The 2019 American Society of Hematology (ASH) guidelines for immune thrombocytopenia (ITP) included recommendations on the management of adults (recommendations 1-9) and children (recommendations 10-21) with primary ITP . We describe here the results of a review of the 2019 guidelines by a working group of experts requested by ASH to inform decision-making about the need for and timing of a guideline revision. An updated Medline and Embase search applied the same search terms as in the 2019 ASH guidelines, limited to systematic reviews and clinical trials, from May 2017 to July 2022. There were 193 studies identified, 102 underwent abstract reviews, and 54 full reviews. Each study was assessed based on relevance to the previous recommendation with regard to the population, prioritized outcomes, new outcomes, and study design. Reviewers assessed if the data would change the strength or the directionality of the existing recommendation or merit development of a new recommendation. Based on this review, the ASH Committee on Quality endorsed a focused update on second-line management for adults with ITP. In addition, there will be continued annual monitoring and reviewing of the 2019 ASH guidelines on ITP in full to evaluate when there is sufficient new evidence to warrant additional revisions.
Introduction
The 2019 American Society of Hematology (ASH) guidelines for immune thrombocytopenia (ITP) included recommendations on the management of adults (recommendations 1-9) and children (recommendations 10-21) with primary ITP.1 The guidelines addressed the treatment of newly diagnosed ITP as well as persistent and chronic ITP with regard to hospitalization, observation, and treatment selection of both first- and second-line agents. The guidelines also carried forward recommendations from the 2011 ASH guidelines for ITP related to diagnostic testing in patients with ITP, management of Helicobacter pylori, hepatitis C, human immunodeficiency virus, and measles mumps rubella vaccine–associated ITP.2 This review of the 2019 guidelines by a working group of experts was requested by ASH to inform decision-making about the need for and timing of a guideline revision.
Literature review methods
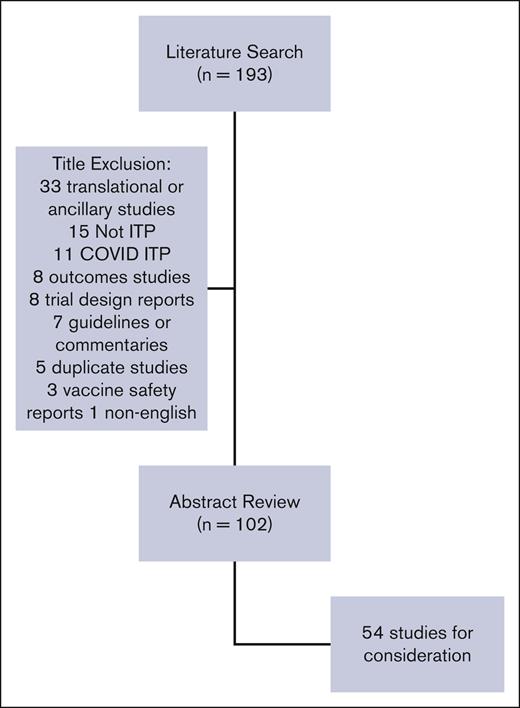
ASH contracted with the University of Oklahoma Health Sciences Center to refresh the literature searches conducted for the 2019 guidelines. The ASH Guideline Oversight Subcommittee provided with project oversight. D.R.T. vetted and retained researchers to conduct the updated search. The updated Medline and Embase search applied the same search terms as in the 2019 ASH guidelines. The search was limited to systematic reviews and clinical trials from May 2017 to July 2022. Non-English language and duplicates were removed. Identified studies (n = 193) were screened by title by 1 reviewer (C.E.N.) for relevance to the scope of the guidelines (see Figure 1). Studies deemed out of the scope were reviewed by title by all reviewers (C.E.N., D.M.A., R.F.G., T.K., and K.R.M.) and excluded. The remaining 102 studies underwent abstract review by 2 reviewers for several patients, study design, and relevance. Reviewers ranked studies 1 to 3 (1 include, 2 possibly include, 3 exclude), and the average score between the 2 reviewers was calculated. Studies with an average score of 1 were included and those with a score of 3 were excluded. The remaining studies were discussed by the entire group and were included or excluded by consensus. Studies selected for full review (n = 54) were grouped by their relevance to an existing guideline recommendation (both 2011 and 2019) or the need for a new recommendation (Table 1) and reviewed by 2 reviewers. Each study was assessed based on relevance to the previous recommendation with regard to the population, prioritized outcomes, new outcomes, and study design. Reviewers assessed if the data would change the strength or the directionality of the existing recommendation or merit the development of a new recommendation.
