The ordinal data are reported as mean ± standard deviation when they have a normal distribution or as median (Q1-Q3) when they do not have a normal distribution, and nominal data are presented as absolute value and percentage.
LVH, left ventricular hypertrophy; max, maximum; min, minimum; PAC, premature atrial complex; Q, quartile; .
Cardiac rhythmic data
In total, 35% of the patients were already treated with antiarrhythmic drugs (mainly beta-blockers) for various overlapping indications, including atrial hyperexcitability (49%), and 15 (12%) were under chronic anticoagulation therapy for a history of AF (Table 3). AA was observed in 34 patients (26%), 20 of whom had ESVEA observed during ECG-Holter; 11 had a recent history of paroxysmal AF and 5 were in permanent or persistent AF (Table 3). In addition, ECG-Holter revealed unknown AF in 2 patients. Among patients with AA, the median PACs per 24 hours was 966 (interquartile range, 122-3169), with 7 patients (25%) above 3000 PACs per 24 hours.
Determinants of AA in SCA: univariate analysis
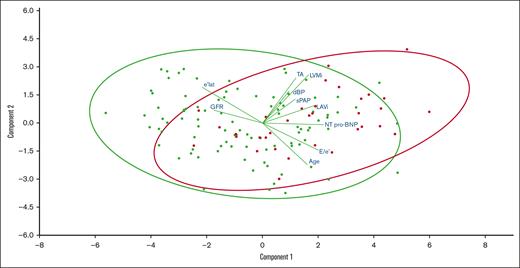
The demographic characteristics of patients with SCA with or without significant AA (n = 34 vs 96) are compared in Table 4, and the projection onto 2 dimensions of the main continuous variables associated with AA is provided in Figure 1. The 2 groups were comparable in terms of SCD treatment (hydroxyurea and erythrocytapheresis). Patients with SCA who exhibited AA were older (52 ± 9 vs 42 ± 12 years; P < .001) and had a similar sex ratio as those without AA. They had a higher diastolic blood pressure (79 ± 11 vs 74 ± 12 mm Hg; P = .01) and more frequently had a history of chronic kidney disease (50% vs 28%; P = .02). Patients with AA had worse kidney function (glomerular filtration rate [GFR], 88 mL/min per 1.73 m2 [48-107] vs 111 mL/min per 1.73 m2 [80-123]; P = .02) and higher plasma levels of Nt Pro-BNP (390 ng/L [131-1243] vs 125 ng/L [44-276]; P < .001) with no difference in hemoglobin or hemolysis markers. Although the total number of stroke episodes was comparable between the 2 groups, the ones potentially linked to ARD or of undetermined cause (n = 14, 56%) were more frequent in the group with AA (26% vs 5%; P = .001).
Univariate and multivariate analyses on primary end point
| Characteristics . | AA (n = 34) . | No AA (n = 96) . | P value in univariate . | P value in multivariate . | OR (95% CI) . |
|---|---|---|---|---|---|
| Demographic and clinical | |||||
| Men | 17 (50) | 45 (47) | .8 | ||
| Age (y) | 52 ± 9 | 42 ± 12 | <.001 | .002∗ | 1.07 (1.02-1.12)∗ |
| Body mass index (kg/m2) | 24 ± 4 | 24 ± 4 | .5 | ||
| Systolic blood pressure (mm Hg) | 133 ± 15 | 128 ± 18 | .1 | ||
| Diastolic blood pressure (mm Hg) | 79 ± 11 | 74 ± 12 | .01 | ||
| Heart rate (bpm) | 69 ± 14 | 73 ± 12 | .1 | ||
| Dyspnea equal to or greater than NYHA class 2, n (%) | 19 (56) | 50 (52) | .7 | ||
