Key Points
Slower thrombin generation and subdued enhancement by platelets were detected in WM samples.
WM-derived IgM impaired HD platelet spreading and aggregation, and plasma thrombin generation.
Visual Abstract
Clinical features in patients with the B-cell lymphoma, Waldenström macroglobulinemia (WM), include cytopenias, immunoglobulin M (IgM)–mediated hyperviscosity, fatigue, bleeding, and bruising. Therapeutics such as Bruton's tyrosine kinase inhibitors (BTKis) exacerbate bleeding risk. Abnormal hemostasis arising from platelet dysfunction, altered coagulation or vascular impairment have not yet been investigated in patients with WM. Therefore, this study aimed to evaluate hemostatic dysfunction in samples from these patients. Whole blood (WB) samples were collected from 14 patients with WM not receiving therapy, 5 patients receiving BTKis and 15 healthy donors (HDs). Platelet receptor levels and reticulation were measured by flow cytometry, plasma thrombin generation with or without platelets by fluorescence resonance energy transfer assay, WB clotting potential by rotational thromboelastometry, and plasma soluble glycoprotein VI (sGPVI) and serum thrombopoietin (TPO) by enzyme-linked immunosorbent assay. Donor platelet spreading, aggregation, and ability to accelerate thrombin generation in the presence of WM-derived IgM were assessed. WM platelet receptor levels, responses to physiological agonists, and plasma sGPVI were within normal ranges. WM platelets had reduced reticulation (P = .0012) whereas serum TPO levels were increased (P = .0040). WM plasma displayed slower thrombin generation (P = .0080) and WM platelets contributed less to endogenous thrombin potential (ETP; P = .0312). HD plasma or platelets incubated with IgM (50-60 mg/mL) displayed reduced spreading (P = .0002), aggregation (P < .0001), and ETP (P = .0081). Thus, alterations to thrombin potential and WB coagulation were detected in WM samples. WM IgM significantly impaired hemostasis in vitro. Platelet and coagulation properties are disturbed in patients with well-managed WM.
Introduction
Waldenström macroglobulinemia (WM) is a rare incurable low-grade B-cell lymphoma, with lymphoplasmacytic infiltration in the bone marrow (BM) and immunoglobulin M (IgM) paraprotein. WM affects ∼3 million people per year,1 however this estimate inadequately captures asymptomatic WM.2 Patients can present with symptoms in later stages of the disease including fatigue, recurrent infections, thrombocytopenia, hyperviscosity, and bleeding.3 Platelet counts of <100 × 109/L are independently associated with worse progression-free survival in patients with WM,4 and bleeding symptoms are often disproportionate to the observed platelet counts. Whether platelet or coagulation defects, or elevated IgM contribute to bleeding propensity in WM remain important and incompletely resolved questions.5,6
Platelets, as primary hemostatic agents, rapidly adhere, activate, and spread upon detection of vascular injury or infection, via coordinated ligand-mediated activation of glycoprotein (GP) receptor signaling pathways. Platelet degranulation, calcium flux, phosphatidylserine (PS) exposure, and thrombin generation ensue, culminating in fibrinogen-αIIbβ3 binding and aggregate formation.7 GPIbα of the GPIb-IX-V complex binds von Willebrand factor (VWF) and other vascular ligands,8,9 whereas GPVI binds collagen and fibrin.10 Younger “reticulated” platelets are more responsive to agonists compared with their older counterparts11 and contain megakaryocyte (MK)–derived RNA.12 As platelets circulate, receptor ectodomains are metalloproteolyzed and intraplatelet RNA decreases. Platelet surface assembly of coagulation factors and capacity to generate thrombin may diminish.9,10 Loss of platelet receptors occurs in patients with bleeding diatheses13-15 and lymphoproliferative diseases,16 and is speculated to be caused by a platelet production defect.16 Monitoring of platelet and coagulation indices in patients with hematological malignancies may help detect disease progression.17
In WM, hyperviscosity is caused by high concentrations of IgM (>50-60 mg/mL) produced by the malignant B cells, which can electrostatically bind and agglutinate red blood cells (RBCs).18 The subsequent increase in rheological drag can result in the physical tearing of capillaries and dysregulated unfolding and proteolysis of high-molecular-weight VWF multimers, resulting in acquired von Willebrand syndrome.19 Elevated levels of IgM in patients with WM can cause cryoglobulinemia,20 amyloidosis,21 specific functional deficiencies in VWF22 and factor VIII,23 accelerated clearance of coagulation factors,24 and functional impairment of platelets.25 In other paraproteinemias such as monoclonal gammopathy of undetermined significance or multiple myeloma, paraproteins have been shown to engage with platelet surface proteins,26,27 plasma proteins,28,29 and coagulation factors30-32 to perturb platelet aggregation and coagulation. All of these sequelae may contribute to bleeding phenotypes and are rapidly reversed with plasmapheresis.33
Bruton's tyrosine kinase (BTK) is widely expressed in hematopoietic cells and participates in signaling pathways mediating B-cell proliferation and platelet function, through activation of nuclear factor-κB.34 BTK inhibitors (BTKis) ibrutinib, zanubrutinib, and acalabrutinib are effective and well-tolerated treatments for B-cell lymphomas,35,36 helping achieve deep and durable responses.37,38 BTKis disrupt signaling pathways initiated by the canonical B-cell and Toll-like receptors, thus reducing B-cell proliferation. Early studies using ibrutinib recorded an attendant risk of bleeding and, importantly, noted defective platelet function in patients with B-cell malignancies before BTKi treatment.39 Newer BTKis with greater selectivity that target different regions of BTK have demonstrated reduced (but not absent) rates of hemorrhage in patients with leukemias and lymphomas.40,41
The cause of the bleeding phenotype in WM has not yet been explored but is likely to involve several intersecting mechanisms. Here, we present data evaluating coagulation potential and platelet function in samples from patients with WM with stable and well-managed disease both on and off treatment, and from healthy donors (HDs) treated with high concentrations of WM-derived IgM in vitro. We identify a reduction in thrombin generation capacity and platelet contribution to thrombus formation in patients with WM, and significantly reduced platelet spreading and aggregation and plasma thrombin generation in IgM-treated HD samples. Laboratory assessment of platelet function and coagulation in patients before receipt of BTKis or other medical interventions may help identify those with strong bleeding potential and stratify patients for bleeding risk.
Materials and methods
Details of extra materials and methods are included in the supplemental Methods.
