Visual Abstract
Venetoclax selectively inhibits B-cell lymphoma 2 (BCL-2) and restores apoptotic signaling of hematologic malignant cells. Venetoclax, in combination with hypomethylating and low-dose cytotoxic agents, has revolutionized the management of older patients affected by acute myeloid leukemia (AML) and that of patients unfit to receive intensive chemotherapy. In a single phase 1 pediatric trial conducted on relapsed or refractory AML, the combination of venetoclax and intensive chemotherapy was shown to be safe and yielded promising response rates. In addition, several retrospective studies in children with AML reported that venetoclax, when combined with hypomethylating agents and cytotoxic drugs, seems to be a safe and efficacious bridge to transplant. The promising results on the use of venetoclax combinations in advanced myelodysplastic syndromes (MDS) and therapy-related MDS/AML have also been reported in small case series. This review summarizes the available current knowledge about venetoclax use in childhood high-risk myeloid neoplasms and discusses the possible integration of BCL-2 inhibition in the current treatment algorithm of these children. It also focuses on specific genetic subgroups potentially associated with response in preclinical and clinical studies.
Introduction
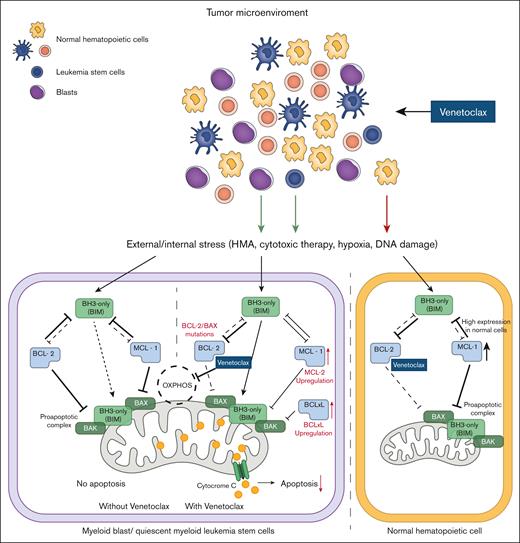
Overexpression of anti-apoptotic proteins of the B-cell lymphoma 2 (BCL-2) family, including BCL-2, BCL-extra large (XL), and myeloid cell leukemia-1 (MCL-1), is one of the primary mechanisms hematologic cancers employ to escape cell death signaling.1 Venetoclax (ABT-199) is a compound that selectively inhibits BCL-2, thereby mimicking the function of BH3-only proteins (a BH3 mimetic) and restoring apoptosis signaling.2 After its first clinical application in chronic lymphocytic leukemia,3 venetoclax has shown efficacy in acute myeloid leukemia (AML) models. Myeloid blasts rely on Bcl-2 for survival, and the overexpression of Bcl-2 is responsible for resistance to chemotherapy.4 Conversely, normal hematopoietic stem cells are dependent on MCL-1, making venetoclax an agent that is capable of more selective killing of AML cells while sparing healthy bone marrow components.4,5 Venetoclax, in combination with hypomethylating agents (HMAs) such as azacytidine or decitabine, eradicates quiescent myeloid leukemia stem cells that overexpress BCL-2 by abrogating cellular oxidative phosphorylation, suggesting that it has an effect on cell metabolism beyond the classical proapoptotic signal6,7 (Figure 1). Venetoclax with azacytidine and low-dose cytarabine was demonstrated to be effective with an acceptable safety profile in patients with newly diagnosed AML,8,9 which led to its US Food and Drug Administration approval for use in the United States for this indication in 2020. In a few years, venetoclax-containing regimens have significantly modified the management of older patients with AML and that of patients who are unfit to receive intensive therapies, which led to similar survival rates as CPX-351 (a dual-drug liposomal encapsulation of cytarabine and daunorubicin) and lower infection rates and shorter inpatient hospital stays in a real-world observational analysis.10 After these results, interest emerged in testing venetoclax therapies in younger patients. Several experiences have been reported so far, and clinical trials are currently ongoing in pediatric myeloid neoplasms.
Mechanism of action of venetoclax (ABT-199) in myeloid malignant cells. In blast cells, venetoclax inhibits BCL-2 protein, thereby reducing the inhibitory effect of BCL-2 on the proapoptotic complex. Venetoclax also inhibits oxidative phosphorylation (OXPHOS) in the mitochondria. Upregulation of MCL-1 or BCL-XL, metabolic reprogramming, or BCL-2/BAX mutations can occur in blast cells as a compensatory effect and escape mechanism (red text and arrows). Normal cells, which rely on MCL-1 signaling, are less sensitive to venetoclax inhibition. BAX, BCL-2–associated X protein.
Mechanism of action of venetoclax (ABT-199) in myeloid malignant cells. In blast cells, venetoclax inhibits BCL-2 protein, thereby reducing the inhibitory effect of BCL-2 on the proapoptotic complex. Venetoclax also inhibits oxidative phosphorylation (OXPHOS) in the mitochondria. Upregulation of MCL-1 or BCL-XL, metabolic reprogramming, or BCL-2/BAX mutations can occur in blast cells as a compensatory effect and escape mechanism (red text and arrows). Normal cells, which rely on MCL-1 signaling, are less sensitive to venetoclax inhibition. BAX, BCL-2–associated X protein.
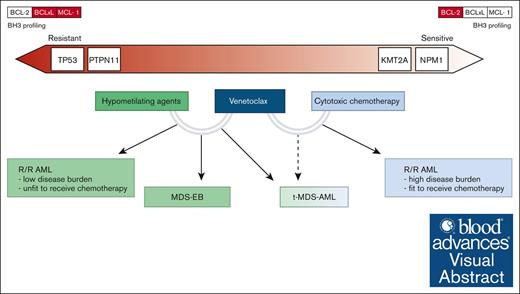
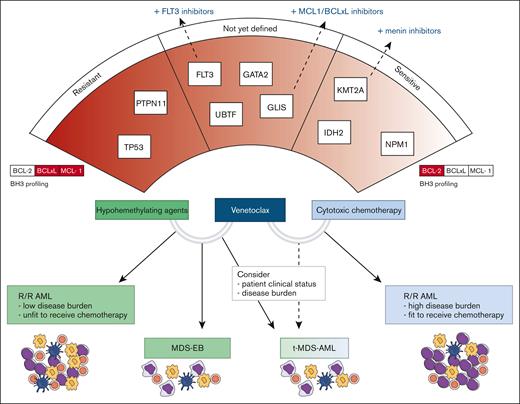
In this review, we critically evaluated and summarized the current evidence regarding the treatment of pediatric high-risk myeloid diseases, including relapsed/refractory AML (r/r AML), therapy-related myelodysplastic syndromes (MDS)/AML (t-MDS/AML), and advanced MDS (or MDS with an excess of blasts [MDS-EB]). We also discussed how venetoclax combination therapies can be integrated into the management of these disorders, often outside defined recommendations (Figure 2). In addition, we focused on identifying specific subgroups of interest and speculated on genetic lesions associated with peculiar venetoclax sensitivity or resistance in pediatric myeloid diseases.
