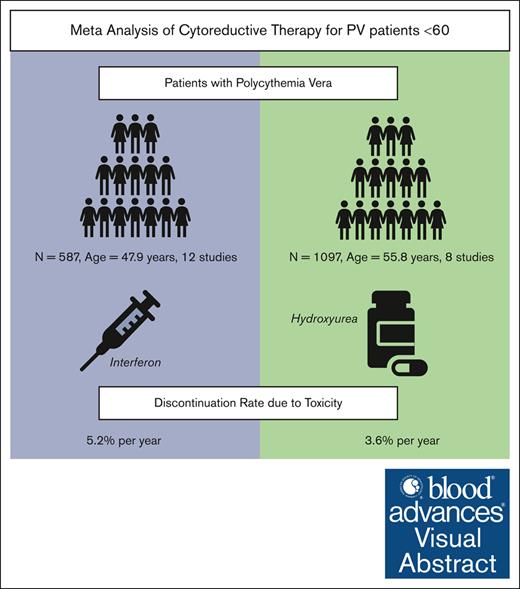
Visual Abstract
Cytoreductive therapy is not routinely recommended for younger patients with polycythemia vera (PV) due to concern that treatment toxicity may outweigh therapeutic benefits. However, no systematic data support this approach. To support objective risk/benefit assessment of cytoreductive drugs in patients with PV aged <60 years (PV<60), this systematic review and meta-analysis was conducted to evaluate toxicity and disease-related complications in PV<60 treated with interferon alfa (rIFN-α) or hydroxyurea (HU). A search of PubMed, Scopus, Web of Science and Embase identified 693 unique studies with relevant keywords, of which 14 met inclusion criteria and were selected for analysis. The weighted average age of patients treated with rIFN-α was 48 years (n = 744 patients; 12 studies) and for HU was 56 years (n = 1397; 8 studies). The weighted average duration of treatment for either drug was 4.5 years. Using a Bayesian hierarchical model, the pooled annual rate of discontinuation due to toxicity was 5.2% for patients receiving rIFN-α (n = 587; 95% confidence interval [CI], 2.2-8.2) and 3.6% for HU (n = 1097; CI, 1-6.2). The average complete hematologic response for rIFN-α and HU was 62% and 52%, respectively. Patients experienced thrombotic events at a pooled annual rate of 0.79% and 1.26%; secondary myelofibrosis at 1.06% and 1.62%; acute myeloid leukemia at 0.14% and 0.26%; and death at 0.87% and 2.65%, respectively. No treatment-related deaths were reported. With acceptable rates of nonfatal toxicity, cytoreductive treatment, particularly with disease-modifying rIFN-α, may benefit PV<60. Future randomized trials prioritizing inclusion of PV<60 are needed to establish a long-term benefit of early cytoreductive treatment in these patients.
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm (MPN) characterized by the clonal proliferation of JAK2-mutated hematopoietic stem, progenitor, and precursor cells, causing blood count abnormalities, associated symptoms, and potentially fatal complications. Currently, PV is frequently diagnosed at a younger adult age (<60 years)1 and occasionally in children and adolescents. Irrespective of age, >60% of patients with PV are symptomatic.2,3 PV symptoms such as pruritus, fatigue, headaches, difficulty concentrating, and erythromelalgia can significantly impair quality of life.2 The course of PV is further complicated by thrombosis (11%-36%)4,5; hemorrhage (4%)4; and progression to more aggressive malignancies including secondary myelofibrosis (sMF) (7%-50%),1,4,6 myelodysplastic syndrome, and acute myeloid leukemia (AML) (3%-6%).4,7 The lifetime risk of these complications for patients aged <60 years (PV<60) is similar to if not greater than older patients.1,4 Relative to the age-matched general population, PV<60 have a higher excess mortality than older patients.8 Despite the facts, cytoreductive treatment is often deferred in PV<60.