Patient samples
A total of 18 patients with WM were prospectively recruited into this study along with 15 contemporaneously collected HDs, who were not age- or sex-matched (Table 1). Patients were diagnosed with WM according to the International Workshop on WM guidelines.35,42 All blood samples were collected after obtaining informed consent, according to protocols approved by the Australian National University (2020/102 and 2022/372) and the Canberra Hospital (2023.LRE.00053) human research ethics committees. All work was carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki). Overall, 72% (13/18) of patients with WM were not on active therapy, whereas 28% (5/18) were prescribed BTKis (ibrutinib or zanubrutinib; Table 1). Mean characteristics between patients not on therapy and patients treated with BTKis were: 73 vs 74 years of age, 62% (8/13) vs 40% (2/5) male, 23 vs 7 g/L IgM, and 43% vs 22% BM infiltration (Table 1). Most patients were heavily pretreated and possessed a wide range of comorbidities (Table 1), likely requiring simultaneous anticoagulant or antiplatelet therapy (Table 1). Generally, patients with WM showed lower levels of RBCs, hemoglobin, white blood cells (WBCs) and platelets (Table 1; supplemental Figure 4; Figure 1A). No patients had bleeding symptoms at the time of blood collection.
Information on patients with WM and HDs
| . | HD . | WM + no BTKi . | WM + BTKi . |
|---|---|---|---|
| Number | 15 | 13 | 5 |
| Age (y) | NR (18-60) | 73 (50-87) | 74 (52-91) |
| Sex | |||
| Male | NR | 8 (62%) | 2 (40%) |
| Female | NR | 5 (38%) | 3 (60%) |
| BM involvement (% cellularity) | NT | 43 (5-80) | 22 (2-70) |
| IgM (g/L) | NT | 23 (3-78) | 7 (2-11) |
| Active treatments | |||
| None | 15 (100%) | 13 (100%) | 0 (0%) |
| Ibrutinib | 0 (0%) | 3 (60%) | |
| Zanubrutinib | 0 (0%) | 2 (40%) | |
| Past treatments∗ | |||
| None | NR | 4 (33%) | 2 (40%) |
| R-CVP | 6 (50%) | 1 (20%) | |
| BR | 3 (25%) | 1 (20%) | |
| DRC | 2 (17%) | 0 (0%) | |
| Ibrutinib | 1 (8%) | 1 (20%) | |
| Rituximab | 2 (17%) | 0 (0%) | |
| R-FC | 1 (17%) | 0 (0%) | |
| CR | 1 (8%) | 0 (0%) | |
| Chlorambucil | 1 (8%) | 0 (0%) | |
| CP | 1 (8%) | 0 (0%) | |
| Comorbidities | |||
| None | NT | 2 (17%) | 1 (20%) |
| Cardiovascular | 4 (33%) | 2 (40%) | |
| Hypertension | 2 (17%) | 2 (40%) | |
| Atrial fibrillation | 2 (17%) | 0 (0%) | |
| Ischemic heart disease + stent | 1 (8%) | 1 (20%) | |
| Dyslipidemia | 1 (8%) | 0 (0%) | |
| Cerebral vascular accident | 1 (8%) | 0 (0%) | |
| Aortic stenosis | 1 (8%) | 0 (0%) | |
| Dilated cardiomyopathy | 1 (8%) | 0 (0%) | |
| Patent ductus arteriosus | |||
| Infection/inflammation | 3 (25%) | 0 (0%) | |
| Arthritis | 3 (25%) | 0 (0%) | |
| Recurrent infections | 2 (17%) | 0 (0%) | |
| Sepsis | 1 (8%) | 0 (0%) | |
| Asbestosis | 1 (8%) | 0 (0%) | |
| Glomerulonephritis | 1 (8%) | 0 (0%) | |
| Meningitis | 1 (8%) | 0 (0%) | |
| Sarcoidosis | 1 (8%) | 0 (0%) | |
| Chronic obstructive airway disease | |||
| Vasculitis | 1 (8%) | 0 (0%) | |
| Shingles | 0 (0%) | 1 (20%) | |
| Additional malignancy | |||
| Cryoglobulinemia | 2 (17%) | 0 (0%) | |
| Prostate cancer | 1 (8%) | 0 (0%) | |
| Thoracic mass | 1 (8%) | 0 (0%) | |
| Schwannoma of lumbar spine | 1 (8%) | 0 (0%) | |
| Lower abdomen mass | 1 (8%) | 0 (0%) | |
| Squamous cell carcinoma | 0 (0%) | 2 (40%) | |
| Myelodysplastic syndrome | 0 (0%) | 1 (20%) | |
| RBC count (×1012/L) | 4.1 (3.5-5.8) | 3.0 (1.8-4.4) | 3.4 (2.8-3.9) |
| Hemoglobin (g/L) | 132 (113-199) | 105 (749-142) | 110 (74-128) |
| WBC count (×109/L) | 5.6 (3.8-8.0) | 4.3 (2.0-6.3) | 5.6 (2.3-8.5) |
| Lymphocyte count (×109/L) | 1.9 (1.0-2.5) | 1.3 (0.3-2.1) | 0.5 (0.7-2.6) |
| Platelet count (×109/L) | 207 (73-264) | 151 (35-285) | 143 (72-202) |
| MPV (fL) | 7.4 (6.7-8.7) | 7.6 (6.7-9.4) | 8.5 (7.3-9.7) |
| . | HD . | WM + no BTKi . | WM + BTKi . |
|---|---|---|---|
| Number | 15 | 13 | 5 |
| Age (y) | NR (18-60) | 73 (50-87) | 74 (52-91) |
| Sex | |||
| Male | NR | 8 (62%) | 2 (40%) |
| Female | NR | 5 (38%) | 3 (60%) |
| BM involvement (% cellularity) | NT | 43 (5-80) | 22 (2-70) |
| IgM (g/L) | NT | 23 (3-78) | 7 (2-11) |
| Active treatments | |||
| None | 15 (100%) | 13 (100%) | 0 (0%) |
| Ibrutinib | 0 (0%) | 3 (60%) | |
| Zanubrutinib | 0 (0%) | 2 (40%) | |
| Past treatments∗ | |||
| None | NR | 4 (33%) | 2 (40%) |
| R-CVP | 6 (50%) | 1 (20%) | |
| BR | 3 (25%) | 1 (20%) | |
| DRC | 2 (17%) | 0 (0%) | |
| Ibrutinib | 1 (8%) | 1 (20%) | |
| Rituximab | 2 (17%) | 0 (0%) | |
| R-FC | 1 (17%) | 0 (0%) | |
| CR | 1 (8%) | 0 (0%) | |
| Chlorambucil | 1 (8%) | 0 (0%) | |
| CP | 1 (8%) | 0 (0%) | |
| Comorbidities | |||
| None | NT | 2 (17%) | 1 (20%) |
| Cardiovascular | 4 (33%) | 2 (40%) | |
| Hypertension | 2 (17%) | 2 (40%) | |