Clinical indications of venetoclax combination therapies and possible predictors of response. The lower section of the figure illustrates the clinical settings in which various venetoclax combinations may be applied. In the upper section, the relationship between genetic drivers, BH3 profiling results, and sensitivity to therapy is depicted. On the left, genes with higher resistance potential are presented, whereas on the right, genes associated with higher sensitivity are highlighted.
Clinical indications of venetoclax combination therapies and possible predictors of response. The lower section of the figure illustrates the clinical settings in which various venetoclax combinations may be applied. In the upper section, the relationship between genetic drivers, BH3 profiling results, and sensitivity to therapy is depicted. On the left, genes with higher resistance potential are presented, whereas on the right, genes associated with higher sensitivity are highlighted.
Venetoclax as bridge to transplant in pediatric r/r AML
Childhood AML is a genetically heterogeneous disease with significant biologic differences when compared with adults. Genomic characterization plays a critical role in the management of pediatric AML, ensuring a more precise risk stratification and tailored treatment. Standard AML chemotherapy is not selective and does not ensure adequate response in all patients because of the biologic heterogeneity of this disease, and it is also associated with a high rate of treatment-related toxicities.11 Integration of genomic characterization and measurable residual disease (MRD) assessment in the treatment of pediatric AML have improved clinical outcomes with overall survival (OS) rates now approaching 70%.12,13 Unfortunately, disease relapse represents the major cause of treatment failure, affecting 30% to 40% of patients.12 No universal standard of care exists regarding the management of r/r AML, and poor OS rates that ranged from 20% to 40% have been reported.14,15 Standard intensive chemotherapy, with or without the addition of gemtuzumab ozogamicin (GO), is often employed during reinduction therapy of children with r/r AML with response rates ranging between 20% and 80% and OS rates of 20% to 40%.16,17 Remarkably, AML cells develop resistance to anticancer drugs through a series of cytogenetic events upon exposure to chemotherapy, demonstrating the importance of adopting drugs that cause total remission in an early phase of disease and preventing the development of refractory clones.18 In recent years, major efforts to modify treatment protocols have been made by incorporating novel targeted therapies and redesigning existing therapeutics, as in the case of CPX-351.19 Administration of 1 CPX-351 cycle, followed by standard chemotherapy in patients with r/r AML, led to complete response (CR) rates of 81% with encouraging OS at 2 years of 52.7%.20 Other novel strategies are currently being tested with promising results, including monoclonal antibodies and cellular therapies, such as chimeric antigen receptor targeting CD123–natural killer (CAR.CD123-NK) cells.21,22 Moreover, genomic analysis at the time of relapse with extensive characterization of clonal evolution of AML can help to identify novel molecular therapeutic target.23,24
Regimens containing venetoclax plus HMAs or low-dose chemotherapy are rarely used in newly diagnosed childhood AML as a first-line approach, because children are generally fit to tolerate more intensive regimens. However, these approaches increasingly have been applied to children, especially in heavily pretreated r/r AML cases in which they can benefit from less intensive regimens. In children, venetoclax has been tested in combination with the intensive chemotherapy regimens typically adopted in pediatric hematology.25 First, robust data on the safety and efficacy of venetoclax with high-dose chemotherapy came from the VENAML phase 1 dose-escalating trial (NCT03194932) that enrolled 38 patients with r/r AML and identified the recommended phase 2 dose (RP2D) of 360 mg/m2 (600 mg maximum) of venetoclax in combination with high-dose cytarabine with or without idarubicin. Of the patients treated with the RP2D, 70% and 80% achieved CR and CR/partial response (PR), respectively, with treatment-related death occurring in only 1 patient.26 Following the publication of these data, several retrospective series and individual cases, predominantly from 2023, have evaluated the role of venetoclax combinations in young patients with myeloid diseases, particularly r/r AML, as summarized in Table 1.27-36 Variable response rates were reported with CR ranging from 10% to 92% and overall response rates ranging from 42% to 92%. Patient populations varied largely across the different studies and in each study cohort but generally included heavily pretreated patients. Different dosage and length cycles of venetoclax were reported with suboptimal responses recorded in studies adopting doses lower than the RP2D.32 Moreover, different drug partners have been described, making it difficult to compare these studies and to draw solid conclusions. An analysis of selected venetoclax combinations, particularly with HMAs, is highly anticipated because of the high efficacy of this approach, the generally good toxicity profile of this combination, and the applicability in an outpatient setting.32,33 This combination could be of particular interest as a bridge for hematopoietic cell transplantation (HCT) by reducing toxicities in the immediate pretransplant phase.27,31-33 Other agents have also been combined with these regimens, including mostly GO and fms-like tyrosine kinase 3 (FLT3) inhibitors, confirming preclinical studies on the synergistic effect of FLT3 and BCL-2 inhibition.34,37 When available, analysis on the blast percentage at the time of therapy showed that these approaches had satisfactory efficacy, even in the presence of a high disease burden.26,31,33 Notably, some patients with mixed phenotype acute leukemia were included in these studies, demonstrating favorable clinical outcomes when used as a bridge for HCT after venetoclax with both HMAs and cytotoxic agents.26,28,38-41 A variable percentage of patients (25%-100%) was bridged to HCT after achieving the maximum best response with 1 or 2 cycles.27,30,31,33 Considering the dismal prognosis of these diseases, the outcome in patients who underwent transplantation was generally satisfactory with 50% to 70% of them being alive and disease-free at the last follow-up.30,31,33 In 1 study, venetoclax was incorporated with daratumumab in the preparative myeloablative regimen of children with refractory AML who received αβ T-cell–depleted haploidentical HCT. No increased transplant-related mortality was reported, and 2-year OS and event-free survival was 65% and 44%, respectively.42 When analyzed, no detrimental effect on engraftment/kinetics of neutrophil recovery or on the occurrence of graft-versus-host disease after HCT was reported.30,42 In all studies, toxicities were generally manageable with no reported toxicity-related death. Dose-escalation during the first 2 days of the first cycle was generally adopted to avoid tumor lysis syndrome. The most frequent adverse event was neutropenia, often prolonged or profound and associated with severe infection,29,32 that required premature venetoclax interruption.