| Cardiovascular risk factors, n (%) | |||||
| Arterial hypertension | 25 (74) | 49 (51) | .02 | ||
| Diabetes | 1 (3) | 3 (3) | .9 | ||
| Dyslipidemia | 1 (3) | 0 (0) | — | ||
| Obesity | 2 (6) | 6 (6) | .9 | ||
| Obstructive sleep apnea syndrome | 5 (15) | 8 (8) | .3 | ||
| Chronic kidney disease† | 17 (50) | 27 (28) | .02 | ||
| Active smoking | 5 (15) | 11 (11) | .6 | ||
| Thromboembolic history (PE/DVT) | 9 (26) | 26 (27) | .9 | ||
| History of stroke | 9 (26) | 16 (17) | .3 | ||
| Possible ARD-related stroke or undetermined cause‡ | 9 (26) | 5 (5) | .001 | .009∗ | 6.6 (1.4-30.3)∗ |
| Treatment of SCD, n (%) | |||||
| Hydroxyurea | 18 (53) | 63 (66) | .2 | ||
| Erythrocytapheresis | 5 (15) | 6 (6) | .1 | ||
| Hydroxyurea + erythrocytapheresis | 8 (24) | 16 (17) | .4 | ||
| Bloodletting | 0 (0) | 2 (2) | - | ||
| Biological characteristics | |||||
| Hemoglobin (g/dL) | 8.8 ± 1.7 | 8.7 ± 1.4 | .5 | ||
| Leukocytes (×109/L) | 7.7 ± 2.5 | 7.7 ± 2.7 | .9 | ||
| Platelets (×109/L) | 275 ± 90 | 326 ± 142 | .06 | ||
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | .8 | ||
| Creatinine (μmol/L) | 87 (57-129) | 59 (51-81) | .7 | ||
| GFR (CKD-EPI, mL/min per 1.73m2) | 88 (48-107) | 111 (80-123) | .02 | ||
| ASAT (IU/L) | 49 (36-68) | 41 (31-60) | .1 | ||
| ALAT (IU/L) | 22 (16-29) | 25 (18-38) | .5 | ||
| LDH (IU/L) | 498 (375-645) | 438 (341-589) | .2 | ||
| Total bilirubin (μmol/L) | 32 (18-46) | 30 (21-48) | .7 | ||
| Free bilirubin (μmol/L) | 20 (10-31) | 22 (13-33) | .5 | ||
| Nt Pro-BNP (ng/L) | 390 (131-1243) | 125 (44-276) | <.001 | ||
| Nt Pro-BNP ≥160 ng/L, n (%) | 23 (68) | 38 (40) | .006 | ||
| Echocardiography data | |||||
| LV function and remodeling | |||||
| LVEF in Simpson biplane (%) | 57 ± 7 | 58 ± 6 | .8 | ||
| GLS (%) | −17.4 ± 3.4 | −18.1 ± 2.4 | .2 | ||
| Cardiac output (L/min) | 6.4 ± 2.1 | 6.6 ± 1.6 | .6 | ||
| Indexed LV volume (mL/m2) | 83 ± 26 | 82 ± 21 | .9 | ||
| Indexed LV mass (g/m2) | 122 ± 41 | 103 ± 33 | .009 | ||
| Diastolic function | |||||
| E (cm/s) | 95 ± 22 | 84 ± 24 | .02 | ||
| EDT (ms) | 197 ± 50 | 187 ± 47 | .3 | ||
| A (cm/s), n = 124 | 67 ± 19 | 64 ± 23 | .6 | ||
| E/A, n = 124 | 1.6 ± 0.7 | 1.4 ± 0.6 | .3 | ||
| E' lateral (cm/s) | 11 ± 3 | 14 ± 4 | .003 | ||
| E/E'lateral | 9.0 ± 3.1 | 7.1 ± 2.7 | .001 | ||
| Indexed LA volume (mL/m2) | 71 ± 24 | 52 ± 14 | <.001 | <.001∗ | 1.05 (1.02-1.08)∗ |
| Right ventricular function and right pressures | |||||
| TAPSE (mm) | 25 ± 5 | 26 ± 5 | .6 | ||
| S-wave (cm/s) | 14 ± 2 | 15 ± 3 | .4 | ||
| TRV (m/s), n = 124 | 2.7 ± 0.3 | 2.6 ± 0.3 | .07 | ||
| TRV ≥3 m/s | 9 (27) | 14 (15) | .1 | ||
| Pulmonary acceleration time (ms) | 123 ± 35 | 135 ± 30 | .08 | ||
| Tricuspid ring diameter (mm) | 38 ± 6 | 34 ± 5 | .002 | .2 | |
| RV end-diastolic area (cm2) | 22 ± 5 | 22 ± 36 | .9 | ||
| Telesystolic area of the right atrium (cm2) | 23 ± 7 | 19 ± 5 | .003 | ||