| Atrial fibrillation | 2 (17%) | 0 (0%) | |
| Ischemic heart disease + stent | 1 (8%) | 1 (20%) | |
| Dyslipidemia | 1 (8%) | 0 (0%) | |
| Cerebral vascular accident | 1 (8%) | 0 (0%) | |
| Aortic stenosis | 1 (8%) | 0 (0%) | |
| Dilated cardiomyopathy | 1 (8%) | 0 (0%) | |
| Patent ductus arteriosus | |||
| Infection/inflammation | 3 (25%) | 0 (0%) | |
| Arthritis | 3 (25%) | 0 (0%) | |
| Recurrent infections | 2 (17%) | 0 (0%) | |
| Sepsis | 1 (8%) | 0 (0%) | |
| Asbestosis | 1 (8%) | 0 (0%) | |
| Glomerulonephritis | 1 (8%) | 0 (0%) | |
| Meningitis | 1 (8%) | 0 (0%) | |
| Sarcoidosis | 1 (8%) | 0 (0%) | |
| Chronic obstructive airway disease | |||
| Vasculitis | 1 (8%) | 0 (0%) | |
| Shingles | 0 (0%) | 1 (20%) | |
| Additional malignancy | |||
| Cryoglobulinemia | 2 (17%) | 0 (0%) | |
| Prostate cancer | 1 (8%) | 0 (0%) | |
| Thoracic mass | 1 (8%) | 0 (0%) | |
| Schwannoma of lumbar spine | 1 (8%) | 0 (0%) | |
| Lower abdomen mass | 1 (8%) | 0 (0%) | |
| Squamous cell carcinoma | 0 (0%) | 2 (40%) | |
| Myelodysplastic syndrome | 0 (0%) | 1 (20%) | |
| RBC count (×1012/L) | 4.1 (3.5-5.8) | 3.0 (1.8-4.4) | 3.4 (2.8-3.9) |
| Hemoglobin (g/L) | 132 (113-199) | 105 (749-142) | 110 (74-128) |
| WBC count (×109/L) | 5.6 (3.8-8.0) | 4.3 (2.0-6.3) | 5.6 (2.3-8.5) |
| Lymphocyte count (×109/L) | 1.9 (1.0-2.5) | 1.3 (0.3-2.1) | 0.5 (0.7-2.6) |
| Platelet count (×109/L) | 207 (73-264) | 151 (35-285) | 143 (72-202) |
| MPV (fL) | 7.4 (6.7-8.7) | 7.6 (6.7-9.4) | 8.5 (7.3-9.7) |
Values are displayed as number (%) or mean (range). P values were determined by Mann-Whitney t test. Data for HD age, sex, and any past treatments were not collected due to ethics protocol requirements. All current HD medications were screened before blood collection. Patients may have had >1 prior treatment or comorbidity.
BR, bendamustine and rituximab; CR, chlorambucil and rituximab; CP, chlorambucil and prednisolone; DRC, dexamethasone, rituximab, and cyclophosphamide; MPV, mean platelet volume; NR, not recorded; NT, not tested; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisolone; R-FC, rituximab, fludarabine, and cyclophosphamide.
Not all medications were recorded, including but not limited to historical use of anticoagulant, antiplatelet and nonsteroidal anti-inflammatory drug therapies to treat comorbidities.
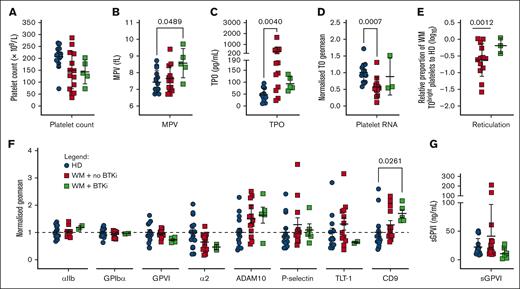
WM platelets have altered thrombopoietic and age-related phenotypic markers. (A) Platelet count, (B) mean platelet volume (MPV), (C) serum thrombopoietin (TPO), (D) normalized thiazole orange (TO) staining of platelets in WB (divided by HD mean), (E) relative proportion of TObright WM platelets falling within the gate encompassing the top 10% of HD platelets (log10 WM/HD %), (F) platelet surface receptor levels in PRP and (G) sGPVI levels in platelet-poor plasma (PPP) from HDs (n = 9-15, blue), patients with WM not on therapy (n = 9-13, red), or receiving BTKis (n = 0-5, green). For panels A-C,E,G, mean +/± SD. For panels D,F, normalized geomeans (divided by mean HD geomean) ± standard error of the mean (SEM). For panels A-C, P values were determined by Kruskal-Wallis 1-way analysis of variance (ANOVA) with Dunn's multiple comparisons test, performed on the raw data; for panel E, Mann-Whitney t test; or for panel E, 1 sample t test.
WM platelets have altered thrombopoietic and age-related phenotypic markers. (A) Platelet count, (B) mean platelet volume (MPV), (C) serum thrombopoietin (TPO), (D) normalized thiazole orange (TO) staining of platelets in WB (divided by HD mean), (E) relative proportion of TObright WM platelets falling within the gate encompassing the top 10% of HD platelets (log10 WM/HD %), (F) platelet surface receptor levels in PRP and (G) sGPVI levels in platelet-poor plasma (PPP) from HDs (n = 9-15, blue), patients with WM not on therapy (n = 9-13, red), or receiving BTKis (n = 0-5, green). For panels A-C,E,G, mean +/± SD. For panels D,F, normalized geomeans (divided by mean HD geomean) ± standard error of the mean (SEM). For panels A-C, P values were determined by Kruskal-Wallis 1-way analysis of variance (ANOVA) with Dunn's multiple comparisons test, performed on the raw data; for panel E, Mann-Whitney t test; or for panel E, 1 sample t test.
Platelet flow cytometry
Platelet surface proteins GPIbα, GPVI, CD9, αIIb, and α2 integrin subunits, a disintegrin and metalloproteinase 10 (ADAM10), P-selectin and Trem-like transcript 1, and estimates of platelet RNA content were measured by flow cytometry using citrated platelet-rich plasma (PRP) or whole blood (WB), as described in supplemental Methods.
Platelet function assays
Unstimulated and agonist-induced αIIbβ3 activation and P-selectin exposure were measured by flow cytometry as described in supplemental Methods.