Summary of primary systematic review findings
| Subject . | Update or new recommendation . | Previous recommendation . | Number of studies . | Comments . |
|---|---|---|---|---|
| Adults with newly diagnosed ITP | ||||
| Prednisone or dexamethasone | Update | 2019 recommendation 4 | 3 | |
| H. pylori eradication | Update | 2011 recommendations 7.3.A and 7.3.B | 2 | |
| IVIg | Update | 2011 recommendation 4.3A | 2 | |
| Combination therapy | New | 3 | Agents used in combination with steroids: all-trans retinoic acid mycophenolate mofetil rhTPO | |
| Children with newly diagnosed ITP | ||||
| Observation or IVIg | Update | 2019 recommendation 12 | 3 | |
| Prednisone or dexamethasone | Update | 2019 recommendation 15 | 1 | |
| Anti-D immunoglobulin or IVIg | Update | 2019 recommendation 17 | 2 | |
| IVIg with or without IV methylprednisolone | New | 1 | ||
| Adults with ITP for ≥3 mo who are corticosteroid dependent or failed corticosteroids | ||||
| TPO-RAs (avatrombopag, eltrombopag, hetrombopag and romiplostim) | Update | 2019 recommendations 7 and 9 | 17 | |
| Low-dose Rituximab | Update | 2019 recommendations 8 and 9 | 1 | |
| Splenectomy | Update | 2019 recommendations 7 and 8 | 1 | |
| Fostamatinib | New | 3 | ||
| Rituximab with or without cyclophosphamide | New | 1 | ||
| Rituximab with or without all-trans retinoic acid | New | 1 | ||
| Danazol with or without all-trans retinoic acid | New | 1 | ||
| Hydroxychloroquine | New | 1 | ||
| IVIg or eltrombopag for surgery | New | 1 | ||
| Children with ITP who have non-threatening mucosal bleeding and/or diminished quality of life and do not respond to first-line treatment | ||||
| TPO-RAs (eltrombopag and romiplostim) | Update | 2019 recommendations 19 and 20 | 3 | 1 included in both adult and peds |
| TPO-RAs and Rituximab | Update | Recommendation 19 | 1 | |
| Splenectomy | Update | 2019 recommendations 20 and 21 | 1 | Included in both adult and peds |
| Sirolimus vs cyclosporine | New | 1 | ||
| Hydroxychloroquine | New | 1 | ||
| Multiple comparison studies | ||||
| Comparative effectiveness in children with ITP | 1 | |||
| Network meta-analysis in adults with ITP | 2 | |||
| Multiple agents single-center experience | 1 | |||
| Subject . | Update or new recommendation . | Previous recommendation . | Number of studies . | Comments . |
|---|---|---|---|---|
| Adults with newly diagnosed ITP | ||||
| Prednisone or dexamethasone | Update | 2019 recommendation 4 | 3 | |
| H. pylori eradication | Update | 2011 recommendations 7.3.A and 7.3.B | 2 | |
| IVIg | Update | 2011 recommendation 4.3A | 2 | |
| Combination therapy | New | 3 | Agents used in combination with steroids: all-trans retinoic acid mycophenolate mofetil rhTPO | |
| Children with newly diagnosed ITP | ||||
| Observation or IVIg | Update | 2019 recommendation 12 | 3 | |
| Prednisone or dexamethasone | Update | 2019 recommendation 15 | 1 | |
| Anti-D immunoglobulin or IVIg | Update | 2019 recommendation 17 | 2 | |
| IVIg with or without IV methylprednisolone | New | 1 | ||
| Adults with ITP for ≥3 mo who are corticosteroid dependent or failed corticosteroids | ||||
| TPO-RAs (avatrombopag, eltrombopag, hetrombopag and romiplostim) | Update | 2019 recommendations 7 and 9 | 17 | |
| Low-dose Rituximab | Update | 2019 recommendations 8 and 9 | 1 | |
| Splenectomy | Update | 2019 recommendations 7 and 8 | 1 | |
| Fostamatinib | New | 3 | ||
| Rituximab with or without cyclophosphamide | New | 1 | ||
| Rituximab with or without all-trans retinoic acid | New | 1 | ||
| Danazol with or without all-trans retinoic acid | New | 1 | ||
| Hydroxychloroquine | New | 1 | ||
| IVIg or eltrombopag for surgery | New | 1 | ||
| Children with ITP who have non-threatening mucosal bleeding and/or diminished quality of life and do not respond to first-line treatment | ||||
| TPO-RAs (eltrombopag and romiplostim) | Update | 2019 recommendations 19 and 20 | 3 | 1 included in both adult and peds |
| TPO-RAs and Rituximab | Update | Recommendation 19 | 1 | |
| Splenectomy | Update | 2019 recommendations 20 and 21 | 1 | Included in both adult and peds |
| Sirolimus vs cyclosporine | New | 1 | ||
| Hydroxychloroquine | New | 1 | ||
| Multiple comparison studies | ||||
| Comparative effectiveness in children with ITP | 1 | |||
| Network meta-analysis in adults with ITP | 2 | |||
| Multiple agents single-center experience | 1 | |||
Relevant findings
The primary findings related to updating the guideline are highlighted below. Table 1 outlines all areas where articles were identified, and revisions were considered.