| sPAP (mm Hg), n = 124 | 35 ± 8 | 32 ± 9 | .03 | .3 | |
| Right atrium pressure (mm Hg), n = 129 | 6 ± 4 | 4 ± 3 | .004 | ||
| Rhythmologic characteristics | |||||
| Corrected QT on surface ECG (ms) | 416 ± 31 | 417 ± 28 | .9 | ||
| Corrected QT >440 ms, n (%) | 4 (12) | 20 (21) | .3 | ||
| Antiarrhythmic treatment, n (%) | 21 (62) | 24 (25) | <.001 | ||
| Beta-blockers | 19 (56) | 24 (25) | .001 | ||
| Cordarone | 4 (12) | 1 (1) | .02 | ||
| Flecaine | 4 (12) | 0 (0) | .004 | ||
| 24-Holter results | |||||
| Ventricular arrhythmia, n (%) | 11 (32) | 15 (16) | .04 | ||
| Walking test data (n = 100) | |||||
| Test performed, n (%) | 21 (62) | 79 (82) | - | ||
| Walking distance (m) | 457 ± 148 | 480 ± 125 | .5 | ||
| Percentage of predicted distance (%) | 78 ± 18 | 75 ± 17 | .4 | ||
| Baseline saturation | 94 ± 3 | 95 ± 3 | .7 | ||
| Average saturation | 91 ± 6 | 89 ± 7 | .4 | ||
| Percentage of time spent <88% (%) | 9 (0-62) | 5 (0-58) | .8 |
| Characteristics . | AA (n = 34) . | No AA (n = 96) . | P value in univariate . | P value in multivariate . | OR (95% CI) . |
|---|---|---|---|---|---|
| Demographic and clinical | |||||
| Men | 17 (50) | 45 (47) | .8 | ||
| Age (y) | 52 ± 9 | 42 ± 12 | <.001 | .002∗ | 1.07 (1.02-1.12)∗ |
| Body mass index (kg/m2) | 24 ± 4 | 24 ± 4 | .5 | ||
| Systolic blood pressure (mm Hg) | 133 ± 15 | 128 ± 18 | .1 | ||
| Diastolic blood pressure (mm Hg) | 79 ± 11 | 74 ± 12 | .01 | ||
| Heart rate (bpm) | 69 ± 14 | 73 ± 12 | .1 | ||
| Dyspnea equal to or greater than NYHA class 2, n (%) | 19 (56) | 50 (52) | .7 | ||
| Cardiovascular risk factors, n (%) | |||||
| Arterial hypertension | 25 (74) | 49 (51) | .02 | ||
| Diabetes | 1 (3) | 3 (3) | .9 | ||
| Dyslipidemia | 1 (3) | 0 (0) | — | ||
| Obesity | 2 (6) | 6 (6) | .9 | ||
| Obstructive sleep apnea syndrome | 5 (15) | 8 (8) | .3 | ||
| Chronic kidney disease† | 17 (50) | 27 (28) | .02 | ||
| Active smoking | 5 (15) | 11 (11) | .6 | ||
| Thromboembolic history (PE/DVT) | 9 (26) | 26 (27) | .9 | ||
| History of stroke | 9 (26) | 16 (17) | .3 | ||
| Possible ARD-related stroke or undetermined cause‡ | 9 (26) | 5 (5) | .001 | .009∗ | 6.6 (1.4-30.3)∗ |
| Treatment of SCD, n (%) | |||||
| Hydroxyurea | 18 (53) | 63 (66) | .2 | ||
| Erythrocytapheresis | 5 (15) | 6 (6) | .1 | ||
| Hydroxyurea + erythrocytapheresis | 8 (24) | 16 (17) | .4 | ||
| Bloodletting | 0 (0) | 2 (2) | - | ||
| Biological characteristics | |||||
| Hemoglobin (g/dL) | 8.8 ± 1.7 | 8.7 ± 1.4 | .5 | ||
| Leukocytes (×109/L) | 7.7 ± 2.5 | 7.7 ± 2.7 | .9 | ||
| Platelets (×109/L) | 275 ± 90 | 326 ± 142 | .06 | ||
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | .8 | ||
| Creatinine (μmol/L) | 87 (57-129) | 59 (51-81) | .7 | ||
| GFR (CKD-EPI, mL/min per 1.73m2) | 88 (48-107) | 111 (80-123) | .02 | ||
| ASAT (IU/L) | 49 (36-68) | 41 (31-60) | .1 | ||