Thrombin generation
A calibrated automated thrombogram protocol43 was modified for measurement in platelet-poor plasma (PPP) with or without washed platelets (WPs). Briefly, duplicate wells of 96-well plates contained 20 μL ethylenediaminetetraacetic acid (EDTA)- or trisoidium citrate-anticoagulated PPP, 10 μL tris-buffered saline, 10 μL WPs (107, preactivated with 10 μM thrombin receptor activator peptide 6 [TRAP-6] at 37°C for 30 minutes), 10 μL trigger (containing tissue factor and phospholipids) and 10 μL thrombin substrate (Z-Gly-Gly-Arg-7-amino-4-methylcoumarin, Diagnostica Stago S.A.S., Asnières-sur-Seine, France). Separate standard curves were prepared for each donor using a thrombin calibrator. Fluorescence was measured at 390/460 nm at 37°C for 30 minutes using an Infinite 200 PRO microplate reader (Tecan, Zürich, Switzerland), with 6-second shaking at 432 rpm between each cycle. Thrombin concentrations were interpolated from the standard curve and transformed to generate thrombograms.
Thromboelastometry
WB coagulation and platelet contributions to thrombus formation were evaluated by rotational thromboelastometry (ROTEM) according to the manufacturer’s instructions. The INTEM test measures intrinsic coagulation, EXTEM measures extrinsic coagulation, and FIBTEM and PLTEM measure the fibrin and platelet contributions to extrinsic coagulation, respectively.
Plasma and serum proteins
Soluble GPVI (sGPVI) ectodomain fragments were quantified by enzyme-linked immunosorbent assay.44 Serum TPO was quantified in a human TPO Quantikine enzyme-linked immunosorbent assay ELISA (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions. Concentrations were interpolated from standard curves.
Platelet spreading
IgM was enriched from the plasma of a patient with WM (WM13; supplemental Methods). WPs (107) were incubated with IgM (60 mg/mL) or an equimolar amount of bovine serum albumin (BSA; 4.11 mg/mL) or vehicle (6 mM K2HPO4, pH 8.0, 0.006% weight per volume NaN3) for 30 minutes at 37°C and allowed to spread on coverslips for 20 to 60 minutes at 37°C, as previously described.45 A total of 5 images were taken per coverslip using a SP5 Confocal Microscope (Leica, Wetzlar, Germany) and analyzed using ImageJ. Circularity describes how closely the platelet shape approximates a circle, reflecting its degree of surface invaginations, whereas the aspect ratio is calculated as the ratio of the width to the height of the platelet, reflecting its degree of elongation.
Statistical analyses
Data were analyzed using GraphPad Prism version 10.1.0. Additional information is included in figure legends and the supplemental Methods.
Results
Platelet production is dysregulated in WM
We assessed a range of platelet parameters in the WM cohort. Patients with WM displayed reduced mean platelet counts (Figure 1A; Table 1) whereas only patients treated with BTKis displayed significantly enlarged platelets (Figure 1B; Table 1). Median TPO levels were increased 3.8-fold and 1.7-fold, respectively, in patients with WM not receiving therapy or receiving BTKis, compared with HDs (Figure 1C). Platelet RNA content was evaluated by flow cytometry to provide an estimate of circulating platelet age. Samples from patients with WM not receiving therapy showed significantly reduced total platelet RNA (Figure 1D) and percentage of TObright reticulated platelets (Figure 1E) compared with HDs, suggesting that circulating WM platelets were significantly aged. Interestingly, proportions of reticulated platelets in patients with WM receiving BTKis were similar to those in HDs (Figure 1E). In the patients with WM not receiving therapy, we observed a mean 43% BM involvement and 23 g/L IgM (Table 1), which are 43% and 15-fold higher than HD means, respectively, both infiltrate the BM and possibly disrupt the exquisite microenvironment in which the MKs reside to produce platelets.
By flow cytometry, levels of αIIb and α2 integrin subunits, GPIbα, GPVI, ADAM10, P-selectin and Trem-like transcript 1 were all within ranges observed in HDs (Figure 1F). Plasma sGPVI was also within normal range (Figure 1G). Two patients with WM displayed elevated sGPVI levels (>100 ng/mL; Figure 1G), however these levels were not associated with changes to any other markers measured (data not shown). Tetraspanin CD9 was significantly increased on the surface of platelets from patients with WM receiving BTKis (Figure 1F).
TPO levels and platelet counts were significantly inversely correlated in HDs (P = .0368) and in patients with WM (P = .0072, data not shown). There were no other significant correlations between TPO, TO–bound RNA, GPIbα, IgM levels, BM involvement, or platelet counts in HDs or patients with WM (data not shown). Taken together, the slightly lower platelet counts, elevated TPO, and higher proportions of aged platelets in patients with WM not receiving therapy suggest dysregulated platelet production in WM.
WM platelets respond appropriately to agonists
To evaluate WM platelet responsiveness to agonists, αIIbβ3 activation (Figure 2A) and P-selectin exposure (Figure 2C) were measured by flow cytometry. The patient sample volumes collected were insufficient to permit light transmission aggregometry. Levels of active αIIbβ3 (Figure 2B) and P-selectin (Figure 2D) on unstimulated WM platelets were similar to levels in HD samples. Treatment of WM or HD platelets with various doses of crosslinked collagen-related peptide (CRP-XL), TRAP-6 or adenosine diphosphate (ADP) resulted in increased Oregon-green 488 fibrinogen (OgFg) binding and P-selectin exposure, suggesting that WM platelets responded normally in this assay and had normal amounts of protein packaged in their α granules by MKs (Figure 2B,D). As expected, patients with WM receiving BTKis showed minimal responses to 0.5-30 μg/mL CRP-XL and normal responses to all other agonists (Figure 2B,D).
WM platelets respond appropriately to standard agonists. (A-B) OgFg binding and (C-D) P-selectin exposure in WB and PRP samples, respectively, from HDs (n = 7-14, blue), patients with WM not on therapy (n = 8-13, red), or receiving BTKis (n = 0-5, green). Representative histograms from a HD showing (A) OgFg- or (C) P-selectin–positive events. Data (mean ± standard deviation [SD]) for (B) OgFg binding or (D) P-selectin exposure (%) in platelets treated with 0.5, 5 or 30 μg/mL crosslinked CRP-XL, 10 μM TRAP-6, or 5 μM ADP. P values were determined by Mann-Whitney t test with multiple comparisons.