Venetoclax therapies in pediatric myeloid disease
| Study . | Pts, n (age, range) . | Disease . | Combination therapies . | Venetoclax . | Response . | HCT post ven . | Survival post-HCT . | Toxicities . |
|---|---|---|---|---|---|---|---|---|
| Karol et al26 | 38 (10 y, 3-22) | rel-AML (33), refr-AML (4), refr-MPAL (1) [KMT2Ar (12); FLT3 (5); TP53 (4)] [median disease burden: 33% (18-68)] | Cytarabine (1000 mg/m2 per dose for 8 doses) ± idarubicin (12 mg/m2 as a single dose) | 240 or 360 mg/m2 per day (28 d)∗ [2 cycles] | In 35 pts evaluable for response: ORR 69% (24), CR (20; 13 MRD neg; 4 CRi); PR (4); NR (11) In 20 pts treated at R2PD: ORR 80%, CR/CRi (14), PR (2), NR (4) | 16 of 20 pts achieving CR | N/A† | Grade 3-4 AEs: febrile neutropenia (25), BSI (6), IFI (6) |
| Winters et al27 | 8 (11 y, 2-20) | MDS-EB (2) t-MDS/AML (1) r/r AML (5) [FLT3 (3), TP53 (2)] | Azacytidine (75 mg/m2 per d; days 1-7) | Adult-equivalent dose of 800 mg (28 d) [median no. cycles: 1 (1-9)] | CR 75% (6; 1 CRi) NR 25% (2) | 4 + 2 pending/8 | N/A | Grade 3-4 neutropenia (8) |
| Bobeff et al28 | 5 (national survey: age <10 y) | MDR-AML (2), rel- AML (1), t-AML (1) refr-MPAL (1) [KMT2Ar (2)] | Cytarabine + idarubicin (2), cytarabine (1), cytarabine + azacitdine (2), azacitdine (1) | 360 mg/m2 per day (28 d) [median no. cycles: 1 (1-2)] | CR 60% (3; 1 CRi), NR 40% (2) | 2/5 | 1 alive disease-free | N/A |
| Marinoff et al29 | 10 (10 y, 1-29) | t-MDS/AML (2), r/r AML (6), MDS (1), MDR-AML (1)‡ [GATA2 (1), KMT2Ar (2), GLIS2 (1), SDS (1)] | Cytarabine (1000 mg/m2 per dose for 8 doses) (5), decitabine (20 mg/m2 per day for 5 d) (4), azacitdine (1) | Adult-equivalent dose of 400 mg [median no. cycles: 1 (1-3)] | CR 10% (1), PR/SD 50% (5), NR/PD 40% (4) | ½0 | 1/2 alive disease-free | Grade 3-4 AEs: cytopenia, infections (5) |
| Pfeiffer et al30 | 28 (13, 1-21) | Refr-AML (5); rel-AML (23) [adverse genetics in 12] | Cytarabine (17), cytarabine + idarubicin (5), cytarabine + azacitdine (3), decitabine (2), azacitidine (1) | 240-360 mg/m2 per day [median no. cycles: 2 (1-7)] | CR 92% (26) (2 CRi), PR/NR 18% (2) | 28/28 | 20/28 alive disease-free, 8 relapse§ | N/A (no impact on GVHD incidence or neutrophil and platelet engraftment) |
| Trabal et al32 | 43 (18, 1-21) | r/r AML (43) [KMT2Ar (17), FLT3-ITD (10), NPM1 (4), TP53 (3), IDH1/2 (2)] | HMA (37), cytotoxic agents (6) [+ trametinib (1), GO (7), TKI (5), MCL-1 inhibitor (1)] | median dose 93 mg/m2 per day (28 d cycles; effective duration median 14 d) [median no. cycles: 2 (1-9)] | CR 37% (16, 6 Cri), PR 5% (2), NR 51% (22) N/E. 7% (3) | 11/43 | 6/11 alive disease-free‖ | Grade 3 neutropenia/febrile neutropenia (49%) |
| Masetti et al31 | 31 (10.2, 1.3-17.4) | MDS-EB (4), rel-AML (11), refr AML (7), t-MDS/AML (9) [KMT2Ar (8), FLT3 (5)] [median disease burden, 20% (0-80)] | HMA (19), cytotoxic agents (9), HMA + cytotoxic (3) [+ gilteritinib (1)] | median 350 mg/m2 per day (28 d) [median no. cycles 2 (1-15)] | CR, 51.6% (16; 6 MRD neg; 5 CRi), PR, 19.4% (6), NR, 25.8% (8) | 20/31 | 15/20 alive disease-free¶ | Grade 3-4 cytopenia (4), IFI (3) (1 TF due to severe pancytopenia) |
| Niswander et al33 | 29 (8, 0-19) | r/r AML (27), MPAL (2) [KMT2Ar (8), GLIS2 (4), FLT3 (1)] [median disease burden, 10.5% (0.01-91.5)] | Azacitidine 100 mg/m2 (days 1-5) [+GO (9)] | Adult-equivalent dose of 800 mg (28 d) [median no. cycles 2 (0-6)] | CR with MRD neg, 44.8% (13) | 12/29 | 7/12 alive disease-free | Severe cytopenia (7), bacteremia (6), IFI (2) |
| Study . | Pts, n (age, range) . | Disease . | Combination therapies . | Venetoclax . | Response . | HCT post ven . | Survival post-HCT . | Toxicities . |
|---|---|---|---|---|---|---|---|---|
| Karol et al26 | 38 (10 y, 3-22) | rel-AML (33), refr-AML (4), refr-MPAL (1) [KMT2Ar (12); FLT3 (5); TP53 (4)] [median disease burden: 33% (18-68)] | Cytarabine (1000 mg/m2 per dose for 8 doses) ± idarubicin (12 mg/m2 as a single dose) | 240 or 360 mg/m2 per day (28 d)∗ [2 cycles] | In 35 pts evaluable for response: ORR 69% (24), CR (20; 13 MRD neg; 4 CRi); PR (4); NR (11) In 20 pts treated at R2PD: ORR 80%, CR/CRi (14), PR (2), NR (4) | 16 of 20 pts achieving CR | N/A† | Grade 3-4 AEs: febrile neutropenia (25), BSI (6), IFI (6) |
| Winters et al27 | 8 (11 y, 2-20) | MDS-EB (2) t-MDS/AML (1) r/r AML (5) [FLT3 (3), TP53 (2)] | Azacytidine (75 mg/m2 per d; days 1-7) | Adult-equivalent dose of 800 mg (28 d) [median no. cycles: 1 (1-9)] | CR 75% (6; 1 CRi) NR 25% (2) | 4 + 2 pending/8 | N/A | Grade 3-4 neutropenia (8) |
| Bobeff et al28 | 5 (national survey: age <10 y) | MDR-AML (2), rel- AML (1), t-AML (1) refr-MPAL (1) [KMT2Ar (2)] | Cytarabine + idarubicin (2), cytarabine (1), cytarabine + azacitdine (2), azacitdine (1) | 360 mg/m2 per day (28 d) [median no. cycles: 1 (1-2)] | CR 60% (3; 1 CRi), NR 40% (2) | 2/5 | 1 alive disease-free | N/A |