| ALAT (IU/L) | 22 (16-29) | 25 (18-38) | .5 | ||
| LDH (IU/L) | 498 (375-645) | 438 (341-589) | .2 | ||
| Total bilirubin (μmol/L) | 32 (18-46) | 30 (21-48) | .7 | ||
| Free bilirubin (μmol/L) | 20 (10-31) | 22 (13-33) | .5 | ||
| Nt Pro-BNP (ng/L) | 390 (131-1243) | 125 (44-276) | <.001 | ||
| Nt Pro-BNP ≥160 ng/L, n (%) | 23 (68) | 38 (40) | .006 | ||
| Echocardiography data | |||||
| LV function and remodeling | |||||
| LVEF in Simpson biplane (%) | 57 ± 7 | 58 ± 6 | .8 | ||
| GLS (%) | −17.4 ± 3.4 | −18.1 ± 2.4 | .2 | ||
| Cardiac output (L/min) | 6.4 ± 2.1 | 6.6 ± 1.6 | .6 | ||
| Indexed LV volume (mL/m2) | 83 ± 26 | 82 ± 21 | .9 | ||
| Indexed LV mass (g/m2) | 122 ± 41 | 103 ± 33 | .009 | ||
| Diastolic function | |||||
| E (cm/s) | 95 ± 22 | 84 ± 24 | .02 | ||
| EDT (ms) | 197 ± 50 | 187 ± 47 | .3 | ||
| A (cm/s), n = 124 | 67 ± 19 | 64 ± 23 | .6 | ||
| E/A, n = 124 | 1.6 ± 0.7 | 1.4 ± 0.6 | .3 | ||
| E' lateral (cm/s) | 11 ± 3 | 14 ± 4 | .003 | ||
| E/E'lateral | 9.0 ± 3.1 | 7.1 ± 2.7 | .001 | ||
| Indexed LA volume (mL/m2) | 71 ± 24 | 52 ± 14 | <.001 | <.001∗ | 1.05 (1.02-1.08)∗ |
| Right ventricular function and right pressures | |||||
| TAPSE (mm) | 25 ± 5 | 26 ± 5 | .6 | ||
| S-wave (cm/s) | 14 ± 2 | 15 ± 3 | .4 | ||
| TRV (m/s), n = 124 | 2.7 ± 0.3 | 2.6 ± 0.3 | .07 | ||
| TRV ≥3 m/s | 9 (27) | 14 (15) | .1 | ||
| Pulmonary acceleration time (ms) | 123 ± 35 | 135 ± 30 | .08 | ||
| Tricuspid ring diameter (mm) | 38 ± 6 | 34 ± 5 | .002 | .2 | |
| RV end-diastolic area (cm2) | 22 ± 5 | 22 ± 36 | .9 | ||
| Telesystolic area of the right atrium (cm2) | 23 ± 7 | 19 ± 5 | .003 | ||
| sPAP (mm Hg), n = 124 | 35 ± 8 | 32 ± 9 | .03 | .3 | |
| Right atrium pressure (mm Hg), n = 129 | 6 ± 4 | 4 ± 3 | .004 | ||
| Rhythmologic characteristics | |||||
| Corrected QT on surface ECG (ms) | 416 ± 31 | 417 ± 28 | .9 | ||
| Corrected QT >440 ms, n (%) | 4 (12) | 20 (21) | .3 | ||
| Antiarrhythmic treatment, n (%) | 21 (62) | 24 (25) | <.001 | ||
| Beta-blockers | 19 (56) | 24 (25) | .001 | ||
| Cordarone | 4 (12) | 1 (1) | .02 | ||
| Flecaine | 4 (12) | 0 (0) | .004 | ||
| 24-Holter results | |||||
| Ventricular arrhythmia, n (%) | 11 (32) | 15 (16) | .04 | ||
| Walking test data (n = 100) | |||||
| Test performed, n (%) | 21 (62) | 79 (82) | - | ||
| Walking distance (m) | 457 ± 148 | 480 ± 125 | .5 | ||
| Percentage of predicted distance (%) | 78 ± 18 | 75 ± 17 | .4 | ||
| Baseline saturation | 94 ± 3 | 95 ± 3 | .7 | ||
| Average saturation | 91 ± 6 | 89 ± 7 | .4 | ||
| Percentage of time spent <88% (%) | 9 (0-62) | 5 (0-58) | .8 |
The univariate analyses used the analysis of variance test for quantitative variables and the χ2 test for qualitative variables. A significance level P ≤ .05 was considered statistically significant. A multivariate analysis was performed using binary logistic regression on univariate significant values (in bold).