WM platelets respond appropriately to standard agonists. (A-B) OgFg binding and (C-D) P-selectin exposure in WB and PRP samples, respectively, from HDs (n = 7-14, blue), patients with WM not on therapy (n = 8-13, red), or receiving BTKis (n = 0-5, green). Representative histograms from a HD showing (A) OgFg- or (C) P-selectin–positive events. Data (mean ± standard deviation [SD]) for (B) OgFg binding or (D) P-selectin exposure (%) in platelets treated with 0.5, 5 or 30 μg/mL crosslinked CRP-XL, 10 μM TRAP-6, or 5 μM ADP. P values were determined by Mann-Whitney t test with multiple comparisons.
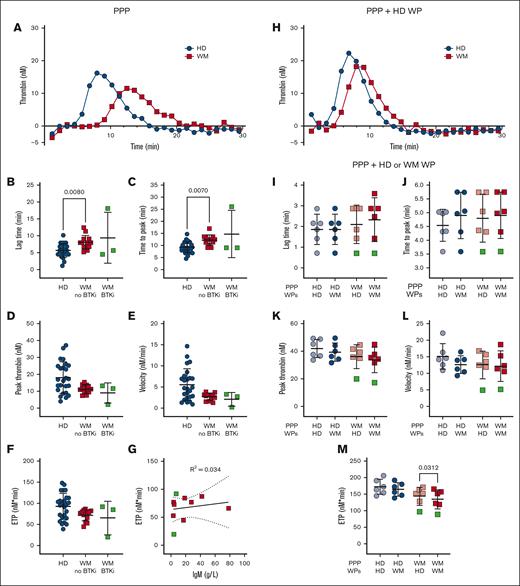
Samples from patients with WM display reduced thrombin generation potential
Platelet aggregation, prothrombin time and activated partial thromboplastin time generally remain within healthy ranges for patients with well-managed WM, and our cohort showed no abnormalities in these assays (data not shown). Plasma thrombin generation studies and platelet contributions to thrombin generation have only been reported in 2 patients with WM and hyperviscosity25 and not in patients with well-managed disease. We developed an assay to quantify plasma thrombin generation in patients with WM and HDs in the absence or presence of HD platelets using a fluorogenic thrombin substrate, in a modified calibrated automated thrombogram protocol, triggered by phospholipids and tissue factor.43 Compared with plasma from HDs, plasma from patients with WM not receiving therapy displayed significantly prolonged lag times (Figure 3A-B) and times to peak thrombin generation (Figure 3C). The peak thrombin (Figure 3D), thrombin generation rate (Figure 3E), and endogenous thrombin potential (ETP; Figure 3F) were all markedly reduced, which became significant if patients with WM were assessed as a single group (data not shown). Plasmas from 3 patients receiving BTKis showed normal rates and extents of thrombin generation (Figure 3B-F). The observed differences were not explained by plasma IgM (Figure 3G). Differences between HD and WM plasma were corrected if protease-activated receptor-1 agonist–activated nonautologous HD platelets were included (Figure 3H). Addition of activated platelets enhanced thrombin generation significantly more in WM plasma than HD plasma (data not shown). When comparing the capacity of WM and HD platelets to accelerate thrombin generation, the ETP in WM plasma was significantly reduced in the presence of WM platelets compared with HD platelets (Figure 3M). No significant differences were observed for the ETP in HD plasma (Figure 3M), nor for the lag time (Figure 3I), time to peak thrombin (Figure 3J), peak thrombin (Figure 3K), or velocity (Figure 3L) in HD or WM plasma.
Patients with WM have delayed and reduced thrombin generation. Thrombin generation in (A-G) EDTA-anticoagulated PPP, (H) EDTA-anticoagulated PPP + PAR-1–activated HD WPs, or (I-M) trisoidium citrate-anticoagulated PPP + PAR1–activated HD or WM WPs in HDs (n = 6-25, blue), patients with WM not on therapy (n = 5-13, red) and receiving BTKis (n = 1-3, green). For panels A,H, representative thrombograms, mean of duplicates. For panels B-F,I-M, mean ± SD. For panel G, correlation between ETP and IgM levels of patients with WM. P values were determined by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test for the panels B-F, or Wilcoxon matched-pairs signed rank test for the panels I-M. R2 values were determined by simple linear regression.
Patients with WM have delayed and reduced thrombin generation. Thrombin generation in (A-G) EDTA-anticoagulated PPP, (H) EDTA-anticoagulated PPP + PAR-1–activated HD WPs, or (I-M) trisoidium citrate-anticoagulated PPP + PAR1–activated HD or WM WPs in HDs (n = 6-25, blue), patients with WM not on therapy (n = 5-13, red) and receiving BTKis (n = 1-3, green). For panels A,H, representative thrombograms, mean of duplicates. For panels B-F,I-M, mean ± SD. For panel G, correlation between ETP and IgM levels of patients with WM. P values were determined by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test for the panels B-F, or Wilcoxon matched-pairs signed rank test for the panels I-M. R2 values were determined by simple linear regression.
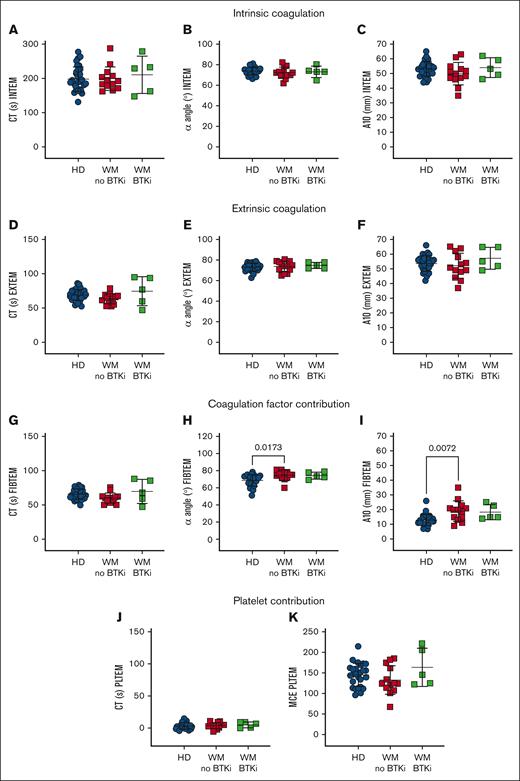
ROTEM reveals an acceleration of clotting rates in WM whole blood
To specifically assess the platelet contribution to WM blood clotting capacity, ROTEM was performed. WM blood displayed normal EXTEM and INTEM measurements, including clotting time (CT; Figure 4A,D), rate (α angle, Figure 4B,E), and amplitude (clot amplitude after 10 minutes [A10]; Figure 4C,F). However, in FIBTEM in which clotting is triggered as for EXTEM but the platelet contribution is minimized by inclusion of the actin polymerization inhibitor cytochalasin D, WM samples returned similar CTs to HDs (Figure 4G) but increased α angles (Figure 4H) and A10s (Figure 4I). These data suggest that WM blood exhibited enhanced clotting activity that was not evident in EXTEM. The platelet contribution (PLTEM) can be extrapolated by subtracting FIBTEM amplitude values from EXTEM.14 However, because the A10 and α angle do not change in a linear fashion and the A10 parameter and platelet count do not have a linear relationship, not all ROTEM parameters can be used to infer platelet contributions.46 Therefore, we performed a PLTEM maximum clot elasticity, which is a function of the maximum clot amplitude and has been shown to express more accurately the platelet component to clot strength compared with other PLTEM amplitude measurements.47 We found no significant difference in PLTEM CT (Figure 4J) or maximum clot elasticity (Figure 4K) between HDs and patients with WM. This shows that WM coagulation factors contributed more to clotting rate and amplitude compared with HDs, whereas WM platelets functioned normally.