| Marinoff et al29 | 10 (10 y, 1-29) | t-MDS/AML (2), r/r AML (6), MDS (1), MDR-AML (1)‡ [GATA2 (1), KMT2Ar (2), GLIS2 (1), SDS (1)] | Cytarabine (1000 mg/m2 per dose for 8 doses) (5), decitabine (20 mg/m2 per day for 5 d) (4), azacitdine (1) | Adult-equivalent dose of 400 mg [median no. cycles: 1 (1-3)] | CR 10% (1), PR/SD 50% (5), NR/PD 40% (4) | ½0 | 1/2 alive disease-free | Grade 3-4 AEs: cytopenia, infections (5) |
| Pfeiffer et al30 | 28 (13, 1-21) | Refr-AML (5); rel-AML (23) [adverse genetics in 12] | Cytarabine (17), cytarabine + idarubicin (5), cytarabine + azacitdine (3), decitabine (2), azacitidine (1) | 240-360 mg/m2 per day [median no. cycles: 2 (1-7)] | CR 92% (26) (2 CRi), PR/NR 18% (2) | 28/28 | 20/28 alive disease-free, 8 relapse§ | N/A (no impact on GVHD incidence or neutrophil and platelet engraftment) |
| Trabal et al32 | 43 (18, 1-21) | r/r AML (43) [KMT2Ar (17), FLT3-ITD (10), NPM1 (4), TP53 (3), IDH1/2 (2)] | HMA (37), cytotoxic agents (6) [+ trametinib (1), GO (7), TKI (5), MCL-1 inhibitor (1)] | median dose 93 mg/m2 per day (28 d cycles; effective duration median 14 d) [median no. cycles: 2 (1-9)] | CR 37% (16, 6 Cri), PR 5% (2), NR 51% (22) N/E. 7% (3) | 11/43 | 6/11 alive disease-free‖ | Grade 3 neutropenia/febrile neutropenia (49%) |
| Masetti et al31 | 31 (10.2, 1.3-17.4) | MDS-EB (4), rel-AML (11), refr AML (7), t-MDS/AML (9) [KMT2Ar (8), FLT3 (5)] [median disease burden, 20% (0-80)] | HMA (19), cytotoxic agents (9), HMA + cytotoxic (3) [+ gilteritinib (1)] | median 350 mg/m2 per day (28 d) [median no. cycles 2 (1-15)] | CR, 51.6% (16; 6 MRD neg; 5 CRi), PR, 19.4% (6), NR, 25.8% (8) | 20/31 | 15/20 alive disease-free¶ | Grade 3-4 cytopenia (4), IFI (3) (1 TF due to severe pancytopenia) |
| Niswander et al33 | 29 (8, 0-19) | r/r AML (27), MPAL (2) [KMT2Ar (8), GLIS2 (4), FLT3 (1)] [median disease burden, 10.5% (0.01-91.5)] | Azacitidine 100 mg/m2 (days 1-5) [+GO (9)] | Adult-equivalent dose of 800 mg (28 d) [median no. cycles 2 (0-6)] | CR with MRD neg, 44.8% (13) | 12/29 | 7/12 alive disease-free | Severe cytopenia (7), bacteremia (6), IFI (2) |
AEs, adverse events; BSI, bloodstream infection; CRi, complete response with incomplete recovery; EFS, event-free survival; GVHD, graft-versus-host disease; IFI, invasive fungal infection; MDR-AML, myelodysplastic related AML; MPAL, mixed-phenotype acute leukemia; N/A, not available; N/E, not evaluable; NR, nonresponse; ORR, overall response rate; PD, progression of disease; pts, patients; refr-AML, refractory AML; rel-AML, relapsed AML; SD, stable disease; SDS, Shwachman-Diamond syndrome; TF, treatment failure; TKI, tyrosine kinase inhibitor; TP53, tumor protein 53.
RP2D of venetoclax plus chemotherapy = 360 mg/m2 per day (600 mg maximum).
1-year OS (whole cohort): 20 of 38 dead.
One patient with SDS who developed AML.
Median follow-up of 344 days (111-1056) from HCT: 1-year OS, 80.5%; 1-year EFS, 69.2%; cumulative incidence of relapse at 1-year post-HCT, 30.8%; and cumulative incidence of relapse at 2 years post-HCT, 43.2%.
Median OS and EFS duration, 8.7 months (range, 0.2-53 months) and 3.7 months (range, 0.1-53), respectively.
30-month estimated OS after the start of venetoclax treatment, 29.9% in the whole cohort and 74.4% for patients who underwent HCT.
Because of the absence of published phase 3 trials, recommending an optimal duration of venetoclax cycles is challenging. Various ongoing trials are currently employing either 21- or 28-day cycles. Regarding the number of cycles, the results of the phase 1 trial indicated that a good response (>50% blast reduction) during the first cycle was associated with a high probability of achieving CR with MRD negativity at the end of second cycle.26 In the study by Niswander and colleagues on azacytidine plus venetoclax combination, 4 of 12 patients who achieved MRD negativity after first cycle did not maintain the remission after the second cycle.33 In our experience, patients who achieved CR after the first cycle with different combinations maintained CR in 5 of 8 cases, whereas 7 of 8 with PR after the first cycle successfully achieved CR after the second one.31 Recommended criteria for venetoclax interruption are also lacking. We generally consider drug interruption only in the case of clinically significant infection or other severe adverse events. If possible, we attempt to temporarily interrupt venetoclax and restart as soon as possible when clinical conditions permit it so as to administer at least 21 days of therapy. Interestingly, patients who did not achieve complete hematologic recovery after therapy underwent successful transplantation with a generally favorable outcome.30,31,33 Regarding anti-infective support measures, antibacterial and antiviral prophylaxis is not routinely administered,32 whereas antifungal prophylaxis, active against both yeasts and molds, has to be administered because of the expected prolonged course of neutropenia with dose reduction in the case of azole coadministration.