Abbreviations are explained in Table 1.
Values are statistically significant (P < .05) and the OR is indicated with the 95% confidence interval.
GFR <90 mL/min per 1.73m2 according to CKD-EPI
Stroke of undetermined etiology or compatible with rhythmic cardioembolic mechanism according to an expert consensus
Continuous variables associated with AA. A biplot representation of the relationship between continuous variables (lines) associated with AA while simultaneously displaying the patients (dots) based on their individual characteristics. Results are projected onto the 2 first dimensions yielded by component analysis. Green dots represent patients without AA and red dots represent patients with AA.
Continuous variables associated with AA. A biplot representation of the relationship between continuous variables (lines) associated with AA while simultaneously displaying the patients (dots) based on their individual characteristics. Results are projected onto the 2 first dimensions yielded by component analysis. Green dots represent patients without AA and red dots represent patients with AA.
Interestingly, despite similar LV systolic function but higher LVMi (128 ± 52 vs 105 ± 31 g/m2; P = .02) in both sexes (data not show) among patients with AA, echocardiography showed signs of worsening diastolic function in patients with AA, which was associated with a higher LAVi (71 ± 24 vs 52 ± 14 mL/m2; P < .001) and E/e' ratio (9.0 ± 3.1 vs 7.1 ± 2.7; P = .001) and a lower e'lateral wave (11 ± 3 vs 14 ± 4 cm/s; P = .003). In addition, patients with AA had more dilated right cavities and higher systolic pulmonary pressure (Table 4).
Because chronic and persistent AF itself contributes to heart cavities remodeling and hemodynamic changes, we performed the same univariate analysis after excluding the 5 patients with persistent or permanent AF (data not shown). The same results were found, apart from diastolic blood pressure and GFR for which a trend was observed (P = .06 for both).
Determinants of AA in SCA: multivariate analysis
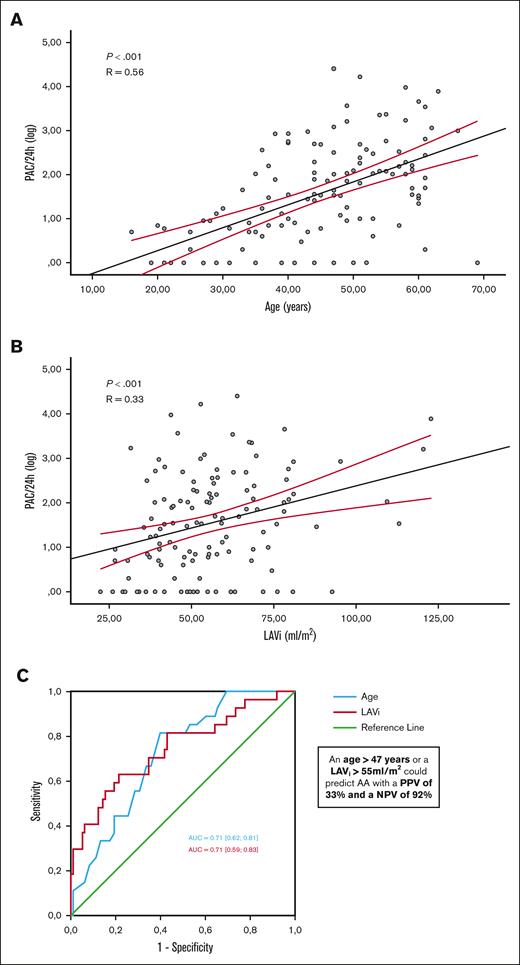
The following variables were included in a multivariate analysis: age, diastolic blood pressure, history of possible ARD-related stroke, GFR, NT pro-BNP, LVMi, E/e’ ratio, LAVi, tricuspid annulus diameter, and sPAP (Table 4). Age (OR, 1.07 [95% CI, 1.02-1.12]; P = .001), a history of plausible ARD-related stroke (OR, 6.6 [95% CI, 1.4-30.3]; P = .009), and LAVi (OR, 1.05 [95% CI, 1.02-1.08]; P < .001) were independently associated with AA. PAC load per 24 hours was correlated with age (R = 0.56; P < .001) and LAVi (R = 0.33; P < .001; Figure 2). Age and LAVi showed good accuracy for identifying patients with AA (AUC, 0.71 [0.62-0.81] and 0.71 [0.59-0.83], respectively) with optimal cutoffs of 47 years (Se = 82%; Sp = 62%) and 55 mL/m2 for LAVi (Se = 71%; Sp = 66%). Meeting at least 1 of these criteria, that is, age >47 years or LAVi >55 mL/m2, could predict AA with a positive predictive value of 33% and a negative predictive value of 92%.