Adults with newly diagnosed ITP
Corticosteroids remain the backbone of therapy for ITP. The 2019 guidelines suggest either prednisone (0.5-2.0/mg/kg per day) or dexamethasone (40 mg per day for 4 days) as the type of corticosteroid for initial therapy in adults with newly diagnosed ITP (recommendation 4). Two new systematic reviews and 1 randomized trial were identified.3-5 The systematic reviews included 1 study not considered in the original 2019 guidelines and 2 non-English studies that were excluded. The inclusion of this manuscript did not change the strength or direction of the current recommendation. A randomized trial favored 3 courses of dexamethasone over prednisone for newly diagnosed and treatment of naïve patients based on higher sustained remission.4 Due to the heterogeneity of the population, the need for 3 courses of dexamethasone, no difference in additional outcomes and <50 patients per study arm, this study was not felt to change the current recommendation.
The 2019 guidelines suggest corticosteroids alone rather than rituximab and corticosteroids for initial therapy (recommendation 5). There were no new trials that addressed this recommendation. However, trials of combination therapy with corticosteroids and recombinant human thrombopoietin receptor agonists (TPO-RAs), all-trans retinoic acid, and mycophenolate mofetil were identified.6-8 We acknowledged that the landscape of upfront management is evolving, however, given the heterogeneity of the studies, did not feel that there was sufficient data to require a new recommendation. Trials in upfront combination therapy should be assessed with an emphasis on cost, patient-related outcomes, and shared decision-making.
Children with newly diagnosed ITP
The 2019 guidelines recommend observation rather than intravenous immunoglobulin (IVIg) in children with newly diagnosed ITP and those who have no or minor bleeding (recommendation 12). The guidelines commented on the randomized trial of IVIg vs observation that was published after the original search.9 The trial included 200 children with newly diagnosed ITP randomized to either IVIg or observation alone. The authors found no difference in the guideline-prioritized outcome of the chronic disease. Higher rates of bleeding were seen in the observation arm. However, the population included children with moderate bleeding. Therefore, the results do not directly apply to the patient population in this recommendation. Bleeding was not increased in children with no or mild bleeding at diagnosis treated with observation, the population included in the guideline and therefore, this trial supports the certainty of the current recommendation.
Adults with persistent and chronic ITP who are corticosteroid dependent or have no response to corticosteroids
The 2019 guidelines suggest a TPO-RA rather than rituximab and suggest either a TPO-RA or splenectomy for adults with persistent and chronic ITP lasting ≥3 months who are corticosteroid dependent or have no response to corticosteroids (recommendations 7 and 9, respectively). TPO-RAs included in the 2019 guidelines included romiplostim and eltrombopag. The guidelines suggested either romiplostim or eltrombopag (recommendation 6) and commented on the recent Food and Drug Administration approval of avatrombopag. Approval of avatrombopag provides a third agent when considering the use of a TPO-RA and any updates to the guidelines would need to consider all available TPO-RAs, including hetrombopag available in China. However, there were no substantial differences among the newer TPO-RAs that would change the recommendations regarding TPO-RAs as a drug class. We further acknowledged that the paired comparisons of TPO-RAs, rituximab, and splenectomy led to discordant recommendations and that alternate strategies for formulating recommendations with multiple comparators may be preferable.