Currently, PV treatment is recommended by some to control symptoms and reduce the risk of thrombosis, whereas others believe that treatment should be initiated from the onset of disease.1,9-12 In addition to phlebotomy and aspirin, cytoreductive drugs such as hydroxyurea (HU) and interferon alfa (rIFN-α) are known to reduce thrombosis risk in PV,13,14 but are not routinely recommended by the European LeukemiaNet (ELN) or the National Comprehensive Cancer Network (NCCN) for PV<60 without a history of thrombosis, a high symptom burden, or intolerance to phlebotomy.9,10 These drugs are readily prescribed to older or patients at “high risk” (PV>60 or history of thrombosis) and have been shown to be safe, tolerable, and effective in the majority.12 A meta-analysis of 44 rIFN-α studies across all age groups, including older patients, revealed an annual discontinuation rate of only 6.5%, with a uniformly low thrombosis rate of 0.5% per patient-year.13 A meta-analysis of 16 studies of patients of all ages also provided insight on long-term outcomes with HU use in “high-risk” PV.15 It is only as recently as 2021 that a phase 2 trial compared the efficacy of cytoreductive therapy with ropeginterferon alfa-2b in patients at “low risk” against the standard treatment with phlebotomy alone.16
Because current recommendations defer cytoreductive therapy for younger, “low-risk” patients with PV, we conducted a systematic review and meta-analysis to support an objective risk/benefit assessment of cytoreductive agents, rIFN-α and HU, in PV<60. The aim of this study was to define cytoreductive treatment toxicity and disease-related complications in PV<60 to support treatment recommendations for younger patients with PV.
Methods
Four databases (PubMed, Scopus, Web of Science, and Embase) were used to identify relevant articles using search terms for PV and cytoreductive drugs (supplemental Methods). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was followed throughout the search. Case reports, case series with ≤20 patients, reviews, abstract-only studies, and any articles that did not include primary data were excluded. Inclusion criteria were predefined as studies of patients with a diagnosis of PV, reporting a median age between 18 and 60 years, in which patients were given HU, rIFN-α, and/or ruxolitinib. Studies reporting results using other cytoreductive agents were excluded (eg, chlorambucil, P32, nitrogen mustard, imatinib, busulfan, or investigational therapy). Studies that did not report drug toxicity data or that focused on sMF, accelerated/blast-phase MPN, or PV during pregnancy were also excluded. Selected literature was archived and reviewed on Covidence.17 The quality of each study design was assessed using the Joanna Briggs Institute (JBI) checklist, in which a higher score from 0 to 1 indicates higher quality.
Data regarding the duration of treatment, dosage, adverse events (AEs), and discontinuation were extracted from each study. The frequency of AEs, the frequency of discontinuation , and the annual rate of discontinuation were calculated using standard methods. Cochran Q test and the heterogeneity index (I2) were used in the assessment of heterogeneity in the reported discontinuation rates between studies. The meta-analysis for discontinuation rates was conducted using a Bayesian hierarchical model. The summary estimates and confidence intervals (CIs) were obtained from the posterior distribution. Forest plots represent estimates for each study and summary estimates. A similar process was followed for extracting efficacy data via the rates of complete hematologic response (CHR), partial hematologic response (PHR), and outcome data including thrombosis, progression to sMF or AML, and death. A weighted average based on sample size and duration (patient-years) was also calculated for outcomes including annual rates of thrombosis, progression to sMF or AML, and death. These data were analyzed using descriptive statistics. All statistical analyses were performed using R version 4.2.2.
Results
A total of 693 unique titles and abstracts were identified through our search. These were independently screened by 2 authors (R.C. and G.A.-Z.); 97 articles were read in full, and ultimately 14 studies11,16,18-29 met inclusion criteria (Figure 1). The concordance rate between the reviewers was 91%. JBI scores for the included studies ranged between 0.54 and 1 (supplemental Table 1). Because only 2 studies reported on ruxolitinib in PV<60, they were not included in the meta-analysis.