Independant variables associated with AA. Correlation observed between PACs (logarithmic scale) and age (A) and indexed LA volume (B). Receiver operating characteristic curves of age and LAVi for identifying AA and determination of the optimal thresholds (with the best sensitivity and specificity) (C).
Independant variables associated with AA. Correlation observed between PACs (logarithmic scale) and age (A) and indexed LA volume (B). Receiver operating characteristic curves of age and LAVi for identifying AA and determination of the optimal thresholds (with the best sensitivity and specificity) (C).
History of stroke and early follow-up data
Because stroke with a plausible ARD mechanism was strongly associated with AA, we retrospectively explored and described all these events (supplemental Table 2). Among the 25 patients with a history of stroke (29% of the cohort), 11 (44%) had an identified cause, mainly cerebral vasculopathy (32%). Regarding the 14 others (56%), only 1 patient had a history of AF preceding the stroke but was not under anticoagulation therapy because of a low CHADS2-VASc score.41 To date, for the 13 other patients, AF was found in 7 of 13 patients (56%), mainly months after a stroke diagnosis (97 days [range, 0-287]), using long-term duration ECG, which enabled us to start anticoagulation therapy (supplemental Table 2). For the 6 remaining patients, rhythmic investigation is ongoing or scheduled.
Discussion
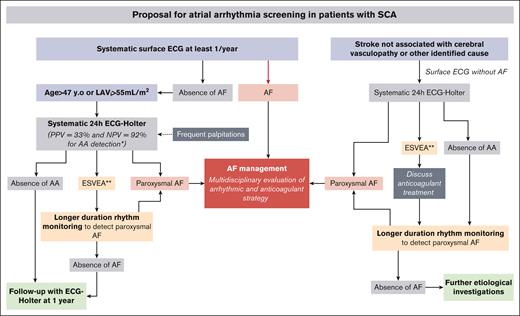
In this pilot study, we prospectively explored, for the first time to our knowledge, the occurrence of AA in patients with SCA by combining clinical, biologic, and echocardiographic evaluation concomitantly with 24-hour ECG monitoring. Our results underline the very high prevalence of AA in SCA, its association with age and LA remodeling, and, more importantly, its association with ischemic strokes unrelated to cerebral vasculopathy. These preliminary data are crucial because they shed new light on AA, an underdiagnosed condition among patients with SCA that could be linked to cardiac and cerebrovascular dramatic complications. Based on these data, derived from a cohort with cardiac risk factors, and along with previous studies,6,9,24,26 we suggest a strategy to implement large-scale systematic screening for AAs among patients with SCA, thereby opening the way to new avenues for larger observational studies before randomized clinical trials (Figure 3).
Proposal for AA screening in patients with SCA. ∗If 1 criterion among age or LAVi is present and ∗∗ESVEA.
Proposal for AA screening in patients with SCA. ∗If 1 criterion among age or LAVi is present and ∗∗ESVEA.