A potential role of venetoclax in pediatric de novo MDS and therapy-related MDS/AML
Childhood MDS present unique biologic characteristics that differ significantly from MDS in older patients.43 Particularly, MDS-EB is defined as the presence of 5% to 19% of blasts according to the recent International Consensus Classification.44 Differentiating between MDS-EB and AML is crucial for selecting the appropriate therapeutic strategies because the treatment approaches vary significantly between the 2 conditions.43,45 However, no standard recommendation exists on the best therapeutic approach for advanced MDS/MDS-EB or AML that evolved from MDS, defined in adults as myelodysplastic-related (MDR)-AML.46 Of note, pediatric MDS can arise from a germ line predisposition condition that frequently presents an excessive and a unique risk for toxicities secondary to treatment.47,48 In advanced MDS, conventional AML-chemotherapy alone led to a high risk for treatment-related toxicities and long-term survival lower than 30%.49,50 More favorable outcomes have been reported with allogeneic HCT, but patients who were given HCT as the first therapy without any bridge had a substantial risk for relapse.51 For clinicians who manage children with advanced MDS/MDS-EB, the role of therapy as a bridge for HCT remains the most controversial issue that has not been investigated in a systematic manner so far.52 In the largest European Working Group study, it was reported that intensive AML chemotherapy before HCT did not have an impact on relapse or transplant-related mortality and led to comparable OS or event-free survival in patients who did or did not receive chemotherapy. In the MDR-AML subgroup, intensive chemotherapy was associated with a lower risk for relapse, leading to improved event-free survival, even if not statistically significant.53 In a recent retrospective study on 36 children with MDS, a blast count ≥5% and having received pre-HCT chemotherapy were both significantly associated with inferior OS (54% vs 87%) because of an increased risk for relapse, whereas patients who achieved MRD-negative status before HCT showed improved OS (63.9% vs 33.3%) in a mixed population of patients with primary and secondary MDS.54,55 Similar to primary MDS, therapy-related or postcytotoxic therapy MDS/AML (t-MDS/AML) represent a difficult-to-treat condition in which the optimal management has not been fully identified.44 Patients with these conditions frequently present a poor biologic response to conventional chemotherapy and a high risk for treatment-related toxicities. The time from diagnosis to HCT has been demonstrated to be the only significant prognostic factor,46 suggesting that novel approaches, such as CPX-351, should be tested as a bridge to HCT.56
These considerations highlight the importance of finding interventions that are able to control disease burden while avoiding intensive chemotherapy to improve the overall outcomes.54 Currently, intensive chemotherapy is not routinely recommended for childhood advanced MDS. However, cytoreduction is often necessary in cases with an excess of blasts, and the role of novel, less intensive agents, such as HMAs or targeted therapies, remains to be fully elucidated.46 Azacytidine was well tolerated with variable response in some retrospective series of pediatric primary MDS cases.57,58 However, results on patients with treatment-naive primary advanced MDS who received azacytidine in the AZA-JMML-001 trial showed poor response, suggesting the ineffectiveness of HMAs as monotherapy.59 The synergistic effect of venetoclax plus HMA was tested in adult MDS with encouraging clinical benefits, and this experience was translated to the pediatric setting.60 For instance, a few cases of childhood primary advanced MDS and MDR-AML who received venetoclax-containing regimens have been reported and are summarized in Table 2.27-29,31,41,61-63 In de novo MDS, results differ in terms of the response rate, but these strategies seem to represent a potential effective bridge to HCT. Patients who do not proceed to HCT after therapy almost invariably relapse, highlighting the need for these patients to undergo transplant at the time of best response.29,31,61,62 Furthermore, when MDS progress to MDR-AML, the efficacy of venetoclax is lower, even when used in combination with cytotoxic therapy.28,29 Interestingly, venetoclax combinations showed activity in patients with MDR-AML in the context of Schwachman-Diamond syndrome and Fanconi anemia, representing a fascinating opportunity to limit toxicities in these peculiar conditions.28,62,63 Among the reported t-MDS/AML cases, 9 or 13 patients achieved CR/PR with venetoclax combinations; 8 were bridged to HCT and were reported to be alive and disease-free. In light of these results, venetoclax plus HMA can represent a valid alternative option to other more intensive therapies.31
Patients with primary advanced MDS or t-MDS/AML treated with venetoclax combination therapies
| Study . | Age (y) . | Diagnosis . | Genetics . | Previous lines . | Venetoclax combination therapy . | Response . | HCT . | Outcome (disease status/cause of death) . |
|---|---|---|---|---|---|---|---|---|
| Winters 2021 | 7 | MDS-EB/RAEB in SDS | Mon7, ETV6, GATA2 | 0 | VEN + AZA (1 cycle) | CR (morphological <5%; cytogenetic monosomy 7 10%) | Yes | Alive; disease-free |
| Marinoff et al29 | 17 | MDS | GATA2 germ line | 1 | VEN + AZA | NR | Yes | Dead; relapse after HCT |
| Masetti et al31 | 15 | MDS-EB | LIG4 and SH2B3 germ line, mon7, RIT1, EZH2, SETBP1, ASXL1, ETV6 | 0 | VEN + cytotoxic (IDA-FLA) (1 cycle) | CR | Yes | Alive; disease-free |
| Masetti et al31 | 14 | MDS-EB | Del7q | 4 | VEN + DEC (5 cycles) | CR∗ | Yes | Dead; TRM |
| Winters 2021 | 8 | MDR-AML/RAEB-t in NF1 | Del17p/loss TP53, ASXL1, TET2 | 0 | VEN + AZA (3 cycles) | CR (morphological <5%; cytogenetic persistence del17p/loss TP53) | Yes | Alive; relapse cytogenetic del17/TP53 and MRD pos† |
| Bobeff et al28 | <6 | MDR-AML in NF1 | Mon7 | 1 | VEN + cytotoxic therapy (IDA-FLA) (1 cycle) | CR | Yes | Dead; relapse post-HCT |
| Bobeff et al28 | 6-10 | MDR-AML in familial platelet disorder | RUNX1 | 4 | VEN + cytotoxic therapy (Idarubicin + ARA-C) (1 cycle) | NR | No | Dead; PD before HCT |
| Marinoff et al29 | 14 | MDR-AML in SDS | IDH1, KMT2A | 0 | VEN + DEC | CR (MRD neg) | Yes | Alive; disease-free |
| Raedler et al61 | 16 | MDR-AML/RAEB-t | Complex karyotype | 2 | VEN + DEC (4 cycles) | CR (for 10 months, then molecular relapse) | No | Alive; molecular relapse‡ |