The 2019 guidelines commented on the syk inhibitor fostamatinib. All clinical trials, however, were published after the conclusion of the original search. The updated search identified 3 studies on the use of fostamatinib.10-12 These studies had the same limitations recognized in the 2019 guideline in that the drug was mostly investigated in highly refractory patients failing multiple agents. Therefore, its role in this population is less clear. The group acknowledged that fostamatinib received Food and Drug Administration indication for patients with chronic ITP who had an insufficient response to a previous treatment. We also recognized the emergence of new data in support of fostamatinib since the publication of the original guideline.
Children with ITP who have non––life-threatening mucosal bleeding and/or diminished Health-Related Quality of Life and do not respond to first-line treatment
The 2019 guidelines suggest TPO-RAs rather than rituximab or splenectomy in children who have non–life–threatening mucosal bleeding and/or diminished Health-Related Quality of Life and do not respond to first-line treatment (recommendations 19-21). Trials of sirolimus, cyclosporine, and hydroxychloroquine have been published since the 2019 guideline. However, the size of the trials does not merit updated recommendations.13,14
Conclusions
The 2019 ASH guidelines on the management of ITP continue to be relevant and important, including recommendations related to second-line therapy for adults. We conclude that there is insufficient evidence to justify a revision of the entire guideline at this time. We also recognize, however, that the 2019 recommendations on second-line therapy for adults were the result of paired comparisons of splenectomy, rituximab, and the TPO-RAs, and were based on a heterogeneous patient population. This resulted in discordant recommendations. Therefore, we recommend that a focused revision on second-line therapy for adults be conducted. We appreciate that due to the absence of comparative randomized trials and the lack of reporting on prioritized patient–related outcomes, recommendations on second-line therapy will likely remain highly dependent on patient values and preferences even with additional clinical trials. Preference for agents may also change with the prioritization of different outcomes and clinical contexts, such as the desire to avoid immunosuppression in the era of COVID-19. The updated search conducted here also identified alternate methodological approaches that would allow comparisons across several treatment strategies.15,16 The GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) approach also outlines a methodology for multiple comparisons that applies the evidence to the decision framework.17 Use of these methods and refinement of the population of interest may clarify the existing recommendations. This would also provide the opportunity to be inclusive of avatrombopag and fostamatinib, discussed above, as well as novel agents currently in clinical trial development.18-20 Based on this review, the ASH Committee on Quality endorsed a focused update on second-line management for adults with ITP. This update will involve the selection of a guideline panel, determination of appropriate questions, conduct of relevant literature searches, application of GRADE methodology, and publication of final recommendations. In addition, there will be continued annual monitoring and reviewing of the 2019 ASH guidelines on ITP in full to evaluate when there is sufficient new evidence to warrant additional revisions.
Acknowledgments
The authors acknowledge the American Society of Hematology for their support of this work.
D.M.A. receives grant support from Canadian Institutes of Health Research, the Public Health Agency of Canada.
Authorship
Contribution: D.R.T. conducted the literature search; C.E.N., D.M.A., R.F.G., T.K., and K.R.M. conducted a systematic review of the identified literature; C.E.N. wrote the first draft of the manuscript; and all authors contributed to revising and finalizing the manuscript.
Conflict-of-interest disclosure: C.E.N. has received research funding from Novartis; has served as a consultant to Novartis, Argenx, and Sanofi; received educational funding from Sobi; received royalties from UpToDate; has served as a medical adviser to UK ITP Support Association; and has voluntarily served ITP Australia. D.M.A. has received research funding from Novartis and Rigel; has served as a consultant to Novartis, Rigel, Amgen, Medison, Daiichi Sankyo, Sobi, Chugai, Principia, Takeda, Cellphire, Alpine, Argenx, and Sanofi; has received royalties from UpToDate; and has served as an honorary medical adviser to Platelet Disorders Support Association. R.F.G. has received research funding from Novartis, Sobi, and Agios; has served on the advisory board of Agios and Sanofi; and has served as an honorary medical adviser to Platelet Disorders Support Association. T.K. has received research funding from Amgen and Novartis; and has served on the advisory board of Argenx, Sobi, and UCB. K.R.M. has served on the data safety board of Argenex and has also served on the advisory board of Novartis and Alpine Bioscience. D.R.T. has served on the advisory board of Sanofi.
Correspondence: Cindy E. Neunert, Columbia University Irving Medical Center, 3959 Broadway, New York, NY 10032; email: cn2401@cumc.columbia.edu.
References
Author notes
Data are available on request from the corresponding author, Cindy E. Neunert (cn2401@cumc.columbia.edu).