A high prevalence of AA among patients with SCA
The first main finding of this study is the high prevalence of AA (26%) in a selected SCA population study based on cardiac risk factors. Such a prevalence is much higher than that reported in the non-SCD population and occurs 1 to 2 decades earlier.26,30,47,48 We hypothesize that the early LA dilation that progresses through life5,12,13 and the development of myocardial fibrosis,21,49 low-grade systemic inflammation, and frequent hydroelectrolytic disorders16,20,50 act as arrhythmogenic triggers in patients with SCA.15,18,27 Consistent with this, we found that age and indexed LA volume were independently associated with AA and thus can be used to optimize risk stratification. Indeed, an age >47 years or an LAVi >55 mL/m2 predicted AA with a positive predictive value of 33% and a negative predictive value of 92%. Furthermore, it is important to underline that in our study, AA was associated with numerous known mortality and heart failure risk factors in patients with SCA, such as impaired diastolic function,4 elevated pulmonary pressures,33 chronic kidney disease,51 and elevated NT pro-BNP.52 Our results require external validation but argue in favor of systematic screening for ARDs using ECG-Holter among patients with SCA who are growing older or presenting with significant atrial remodeling (Figure 3). Such screening would seem to be in line with current practice seeing that most adult patients with SCA already benefit from screening or recurrent echocardiography to detect signs of diastolic dysfunction or pulmonary hypertension.53
Different AA subgroups and potential implications
In our study population, AAs included a majority of patients who presented with ESVEA, some with paroxysmal AF and a few with persistent AF. The link between AF, heart failure, stroke, and mortality is well established in the general population,31,34,41,54,55 but very few studies reported an increased risk for stroke and mortality among patients with SCD with AF.9,25 In contrast, the ESVEA entity (≥720 PACs per day or any episodic runs of ≥20 PACs) has attracted considerable interest in recent years and has led the European Heart Rhythm Association to consider ESVEA as a surrogate marker for paroxysmal AF.40 Because ESVEA has never been investigated in patients with SCA, we may hypothesize that many patients with SCA have undiagnosed ESVEA with a substantial risk for progression to short-term AF, thereby exposing them to subsequent cardiac and embolic complications. Indeed, ESVEA is associated with a risk for incident AF,30 stroke,47 and mortality in the general populations.40,56 In our study, 25% of the patients with ESVEA had >3000 PACs per day, a cutoff value that has been shown to increase the 2-year risk for AF in patients without SCD by 11-fold.57 Consistently, our early follow-up data using longer-duration ECG monitoring or systematic ECG during patients’ visits revealed that, to date, 6 of 16 patients (40%) who presented with ESVEA without a history of AF showed paroxysmal AF in the short-term follow-up (<2 years, data not published). These preliminary data need to be consolidated but underline the importance of screening for AF among patients with SCA with ESVEA using longer-duration rhythm monitoring (Figure 3).
AA is associated with an increased risk of stroke history
A major finding of this study is the independent association between AA and a history of stroke unrelated to cerebral vasculopathy or any other defined cause (OR, 6.6). Stroke is a frequent complication of SCD with a dramatic impact on the quality of life and mortality. Indeed, about 50% of patients with SCA experience a silent cerebral infarct before 30 years of age and 4% experience overt stroke by the age of 40 years.29,58,59 It is now recognized that ischemic strokes in adults with SCA have a variety of causes, including mainly cerebral vasculopathy but also cardioembolism or sometimes an undetermined cause.7,60 In our study, although the overall history of stroke was not different between patients with or without AA, ARDs were independently associated with the majority of ischemic stroke events (56%) after excluding those related to cerebral vasculopathy or other defined cause. For more than half of these strokes, an AF episode was found often concomitantly but mostly months following the event using long-term ECG monitoring (supplemental Table 2). Notably, a paradoxical embolus through the foramen ovale only affected 1 patient. To date, 28% of all strokes in this cohort are compatible with an AF complication. These data are consistent with our previous study that reported a significant prevalence of cardioembolic stroke in adults with SCA6 and underline the critical importance of detecting AA early and initiating the appropriate preventive therapies. In addition, these findings emphasize the relevance of screening for rhythmic abnormalities in patients with SCA with stroke that cannot solely be explained by evolved cerebral vasculopathy. Based on these preliminary data, we advocate for a systematic ECG-Holter in patients with SCA who experienced stroke not related to cerebral vasculopathy and to use longer-duration ECG monitoring (including implantable loop recorder) if the 24-hour monitoring is not contributive (Figure 3). This strategy is already carried out in the etiologic assessment of cryptogenic stroke in the general population and is associated with higher detection rates of AF.40,54,61,62 In face of such a prevalence of clinically overt stroke and AA in adult patients with SCA, improving the screening for AA and particularly paroxysmal AF could have major beneficial consequences in terms of morbidity and mortality.60
Limitations
This was a pilot prospective study and was thus underpowered with AA occurring in only 34 patients. Nevertheless, to our knowledge, this was the largest prospective cohort of patients with SCA with deep cardiac phenotyping that combined systematic arrhythmia monitoring and cardiac imaging. This population study was biased because patients were selected based on clinical criteria indicative of underlying cardiovascular abnormalities and therefore does not reflect patients with SCA in general. The population was relatively old for SCA (45 years), heterogeneous in terms of antiarrhythmic therapy, and overall, at an advanced stage of the disease with frequent organ complications, which altogether potentially contributed to the AA prevalence. Thus, we are cautious in extending our data to all patients with SCA and are convinced that future studies should include younger patients at lower risk. In addition, we showed an association between AAs and an increased risk for stroke history, but given the retrospective nature of this specific analysis, we cannot be definite about the causal link between these 2 events. However, given the systematic and multidisciplinary etiologic approach applied to each neurologic event that enabled us to rule out the best we could another apparent stroke etiology, we limited this bias to a minimum. Finally, >80% of the study population was under specific SCA treatments known to have major and heterogeneous consequences on hematologic laboratory data. This leads to difficulty in interpreting hematologic data and could have masked differences between the 2 groups.