WM coagulation factors contribute robustly in a WB clotting assay. ROTEM measurements in WB samples from HDs (n = 31, blue), patients with WM not on therapy (n = 13, red) or receiving BTKis (n = 5, green). (A-C) INTEM is triggered by calcium, phospholipids, and ellagic acid. (D-F) EXTEM is triggered by calcium and tissue factor, whereas (G-I) FIBTEM additionally inhibits platelet cytoskeletal changes with cytochalasin D and reflects the coagulation factor contribution to thrombus formation. (J-K) EXTEM-FIBTEM (PLTEM) reflects the platelet contribution to thrombus formation. Mean ± SD. P values were determined by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test. MCE, maximum clot elasticity (MCF × 100)/(100 − MCF); MCF, maximal clot firmness.
WM coagulation factors contribute robustly in a WB clotting assay. ROTEM measurements in WB samples from HDs (n = 31, blue), patients with WM not on therapy (n = 13, red) or receiving BTKis (n = 5, green). (A-C) INTEM is triggered by calcium, phospholipids, and ellagic acid. (D-F) EXTEM is triggered by calcium and tissue factor, whereas (G-I) FIBTEM additionally inhibits platelet cytoskeletal changes with cytochalasin D and reflects the coagulation factor contribution to thrombus formation. (J-K) EXTEM-FIBTEM (PLTEM) reflects the platelet contribution to thrombus formation. Mean ± SD. P values were determined by Kruskal-Wallis 1-way ANOVA with Dunn's multiple comparisons test. MCE, maximum clot elasticity (MCF × 100)/(100 − MCF); MCF, maximal clot firmness.
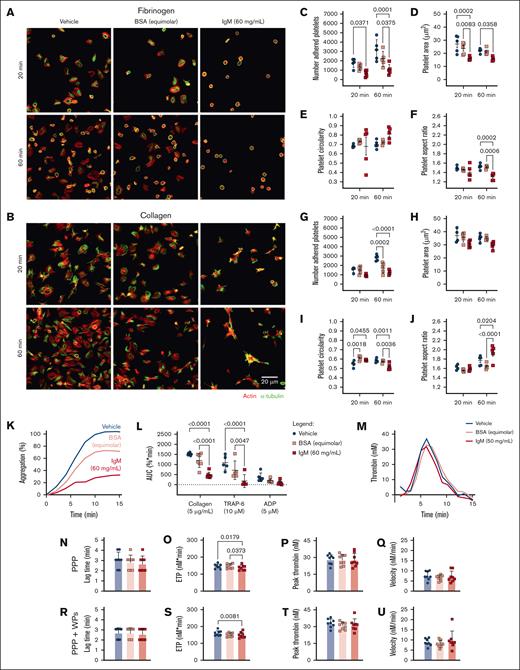
High concentrations of patient-derived IgM impair platelet function in vitro
To evaluate the effects of hyperviscosity on platelet function, IgM was isolated from a plasmapheresis bag generated as part of the treatment of a patient with WM, and used to assess platelet spreading under hyperviscous experimental conditions by mixing WM-derived IgM or an equimolar concentration of BSA with HD plasma or platelets. WM-derived IgM (60 mg/mL) bound to HD platelets (supplemental Figure 5). Unfortunately, this could not be compared with similar concentrations of healthy human IgM due to challenges in obtaining sufficient quantities. Therefore, we used equimolar amounts of BSA as the control for future experiments.
HD platelets, preincubated with IgM and allowed to adhere to fibrinogen-coated surfaces, displayed reduced numbers of adherent platelets (Figure 5A,C), reduced platelet surface area (Figure 5A,D), and reduced aspect ratios (Figure 5A,F) compared with vehicle and BSA-treated platelets. On collagen-coated surfaces, HD platelets incubated with IgM or BSA displayed reduced adhesion after 60 minutes (Figure 5B,G), increased circularity after 20 minutes (Figure 5B,I), and increased aspect ratios after 60 minutes compared with vehicle-treated platelets (Figure 5B,J). This indicates that under static conditions, these concentrations of BSA or IgM interfere with platelet adhesion and initial filopodia extension events on collagen. However, only IgM delayed spreading on collagen, resulting in more invaginated and elongated platelets and impaired lamellipodia formation at 60 minutes. We conclude that under static conditions, IgM interferes with rates and extents of platelet adhesion and spreading on substrates, resulting in fewer and smaller platelets with altered membrane projections.
High concentrations of WM-derived IgM impair platelet spreading, aggregation, and thrombin generation. HD WP spreading (n = 4-5) for 20-60 minutes on (A,C-F) fibrinogen or (B,G-J) collagen, then visualized for actin (red) and α-tubulin (green), in the presence of (C-F,G-J) vehicle (blue), BSA (equimolar to IgM, 4.11 mg/mL, pink), or IgM (60 mg/mL, red). (C,G) Number of adhered platelets; (D,H) platelet surface area; (E,I) circularity; or (F,J) aspect ratio, average of 5 field of views. (K) Representative aggregation trace, mean of duplicates. (L) WP aggregation responses to collagen (5 μg/mL), TRAP-6 (10 μM), and ADP (5 μM). (M) Representative thrombogram, mean of duplicates. Thrombin generation in HD samples (n = 8), with (N,R) lag time, (O,S) ETP, (P,T) peak thrombin generated, and (Q,U) thrombin generation velocity in PPP for panels N-Q or PPP + autologous WPs for panels R-U. For panel T, mean ± SD. P values were determined by 2-way ANOVA with multiple comparisons for panels C-F,G-J,L or Friedman test for panels N-U. AUC, area under the curve.