PRISMA flowchart of study selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
PRISMA flowchart of study selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
A total of 2141 patients from 14 studies were included in the final analysis; 744 patients received rIFN-α (12 studies) and 1397 patients received HU (8 studies). The weighted average age of patients receiving rIFN-α was 48 years, whereas the weighted average age of patients receiving HU was 56 years. The weighted average duration of treatment for either drug was 4.5 years. The dosing of rIFN-α–2a/2b, pegylated rIFN-α–2a, and ropeg rIFN-α–2b varied across studies (supplemental Table 1). The most frequently prescribed HU dose ranged between 0.5g per day and 1.5g per day.
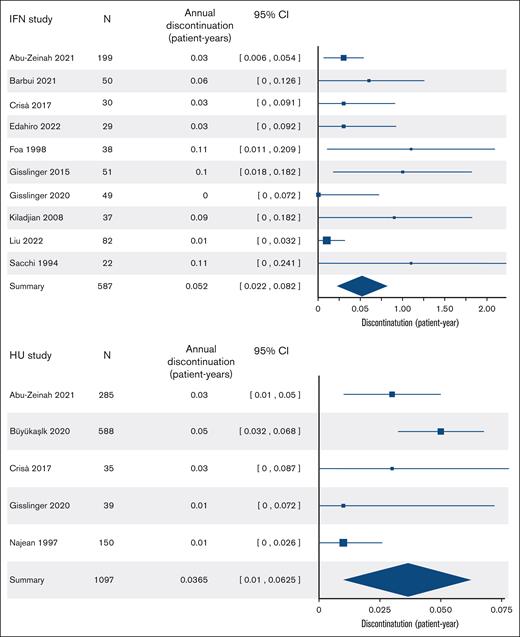
During the treatment period, AEs were reported at different rates across studies. The frequency of grade 3 to 4 toxicity in patients on rIFN-α ranged from 0% to 11%, with flu-like symptoms and liver enzyme abnormality being cited the most (supplemental Table 1). The frequency of grade 3 to 4 toxicity in patients on HU was reported only in 2 studies included in our analysis; hematologic and dermatologic toxicity being the most common. The discontinuation rates of rIFN-α ranged from 4.6% to 37% over median durations of 0.4 to 6.3 years. The discontinuation rates of HU ranged from 2.6% to 17% over median durations of 0.5 to 14 years. There was significant heterogeneity in discontinuation rates across rIFN-α studies (Cochran Q = 29; P < .001; and I2 = 70%), perhaps related to differences in rIFN-α formulation and dosing, but not across HU studies (Q = 6.4; P = .3; and I2 = 27%). Pooling the rIFN-α studies that reported discontinuation (587 patients in 10 studies), the overall frequency was 13% (95% CI, 2.7-23), and the annualized rate was 5.2% (95% CI, 2.2-8.2). Pooling HU studies that reported discontinuation (1097 patients in 5 studies), the overall frequency was 15% (95% CI, 6.9-24), and the annualized discontinuation rate was 3.6% (95% CI, 1-6.2) (Figure 2).
Cytoreductive discontinuation rates. Annual discontinuation rates in patients receiving rIFN-α (top panel) and HU (bottom panel).
Cytoreductive discontinuation rates. Annual discontinuation rates in patients receiving rIFN-α (top panel) and HU (bottom panel).