| Naviglio et al62 | 14 | MDR-AML in SDS | neg | 2 | VEN + AZA (1 cycle) | PR | No | Dead; PD before HCT |
| Wen et al41 | 3 | MDR-AML | Complex karyotype, NRAS | 1 | VEN + AZA | CR (MRD neg) | Yes | Alive; disease-free |
| Ma et al63 | 7 | MDS/MDR-AML in FA§ | NPM1, GATA2, WT1 | 1 | VEN+AZA (2 cycles) | CR (MRD neg) | Yes | Alive; disease-free |
| Masetti et al31 | 17 | MDR-AML | FLT3, WT1 | 1 | VEN + AZA (1 cycle) | NR | Yes | Dead; TRM |
| Masetti et al31 | 14 | MDR-AML | WT1 | 2 | VEN + AZA (1 cycle) + VEN + ARA-C (1 cycle) | CR | Yes | Dead; relapse post-HCT |
| Bobeff et al28 | 6-10 | t-AML | KMT2A, t (9;11) | 1 | VEN + ARA-C (1 cycle) | CR (MRD neg) | Yes | Alive; disease-free |
| Marinoff et al29 | 17 | t-AML | Mon7, t (7;11), PTPN11, SED2, RUNX1, BCOR | 3 | VEN + DEC | PR | No | Dead; PD before HCT |
| Winters 2021 | 11 | t-MDS/AML | RUNX1 | 1 | VEN + AZA (9 cycles) | CR (MRD neg) | No | Alive; disease-free‖ |
| Marinoff et al29 | 9 | t-MDS | PTPN11 | 1 | VEN + DEC | PR (stable disease) | No | Dead; PD before HCT |
| Masetti et al31 | 7 | t-MDS/AML | T (11;17), KMT2A | 1 | VEN + IDA-FLA (2 cycles) | CRi | Yes | Alive; disease-free |
| Masetti et al31 | 5 | t-MDS/AML | t (9;11), SDHC, KMT2A | 1 | VEN + IDA-FLA (2 cycles) | CRi | Yes | Alive; disease-free |
| Masetti et al31 | 1 | t-MDS/AML | t (4;11), KMT2A | 1 | VEN + ARA-C + idarubicin | NR | No | Dead; PD before HCT |
| Masetti et al31 | 10 | t-MDS/AML | Mon7 | 1 | VEN + AZA (15 cycles) | PR (stable disease)¶ | Yes | Alive; disease-free |
| Masetti et al31 | 10 | t-MDS/AML | Del3q, PTPN11, WT1 | 1 | VEN + AZA | NR | Yes | Alive; disease-free |
| Masetti et al31 | 6 | t-MDS/AML | t (11;19), KMT2A | 2 | VEN + AZA (5 cycles) | PR | No | Dead; PD before HCT |
| Masetti et al31 | 9 | t-MDS/AML | Mon7, TP53 | 1 | VEN + AZA (1 cycle) | PR | Yes | Alive; disease-free |
| Masetti et al31 | 14 | t-MDS/AML | Mon7, CBL, KRAS, ASXL2 | 1 | VEN + AZA (2 cycles) | CR | Yes | Alive; disease-free |
| Masetti et al31 | 6 | t-MDS/AML | t (9;11), KMT2A | 1 | VEN + FLA (1 cycle); VEN + AZA (1 cycle) | CR (MRD neg) | Yes | Alive; disease-free |
| Study . | Age (y) . | Diagnosis . | Genetics . | Previous lines . | Venetoclax combination therapy . | Response . | HCT . | Outcome (disease status/cause of death) . |
|---|---|---|---|---|---|---|---|---|
| Winters 2021 | 7 | MDS-EB/RAEB in SDS | Mon7, ETV6, GATA2 | 0 | VEN + AZA (1 cycle) | CR (morphological <5%; cytogenetic monosomy 7 10%) | Yes | Alive; disease-free |
| Marinoff et al29 | 17 | MDS | GATA2 germ line | 1 | VEN + AZA | NR | Yes | Dead; relapse after HCT |
| Masetti et al31 | 15 | MDS-EB | LIG4 and SH2B3 germ line, mon7, RIT1, EZH2, SETBP1, ASXL1, ETV6 | 0 | VEN + cytotoxic (IDA-FLA) (1 cycle) | CR | Yes | Alive; disease-free |
| Masetti et al31 | 14 | MDS-EB | Del7q | 4 | VEN + DEC (5 cycles) | CR∗ | Yes | Dead; TRM |
| Winters 2021 | 8 | MDR-AML/RAEB-t in NF1 | Del17p/loss TP53, ASXL1, TET2 | 0 | VEN + AZA (3 cycles) | CR (morphological <5%; cytogenetic persistence del17p/loss TP53) | Yes | Alive; relapse cytogenetic del17/TP53 and MRD pos† |
| Bobeff et al28 | <6 | MDR-AML in NF1 | Mon7 | 1 | VEN + cytotoxic therapy (IDA-FLA) (1 cycle) | CR | Yes | Dead; relapse post-HCT |
| Bobeff et al28 | 6-10 | MDR-AML in familial platelet disorder | RUNX1 | 4 | VEN + cytotoxic therapy (Idarubicin + ARA-C) (1 cycle) | NR | No | Dead; PD before HCT |
| Marinoff et al29 | 14 | MDR-AML in SDS | IDH1, KMT2A | 0 | VEN + DEC | CR (MRD neg) | Yes | Alive; disease-free |
| Raedler et al61 | 16 | MDR-AML/RAEB-t | Complex karyotype | 2 | VEN + DEC (4 cycles) | CR (for 10 months, then molecular relapse) | No | Alive; molecular relapse‡ |
| Naviglio et al62 | 14 | MDR-AML in SDS | neg | 2 | VEN + AZA (1 cycle) | PR | No | Dead; PD before HCT |
| Wen et al41 | 3 | MDR-AML | Complex karyotype, NRAS | 1 | VEN + AZA | CR (MRD neg) | Yes | Alive; disease-free |
| Ma et al63 | 7 | MDS/MDR-AML in FA§ | NPM1, GATA2, WT1 | 1 | VEN+AZA (2 cycles) | CR (MRD neg) | Yes | Alive; disease-free |
| Masetti et al31 | 17 | MDR-AML | FLT3, WT1 | 1 | VEN + AZA (1 cycle) | NR | Yes | Dead; TRM |
| Masetti et al31 | 14 | MDR-AML | WT1 | 2 | VEN + AZA (1 cycle) + VEN + ARA-C (1 cycle) | CR | Yes | Dead; relapse post-HCT |
| Bobeff et al28 | 6-10 | t-AML | KMT2A, t (9;11) | 1 | VEN + ARA-C (1 cycle) | CR (MRD neg) | Yes | Alive; disease-free |
| Marinoff et al29 | 17 | t-AML | Mon7, t (7;11), PTPN11, SED2, RUNX1, BCOR | 3 | VEN + DEC | PR | No | Dead; PD before HCT |
| Winters 2021 | 11 | t-MDS/AML | RUNX1 | 1 | VEN + AZA (9 cycles) | CR (MRD neg) | No | Alive; disease-free‖ |
| Marinoff et al29 | 9 | t-MDS | PTPN11 | 1 | VEN + DEC | PR (stable disease) | No | Dead; PD before HCT |
| Masetti et al31 | 7 | t-MDS/AML | T (11;17), KMT2A | 1 | VEN + IDA-FLA (2 cycles) | CRi | Yes | Alive; disease-free |
| Masetti et al31 | 5 | t-MDS/AML | t (9;11), SDHC, KMT2A | 1 | VEN + IDA-FLA (2 cycles) | CRi | Yes | Alive; disease-free |
| Masetti et al31 | 1 | t-MDS/AML | t (4;11), KMT2A | 1 | VEN + ARA-C + idarubicin | NR | No | Dead; PD before HCT |
| Masetti et al31 | 10 | t-MDS/AML | Mon7 | 1 | VEN + AZA (15 cycles) | PR (stable disease)¶ | Yes | Alive; disease-free |
| Masetti et al31 | 10 | t-MDS/AML | Del3q, PTPN11, WT1 | 1 | VEN + AZA | NR | Yes | Alive; disease-free |
| Masetti et al31 | 6 | t-MDS/AML | t (11;19), KMT2A | 2 | VEN + AZA (5 cycles) | PR | No | Dead; PD before HCT |
| Masetti et al31 | 9 | t-MDS/AML | Mon7, TP53 | 1 | VEN + AZA (1 cycle) | PR | Yes | Alive; disease-free |
| Masetti et al31 | 14 | t-MDS/AML | Mon7, CBL, KRAS, ASXL2 | 1 | VEN + AZA (2 cycles) | CR | Yes | Alive; disease-free |
| Masetti et al31 | 6 | t-MDS/AML | t (9;11), KMT2A | 1 | VEN + FLA (1 cycle); VEN + AZA (1 cycle) | CR (MRD neg) | Yes | Alive; disease-free |
ARA-C, cytarabine; AZA, azacytidine; CRi, complete response with incomplete recovery; DEC, decitabine; FA, Fanconi anemia; FLA, fludarabine, cytarabine; IDA-FLA, fludarabine, cytarabine, idarubicin; IDH1, isocitrate dehydrogenase 1; Mon7, monosomy 7; N/A, not available; NF1, neurofibromatosis type 1; PD, progression of disease; PTPN11, protein tyrosine phosphatase nonreceptor type 11; RAEB, refractory anemia with excess of blasts; RAEB-t, refractory anemia with excess of blasts in transformation; SDS, Shwachman-Diamond syndrome; TP53, tumor protein 53; TRM, transplant-related mortality; VEN, venetoclax.