High concentrations of WM-derived IgM impair platelet spreading, aggregation, and thrombin generation. HD WP spreading (n = 4-5) for 20-60 minutes on (A,C-F) fibrinogen or (B,G-J) collagen, then visualized for actin (red) and α-tubulin (green), in the presence of (C-F,G-J) vehicle (blue), BSA (equimolar to IgM, 4.11 mg/mL, pink), or IgM (60 mg/mL, red). (C,G) Number of adhered platelets; (D,H) platelet surface area; (E,I) circularity; or (F,J) aspect ratio, average of 5 field of views. (K) Representative aggregation trace, mean of duplicates. (L) WP aggregation responses to collagen (5 μg/mL), TRAP-6 (10 μM), and ADP (5 μM). (M) Representative thrombogram, mean of duplicates. Thrombin generation in HD samples (n = 8), with (N,R) lag time, (O,S) ETP, (P,T) peak thrombin generated, and (Q,U) thrombin generation velocity in PPP for panels N-Q or PPP + autologous WPs for panels R-U. For panel T, mean ± SD. P values were determined by 2-way ANOVA with multiple comparisons for panels C-F,G-J,L or Friedman test for panels N-U. AUC, area under the curve.
We also assessed agonist-induced platelet aggregation in the presence of IgM. HD platelets preincubated with IgM displayed significantly reduced aggregation responses to collagen and TRAP-6 compared with vehicle- or BSA-treated platelets (Figure 5K-L). In thrombin generation assays, HD plasma mixed with WM IgM in the absence or presence of donor platelets, displayed reduced thrombin generation potential compared with vehicle and/or BSA-treated plasma (Figure 5M,O,S), but normal lag times (Figure 5N,R), peak thrombin (Figure 5P,T), and thrombin generation velocity (Figure 5Q,U). The observed muted aggregatory and thrombin generation responses when IgM was present may disrupt ligand-receptor interactions because of steric hindrance due to the large molecular weight of IgM relative to BSA (∼970 vs 66 kDa). Additionally, IgM may interfere with exposure of PS on the membrane surface, which would also reduce thrombin generation.
Discussion
Published data on hemostatic function in WM are lacking, however anecdotally, patients with WM often present to clinics with mild but chronic bleeding symptoms, disproportionate to their platelet counts or medications.5,25 Patients with WM also have an increased risk of venous thrombosis,48 infection,49 and chronic inflammation,49,50 which together are consistent with primary hemostatic failure. BTKis are effective in the long-term treatment of B-cell malignancies,36 so it is important to understand hemostatic function as BTKis can affect platelet function, particularly in susceptible individuals. Here, we characterized platelet and coagulation function in samples from 18 patients with WM and explored the effects of high concentrations of IgM on hemostasis. In our cohort, of which 72% were not receiving active treatment, we identified substantial differences in WB clotting and thrombin generation assays, as well as impairment of donor platelet function in vitro in the presence of high concentrations of WM-derived IgM.
Flow cytometric analysis of platelets from patients with WM revealed no significant differences in levels of αIIb integrin subunit, GPIbα, and GPVI. Plasma sGPVI was also within normal ranges, consistent with levels measured in patients with other lymphoproliferative malignancies.16 Reduced levels of GPIbα and αIIbβ3 on platelets from patients with chronic lymphocytic leukemia (CLL) have been reported,51 and reduced levels of GPIbα and GPVI in treatment-naïve patients with CLL and mantle cell lymphoma have been noted.16,39 Reasons for observed platelet receptor differences between patients with WM and those with CLL remain obscure, and whether the degree of BM involvement affects MK maturation and thrombopoiesis has not yet been studied. WM platelets responded normally to physiological agonists by upregulating active αIIbβ3 and increasing P-selectin exposure. As expected, GPVI-mediated platelet function was abolished in samples from patients with WM receiving BTKis because of on-target effects of the BTKi.52
We observed significant increases in TPO levels in our WM cohort, consistent with other studies in which levels were elevated 2- to 12-fold in patients with hematological malignancies53 and commensurate with the observed irregular platelet count. Increased levels of interleukin-6, which can stimulate TPO production,54 have been reported in patients with WM.55,56 Levels of TPO generally vary inversely with the platelet count,57 and TPO levels are elevated in CLL and correlate with disease progression and prognosis.58 We found that patients with WM not receiving active treatment had reduced proportions of reticulated new platelets displaying elevated CD9 levels, which are normally tightly regulated on MKs during megakaryopoiesis.59 CD9 coordinates MK interactions with BM stromal cells,60 and elevated levels may perturb stromal signals that provide molecular cues for normal megakaryopoiesis and thrombopoiesis. Our patients had clinically elevated BM involvement and IgM levels. Both properties may disrupt MK function and platelet production however this remains to be addressed experimentally. Purified IgG antibodies from thrombocytopenic patients can bind to MKs in vitro61 and inhibit MK maturation and proplatelet formation.62,63 Whether WM paraprotein engages MKs and impairs platelet production remains to be experimentally validated. Nevertheless, the TPO, reticulated platelet, and CD9 irregularities are consistent with disturbed thrombopoietic pathways, possibly due to BM disease-associated changes. It would be of interest to evaluate hematopoietic progenitors and MK numbers, as well as CD9 levels in WM BM and correlate with BM involvement and platelet function.
None of our patient cohort displayed platelet counts <35 × 109/L or significant bleeding symptoms. However, to explore subtle coagulation aberrations, we evaluated thrombin generation potential in WM plasma alone or mixed with donor platelets.64 WM plasma displayed prolonged lag times and times to reach peak thrombin, with trends toward reduced rate and extent of thrombin formation and ETP compared with HD plasma. These findings align with an isolated case study reporting reduced ETP associated with elevated IgM levels in 2 patients with WM.25 Our work shows that patients with WM receiving BTKis have equivalent capacity for thrombin generation compared with those not receiving treatment. In our study, observed deficits in thrombin generation in WM plasma were largely corrected by the inclusion of sensitized donor, but not WM platelets, indicating a thrombocytopathy was present in patients with well-managed WM. The mean serum IgM level in our WM cohort was 22 ± 24 g/L (no treatment) and 7 ± 4 g/L (BTKi therapy) and we found no relationship between IgM levels and ETP in patients with WM. This question should be further explored in WM cohorts with significant hyperviscosity (>60 g/L IgM). Agonist-treated WM platelets exposed P-selectin to an extent similar to HD platelets, suggesting that platelet degranulation pathways were intact. Observed differences between WM and HD platelets in accelerating thrombin generation may reflect dysregulated PS exposure or release of mediators such as VWF, factor V, factor XIII, and polyphosphates from WM platelets. Future work will evaluate this possibility by assessing PS exposure and releasate components from activated WM platelets. Together, our findings suggest that thrombin generation assays using plasma and PRP may better reflect the hemostatic capacity of patients with WM than routine coagulation assays, with utility in clinical decisions around changes to therapy and management of patients through medical procedures.