Perspectives
This pilot study highlights the need to improve the detection of AA among patients with SCA, a population at risk for stroke, heart failure, and sudden death. Our data uncovered parameters that contribute to the identification of a population at risk that may benefit from rhythmic monitoring to precociously detect AA and to start appropriate treatment. To our knowledge, there are currently no data regarding antiarrhythmic and anticoagulant strategies for ESVEA and AF in patients with SCA. We believe that some antiarrhythmics treatments, such as flecainides, should be used with caution because of their known proarrhythmogenic effect in the context of myocardial fibrosis.63 Given the uniqueness of these patients in terms of cardiac remodeling, systemic inflammation, and thrombo-embolic risk, AF guidelines for the general population should not be duplicated.20,21,41,64-66 Considering their high rate of stroke and sudden death and based on our referral center experience, we would advocate for early anticoagulation initiation in patients who present with paroxysmal or persistent AF, disregarding the CHA2DS2-VASc score, if the bleeding risk allows.41 This position needs urgent prospective evaluation. Indeed, identifying therapeutic strategies for ARDs in SCA remains a field to be developed and 1 in which we will play an active part, and we enthusiastically look forward to collaborative international studies. This work focused on patients with SCA, whereas stroke also affects patients with Sickle Hemoglobin-C Disease whose mechanisms are very likely to be different given the distinct underlying pathophysiology.67 Dedicated analyzes will be carried out for this specific population. Overall, these preliminary data are crucial because they highlight an underestimated pathology in adult patients with SCA and lay the foundations for organized screening to ultimately reduce cardiac and cerebrovascular complications.
Conclusion
AAs are very prevalent among middle-aged patients with SCA and are associated with an increased risk for ischemic stroke. Age and LA volume are simple and effective prognostic parameters for arrhythmia risk stratification. This pilot study paves the way for a new era toward a broader and systematic screening for ARDs among high-risk patients with SCA to initiate adapted therapeutic strategies early and to hopefully reduce the cardioembolic stroke burden.
Acknowledgment
This work was supported by FHU-SENEC (grant allocation RSE20003DDA).
Authorship
Contribution: T.d., Z.S., G.D., and P. Bartolucci. designed the research, analyzed the data, and wrote the manuscript; L.S., L.B., H.G., L.A., G.d.L., S.I., P. Balfanz., A.H., Z.A., S.M., T.L.D., D.R., L.T., T.S., C.I., L.A.M., and H.D. performed the research and were involved in the deep cardiac phenotyping of these patients; A.L.P.H.d.d., D.C., and N.L. critically reviewed the manuscript; and E.A. supervised data analysis methodology.
Conflict-of-interest disclosure: P. Bartolucci. received grants from Addmedica, Fabre Foundation, Novartis, and Bluebird in the past 36 months; consulting fees from Addmedica, Novartis, Roche, GBT, Bluebird, Emmaus, Hemanext, and Agios; received honoraria for lectures from Novartis, Addmedica, Jazz Pharmaceuticals; and reports being a member of the Novartis steering committee and cofounder of Innovhem. The remaining authors declare no competing financial interests.
Correspondence: Thomas d’Humières, Physiology Department, Henri Mondor University Hospital, Assistance Publique Hôpitaux de Paris, 51 Ave du Maréchal de Lattre de Tassigny, 94010 Créteil, France; email: thomas.d'humieres@aphp.fr.
References
Author notes
T.d., Z.S., G.D., and P.B. contributed equally to this study.
Data of this study are available upon reasonable request from the corresponding author, Thomas d’Humières (Thomas.dhumieres@aphp.fr).
The full-text version of this article contains a data supplement.