CR after 2 cycles, maintained for 10 months, then relapsed, another 3 cycles with response, bridged to HCT.
Received AZA post-HCT.
Therapy ongoing.
Previous HCT for FA; donor-cell derived leukemia (DCL) 43 months after HCT.
Maintaining MRD-negative status after 9 cycles.
Stable disease maintained for 15 cycles, then relapse, received AML-type induction therapy, bridged to HCT.
Some clinical issues still remain unresolved; these pertain to the use of venetoclax-containing regimens in this peculiar population. The optimal number of venetoclax cycles in these disorders has not been definitively elucidated, and these combinations should be tested in rigorously conducted trials. In case of poor response to a first cycle, a second cycle should be avoided and other alternatives should be explored. In the case of PR or CR, a second cycle before HCT seems to be an option to consider. Lastly, the best clinical end point to assess the efficacy of the different approaches, either blast reduction before HCT or post-HCT outcomes, remain undefined and this limits, to some extent, the ability to clearly define the best treatment option and the best partner drug to be used with venetoclax. MRD-negative remission, morphologic CR (blasts <5%), PR (blasts 5%-20%), or stable disease with a lack of leukemic progression has been adopted as the required criteria to proceed to HCT in different centers.64 Defining the treatment algorithm of these diseases represents an unmet need for the pediatric hematology community.46
Identifying genetic lesions predictive of response in AML and MDS cases
With the wider clinical use of venetoclax in pediatric hematology, it became increasingly important to identify recurrent genetic abnormalities that can help to predict the response to venetoclax therapy.25 Importantly, no mechanistic link between genetic lesions and venetoclax response has been demonstrated so far; however, specific molecular subtypes have been investigated in clinical reports. Rearrangements of the KMT2A gene are frequent in pediatric and infant leukemia and are generally associated with an aggressive clinical course.65,66 In the International Consensus Classification, the presence of ≥10% of blasts is sufficient for the diagnosis of KMT2A-rearranged AML.52 Revumenib, a potent and selective oral inhibitor of the menin-KMT2A interaction, has shown promising remission rates with a favorable toxicity profile in patients with KMT2A-rearranged AML refractory to multiple previous lines.67 Of the 12 patients with KMT2A rearrangements in a phase 1 trial on venetoclax plus chemotherapy, 6 responded to therapy (5 with CR/CR with incomplete recovery).26 In the retrospective study that we published, 8 patients presented KMT2A rearrangements; of them, 6 and 1 achieved CR and PR, respectively.31 A CR of 40% was achieved in 17 KMT2A-rearranged AML cases included in another report.32 Of 8 patients with KMT2A-rearranged acute leukemias who received a cycle of venetoclax plus azacytidine, 2 achieved MRD negativity.33 In vitro models showed high response rates to venetoclax plus azacytidine in lymphoblastic KMT2A-rearranged acute leukemia.68 Moreover, adult KMT2A-rearranged AML seems to be sensitive to this combination.69 The role of the association of venetoclax with menin inhibitors is currently under investigation with preliminary results of a phase 1-2 study on HMA plus venetoclax and revumenib reporting high efficacy in KMT2A-rearranged, nucleophosmin (NPM1)-mutated, and nucleoporin 98 and 96 precursor–rearranged AML.70 These results confirmed preclinical studies that showed a synergistic lethal effect of menin plus BCL-2 inhibition in AML lines.71 Moreover, novel compounds, such as bromodomain inhibitor, I-BET151, sunitinib, or thioridazine, have been shown to decrease BCL-2 expression and significantly synergized with venetoclax, thereby enhancing blast death in KMT2A-rearranged myeloid cell lines.72
The role of venetoclax in AML with FLT3 aberrations is more controversial. FLT3-ITD is common in children with AML with a prognostic negative effect in patients treated with conventional, multiagent chemotherapy.73 FLT3 inhibitors are currently adopted in adult AML and favorable results also have been observed in a Children Oncology Group report on pediatric patients who received sorafenib in combination with chemotherapy.74 Encouraging results have been reported with gilteritinib and quizartinib, and pediatric trials are currently ongoing.75,76 Adult patients with FLT3-ITD AML showed limited response to venetoclax-containing treatments, and these results were confirmed in the pediatric phase 1 trial in which none of the 5 children with FLT3-AML responded to therapy.26 At the same time, preclinical tests showed a synergistic effect between venetoclax and FLT3 inhibitors.37 Some pediatric reports have incorporated these drugs into venetoclax combinations, leading to improved response rates31,32,34 and suggesting evaluation of the triplet approach (venetoclax plus FLT3 inhibitor plus cytotoxic drugs or HMA) in this genetic subgroup.
The CBFA2T3::GLIS2 fusion gene, a consequence of the cryptic inversion of chromosome 16, defines a rare subtype of AML that is peculiarly diagnosed in young children and is characterized by an aggressive clinical course with an OS ranging from 10% to 30%.77 In very recent years, novel therapies have been tested in this setting. Specifically, the identification of folate receptor alpha as target for CAR T cells and monoclonal antibodies (STRO-002) has the potential to modify the management paradigm.78,79 Although 4 patients with AML with CBFA2T3::GLIS2 fusions in 2 different studies29,31 did not respond to therapy, venetoclax plus azacytidine led to effective treatment of molecular relapse of CBFA2T3::GLIS2 AML posttransplant80 and achieving MRD-negative remission in 3 of 4 children with CBFA2T3::GLIS2 AML.33 Interestingly, preclinical tests showed that dual Bcl-2 family protein inhibition is necessary to treat these diseases by combining venetoclax with MCL-1 or BCL-XL inhibitors.81,82 Integration of proapoptotic agents with novel target therapies will represent a fascinating opportunity that warrants future investigations.