Using ROTEM to evaluate WB clotting, we found that WM samples performed similarly to HD samples in intrinsic and extrinsic coagulation assays. In FIBTEM in which clotting activity is triggered extrinsically but the platelet contribution is minimized by inclusion of cytochalasin D, we observed no change to clot time but significant enhancements to the rate and extent of clot formation over HD values, which fell within normal ranges. Although WM plasma had reduced thrombin generation potential, in the presence of a platelet inhibitor, WM blood showed a faster onset and extent of clotting in ROTEM, suggesting increased contribution from fibrinogen in this cohort. WB assays allow contributions from numerous other cells, such as PS-exposing RBCs and tissue factor-bearing neutrophils, which are absent from thrombin generation assays. When focusing on the platelet contribution to ROTEM (the relative differences between FIBTEM and EXTEM values), WM platelets showed a similar per-platelet contribution to clot amplitude compared with HDs.
Elevated IgM is likely to contribute to the exacerbated bleeding phenotype often reported in patients with WM with hyperviscosity.35,65 We observed that purified WM IgM bound to platelets and significantly impaired spreading and aggregation responses when compared with equimolar concentrations of a control protein. WM IgM is reported to coat blood cells and platelets and interfere with platelet filopodia formation25 and thrombin generation.66 Expression of the known IgM receptor FcμR is restricted to lymphocytes,67 so it is likely that IgM engages nonspecifically with platelets, possibly via electrostatic interactions, to interfere with platelet function. Under the experimental conditions used here, IgM had no major effect on coagulation, with only the ETP being slightly reduced. Future experiments will aim to assess the effects of hyperviscosity on endothelial cell function and vascular integrity, and to assess an electrostatic interaction of IgM with platelets by use of different polyanionic compounds. In summary, it is likely that WM-derived IgM interacts with platelets electrostatically and interferes with platelet function and thrombin generation, which may contribute to bleeding.
This study has some limitations. First, the patients were from a single center and require standardization and validation before being adopted more widely. Second, our patient cohort was clinically stable, with only a few patients requiring therapeutic intervention and none with symptomatic hyperviscosity. Future work evaluating hemostatic properties of blood from patients with WM on therapeutic regimens, including expansion of data from patients in receipt of BTKis and/or with hyperviscosity, will be important. Furthermore, a direct comparison with patients with other types of B-cell lymphomas without hyperviscosity symptoms as additional controls, to delineate how specific the observed hemostatic dysfunctions are to WM, would be valuable. Third, our findings could not be linked to clinical outcomes as no bleeding events were observed in our cohort. Fourth, age and other demographic differences between our HDs and WM cohort should be noted when considering the findings. Finally, we had opportunity to isolate IgM from only 1 patient with WM in our cohort with high IgM levels, thus it remains unclear whether the IgM-mediated hemostatic impairment was specific to that patient.
Our study describes significant differences in platelet parameters and hemostatic functions in a clinicallystable WM cohort. Whether these findings can be extended to other lymphomas and leukemias with similar burdens of BM disease6 awaits further investigation. The extent to which hemostatic changes contribute to clinically significant thrombotic and bleeding risks in patients with WM also remains to be evaluated, however monitoring of platelet function and thrombin generation data could be incorporated into a patient’s management plan and reevaluated in the event of clinically significant bleeding or prior to changes in therapy or surgical intervention.
Acknowledgments
The authors thank the John Curtin School of Medical Research Flow Cytometry Facility for their expertise and assistance, and Samina Nazir for helpful discussions. Patient samples were obtained from the Australian Capital Territory Haematology Research Tissue Bank.
This work was supported, in part, by the Australian Capital Territory Department of Health and National Health and Medical Research Council of Australia grant APP1143539.
Authorship
Contribution: S.A.B., D.T., and E.E.G. designed the study and interpreted the data; S.A.B. drafted the manuscript; M.M., M.G.M., P.J.C., and D.T. acquired informed consent from patients, provided patient-specific data and samples, and clinical advice on interpretation of data; S.A.B., S.M.H., J.I.H., S.A.A., V.B., A.K., and Y.L.T. contributed to experimental data and intellectual discussions; J.C.W. provided key reagents; E.E.G. acquired funding for the project; and all authors contributed to the review of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth E. Gardiner, ACRF Division of Genome Science and Cancer, John Curtin School of Medical Research, The Australian National University, 131 Garran Rd, Canberra, ACT 2601, Australia; email: elizabeth.gardiner@anu.edu.au.
References
Author notes
Publication-related data will be shared upon request from the corresponding author, Elizabeth E. Gardiner (elizabeth.gardiner@anu.edu.au).
The full-text version of this article contains a data supplement.

![WM platelets respond appropriately to standard agonists. (A-B) OgFg binding and (C-D) P-selectin exposure in WB and PRP samples, respectively, from HDs (n = 7-14, blue), patients with WM not on therapy (n = 8-13, red), or receiving BTKis (n = 0-5, green). Representative histograms from a HD showing (A) OgFg- or (C) P-selectin–positive events. Data (mean ± standard deviation [SD]) for (B) OgFg binding or (D) P-selectin exposure (%) in platelets treated with 0.5, 5 or 30 μg/mL crosslinked CRP-XL, 10 μM TRAP-6, or 5 μM ADP. P values were determined by Mann-Whitney t test with multiple comparisons.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/21/10.1182_bloodadvances.2024014190/2/m_blooda_adv-2024-014190-gr2.jpeg?Expires=1769105685&Signature=Jn8giT49qc0bZ7uT6Dqb9DWMtSOSS8YTwxKbu8Eqf0nX6Pv1CeUgUAgg8JEQEBcUUwSwf1Mdnns0oRXBu9TaykUhqQzeVrzBipHuYG9Y7sTfkKiEqrD5V4zJmQwC5QDK2QMBlgElG2WkQGkxFQMXVRi-pxwslDbkW9e17-gzKIQWL9A0KkZan1r~PmL9hyUUpnktnmma2KJ6TChQHsSTHLqWZqXS59ih7d1-~aQK3jEXG4Pg9wRp9t8EaTDKZfDCGtzx45ayIevf1UXcdJ21qtZV2taFMCBUDreMm5n9nHjhAkZBpDj~g00MVmSHrYBiTguI~XWXEBBPPWi51xywGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)