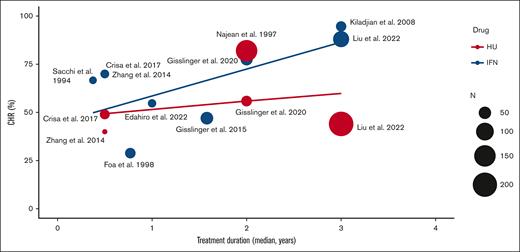
Both rIFN-α and HU were effective in controlling blood counts, with an average CHR of 62% and 52%, respectively; and PHR of 27.9% and 43.0%, respectively (supplemental Table 2). Frequency of hematologic response increased over time in patients treated with rIFN-α but not for patients treated with HU (Figure 3). Thrombotic events were estimated at a frequency of 3.2% with an annual rate of 0.79% on rIFN-α (284 patients in 7 studies) and a frequency of 5.4% with an annual rate of 1.26% on HU (842 patients in 4 studies). Progression to sMF was estimated at a frequency of 11% with an annual rate of 1.06% on rIFN-α (267 patients in 3 studies) and at a frequency of 27% with an annual rate of 1.62% on HU (1206 patients in 5 studies). Progression to AML was infrequent at an annual rate of 0.14% in 267 rIFN-α–treated patients in 3 studies and 0.26% in 908 HU-treated patients in 3 studies. In studies that reported deaths, the annual mortality rate was 0.87% on rIFN-α (592 patients in 9 studies) and 2.65% on HU (1223 patients in 6 studies). No deaths were attributed to treatment toxicity.
The rates of CHR in patients receiving rIFN and HU over time. N, number of patients.
The rates of CHR in patients receiving rIFN and HU over time. N, number of patients.
Of the 14 studies, only 4 compared cytoreduction with the control arm of phlebotomy only (PHL-O).11,16,22,29 The key findings of the limited data available include: improved symptoms and hematologic and histomorphologic responses with rIFN-α compared with PHL-O11,16,29; lower myelofibrosis incidence with rIFN-α compared with PHL-O11; and no significant difference in myelofibrosis incidence between HU and PHL-O.11,22 Overall survival for PV<60 on cytoreduction compared with PHL-O was not reported16,22,29 or was not significantly different.11
Discussion
Younger patients with PV are usually symptomatic and suffer impaired quality of life, reduced work productivity, and excess mortality compared with age-matched controls.2,8 Unfortunately, effective and potentially life-prolonging cytoreductive therapy1,11 is often deferred in younger patients who are considered “low risk” because of their age and lack of thrombosis history.9,10 The rationale for withholding cytoreductive therapy is data-sparse and driven by theoretical concerns for toxicity and unknown benefits from early treatment. Yet, there is some evidence that early treatment is both well tolerated and potentially useful. The Low-PV Study demonstrated that young patients receiving cytoreduction with ropeg IFN-α–2b experienced no more AEs of grade ≥3 than those randomized to treatment with phlebotomy alone and improved quality of life after 1 to 2 years of treatment.16 An evaluation of thrombosis-free, progression-free, and overall survival would require a larger study with long follow-up powered for these important end points, and only retrospective data are currently available. Recently, our retrospective study demonstrated that use of rIFN-α can improve myelofibrosis-free and overall survival, independent of patient age.1,11 These findings motivated this systematic review and meta-analysis to help inform decisions regarding use of cytoreductive agents in PV<60.