Mutations in NPM1 and isocitrate dehydrogenase 1 and 2 are associated with a good response to venetoclax in adult AML.83,84 Isocitrate dehydrogenase 1 and 2 mutations are rare in children, being detected in less than 3% of pediatric AML cases,85 whereas mutations of NPM1 are found in ∼5% to 8% of cases.86 Mutant NPM1 demonstrated a critical oncogenic mechanism in AML in that it was associated with the upregulation of HOX genes in a menin-dependent manner.87 Cooperation with KMT2A complex is responsible for the sensitivity to menin inhibitors observed in NPM1-mutated AML.67,88 Favorable response rates to venetoclax were confirmed in a retrospective pediatric study in NPM1-mutated AML32 and in 1 case of NPM1-mutated MDS/AML in a patient with Fanconi anemia.63 Interestingly, NPM1 mutations have been reported in 14 of 235 pediatric MDS-EB cases in the European Working Group registry with potential implications on the management of these rare entities.89
Mutations in tumor protein 53 confer resistance to venetoclax in adult AML.90 These numbers among the pediatric reports are low with surprisingly favorable results in a phase 1 trial26 that were not confirmed in other reports, showing a general association between tumor protein 53 mutations and diseases resistant to therapy or a high risk for relapse after HCT.27,32 Protein tyrosine phosphatase nonreceptor type 11 mutations were also associated with venetoclax-resistant pediatric AML, confirming adult reports.26,32,91 Venetoclax combinations has to be considered with caution in these genetic subgroups and other therapies, if available, should be preferred.25,92
Regarding MDS-EB, only preliminary results have been reported so far on the susceptibility of specific genetic subgroups to BCL-2 inhibition. A recent study showed particularly high BCL-2 expression in GATA2 MDS-EB cases when compared with GATA2 refractory cytopenia of childhood, suggesting de-regulation of apoptosis as a potential driver of disease progression of GATA2 disease to overt MDS and AML and providing biologic evidence for the use of venetoclax therapies in this disease.93 Moreover, there is increasing understanding of the role of tandem duplications in exon 13 of the upstream binding transcription factor (UBTF-TDs) gene in pediatric myeloid hematology.94 UBTF-mutated AML cases present a distinct genetic profile that is associated with poor outcomes.95 In adult AML, UBTF-TD has been associated with myelodysplastic features, lower response rates to induction therapy, and worse survival when compared with UBTF-wild type.95,96 Preliminary data from a German cohort of children revealed that UBTF-TDs are present in nearly a third of pediatric MDS-EB cases and are associated with worse outcome post-HCT when compared with UBTF-wild type MDS.97 The poor prognosis of patients with UBTF in both the AML and MDS setting, despite the use of allogeneic HCT, suggests that conventional treatment algorithms need to be revised in the management of these patients who carry this molecular abnormality. Preliminary data suggest a role for menin inhibitors in UBTF-TD AML.98 The possible role of BCL-2 inhibition may warrant further exploration in light of the overexpression of HOX genes in these diseases, a biomarker for sensitivity to Bcl-2 inhibitors, and the genetic expression profile that overlap with NPM1-mutated diseases.88,96,99 A single case report has been published recently that described a patient with UBTF-TD MDS who experienced relapse after 2 HCT procedures and who showed a dramatic optimal response to venetoclax plus azacitydine.100
Future directions
Despite the promising results we summarized and discussed, several questions remain to be addressed to optimize the use of venetoclax in pediatric myeloid neoplasms. First, we urgently need prospective studies aimed at obtaining regulatory approval and taking into consideration the lack of a pediatric formulation and the limited effectiveness of intensive chemotherapy alone in children with r/r AML. With this perspective, several clinical trials are currently ongoing (as reviewed in Leśniak et al25), and preliminary findings of venetoclax in combination with intensive chemotherapy plus GO or CPX-351 are certainly promising.101,102 Moreover, factors that predict response should be investigated systematically in large pediatric cohorts and will potentially help clinicians in the future to determine the best therapeutic approach on a single-patient basis. These factors include both the genetic lesions previously discussed and the pharmaco-typing assays that are becoming part of clinical practice in recent years. In this regard, BH3 profiling is a functional assay that measures apoptotic priming and determines dependence on BCL-2, BCL-XL, or MCL-1 by the relative release of cytochrome-c by mitochondria. As an exploratory objective of the pediatric phase 1 trial, AML blasts of patients with BCL-2 dependence presented a major reduction in circulating blasts and higher CR rates when compared with samples with BCL-XL dependance.26 Ex vivo drug sensitivity screening of blasts to venetoclax with exposure to serial drug dilution has also been used in some reports and generally correlates with clinical response to Bcl-2 inhibition in pediatric acute leukemia.35,103 Different mechanisms of resistance to venetoclax have been identified in recent years, including downregulation of the proapoptotic proteins BCL-2 interacting mediator of cell death and BCL-2–associated X protein secondary to venetoclax exposition, acquisition of MCL-1 or BCL-XL dependence of myeloid blasts, and acquisition of BCL-2 mutations.104-106 Possible strategies to overcome venetoclax resistance are currently being tested107-110 and have been reviewed extensively in Griffioen et al.111 Finally, interest in testing venetoclax therapies in other potential pediatric myeloid settings is emerging, including the management of AML molecular relapse after HCT80 and different diseases such as chronic myeloid leukemia112 and juvenile myelomonocytic leukemia.28,113 Future studies will also have to dissect the optimal duration of venetoclax treatment and the number of cycles to be administered.
Conclusions
The integration of venetoclax into clinical practice represents a potential opportunity to enhance the clinical care of pediatric patients with myeloid diseases. BCL-2 inhibition provides a potential option that can be considered in different conditions. In r/r AML and t-MDS/AML, venetoclax, in combination with both cytotoxic therapies and HMAs, can be used as bridge to HCT depending on the clinical condition of the patient and the impact of genetic characterization in predicting response. In advanced MDS, a peculiar setting that lacks largely validated therapeutic options, venetoclax with azacytidine certainly represents a promising approach, potentially reaching the ambitious goal of reducing disease burden pre-HCT while avoiding intensive AML-type chemotherapy.
Acknowledgments
This work was supported by grants from the Fondazione Italiana per la Ricerca sul Cancro (AIRC) (AIRC IG 26039 [R.M.]; AIRC IG 2018 id. 21724 [F.L.]), Accelerator Award–Cancer Research UK/AIRC–Innovative CAR Therapy Platform project (F.L.), Associazione Italiana Ricerca per la Ricerca sul Cancro (AIRC)-Special Project 5×1000 no. 9962 (F.L.), Ministero dell’Università e della Ricerca grants PRIN 2017 and PRIN2020 (F.L.), Italian PNRR CN3 “National Center for Gene Therapy and Drugs based on RNA Technology” (F.L.), and Hub Life Science-Terapie Avanzate Ecosistema innovative della Salute (F.L.).
Authorship
Contribution: R.M. and F.B. designed the study; R.M., F.B., and D.L. performed the review and wrote the manuscript; F.B. and D.L. designed the figures; and F.L. critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Baccelli, Pediatric Hematology and Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, 11, Via Masserenti, 40138 Bologna, Italy; email: francesco.baccelli2@studio.unibo.it.
References
Author notes
R.M. and F.B. contributed equally as first authors.