To our knowledge, this is the first systematic review and meta-analysis evaluating the available evidence regarding the safety of cytoreductive agents in PV<60. Our findings suggest that both rIFN-α and HU are safe and well-tolerated in younger patients with low rates of discontinuation for toxicity. The annualized rates of discontinuation we calculated are similar to those reported for older patients who are routinely prescribed cytoreductive therapy.13 In fact, our institutional experience showed even lower rates of discontinuation at 2.2% and 2.8% for HU and rIFN-α, respectively,11 a finding possibly related to younger age of our patients, dosing, or longer follow-up. With regards to efficacy, nearly all PV<60 patients achieved a hematologic response (CHR + PHR of 90%-95%) with both rIFN-α and HU; a proportion at least as good as reported for older patients or “high-risk” patients with PV.13
This study identified a modest but not insignificant rate of thrombosis, sMF, AML and disease-related mortality in younger patients with PV. There are currently no studies in “low-risk” PV or PV<60 evaluating event-free survival as an end point of cytoreductive therapy. The low event rates observed in this meta-analysis point to the known role of these cytoreductive drugs in reducing thrombosis (rIFN-α & HU) and sMF (rIFN-α).11,13,30 Unfortunately, long-term data reporting the rate of CHR, toxicity, or event-free survival with cytoreduction compared with PHL-O for younger patients are limited or unavailable. In this meta-analysis, event rates were lower with rIFN-α than with HU, but the data available from these studies did not allow for direct comparison of these 2 drugs. It is potentially informative that the CONTI-PV study of ”high-risk” patients with PV showed longer event-free survival in patients treated with rIFN-α compared to HU.31 Similarly, rIFN-α compared with no cytoreduction was associated with a significantly higher myelofibrosis-free survival in a recent study of adolescent and young adult patients with MPN (20-year myelofibrosis-free survival of 100% vs 73%, respectively).32
Although preventing thrombosis remains the core of PV treatment recommendations, our study highlights that PV<60 patients suffer greater risk of disease progression to sMF than thrombosis during a few years of follow-up, despite thrombosis being a recurrent event (rates of 1.06% vs 0.79%, respectively, with rIFN-α, and 1.62% vs 1.26%, respectively, with HU). Progression to AML was a rare event. Although concerns have been raised regarding potential oncogenicity of HU in PV, we found that progression to AML at 0.26% was very uncommon in PV<60 receiving HU. In contrast, fibrotic progression in PV<60 is a major problem considering the long duration of disease expected for these patients.1 Therefore, future studies should evaluate the role of early intervention with cytoreductive therapy in preventing disease progression in PV<60 as well as thrombosis, particularly with rIFN-α, which has been shown to improve survival outcomes in a retrospective analysis.11,31 Such studies are feasible if highly predictive risk models are available to identify patients at greatest risk of events; these can be developed in the era of large data and artificial intelligence.33
This meta-analysis provides objective data demonstrating low toxicity and the potential for therapeutic benefit from early cytoreductive therapy in younger patients with PV. Yet, this analysis shared limitations common to meta-analyses. Patient-level raw data were not available, so summary statistics across heterogenous studies were pooled. Heterogeneous dosing (HU and rIFN-α), type of rIFN-α, and follow-up duration may explain some variability in AE rates and outcomes. Overall, the follow-up duration on treatment was short (4.5 years), limiting an assessment of long-term safety and efficacy. Additionally, our inclusion criterion for age based on a median age <60 years allowed inclusion of some older patients, but this limitation was more likely to overestimate rather than underestimate the discontinuation rate (supplemental Table 4). Available data also did not allow for us to identify those younger patients with a history of thrombosis. Lastly, the limited number of studies on this subject made it difficult to assess the quality of each study according to the same JBI criteria. Nonetheless, in the absence of perfect data, we believe this study provides the best available objective data related to efficacy and toxicity of cytoreductive therapy in patients with PV<60.
The findings of this meta-analysis could influence current clinical practice recommendations and perhaps aid the design of future research studies. The findings suggest that ELN/NCCN recommendations result in undertreatment of patients with PV<60 because there is no clear evidence to support concerns that risk of toxicity exceeds potential benefit. ELN/NCCN risk stratification is only valid for thrombotic events and not validated for the risk of sMF, myelodysplastic syndrome/AML, or death. A history of thrombosis is unlikely to affect treatment tolerance or nonthrombosis outcomes. Most younger patients are appropriately categorized as “low-risk” for thrombosis, but they continue to be at risk for PV progression because of a longer duration of illness. Future investigation of cytoreductive agents should prioritize the inclusion of PV<60, allowing for the development of new, evidence-based clinical guidelines that can improve their management.
Acknowledgments
The authors thank Sa’ad Law from Weill Cornell Medicine-Qatar Library for assistance with the literature search, the American Society of Hematology for providing a travel award for the first author to present this work, and the Johns Family Fund of the Cancer Research and Treatment Fund for funding support. The authors also thank the MPN Research Foundation, MPN Peoria, and Starr Cancer Consortium for research funding.
Authorship
Contribution: G.A.-Z., and R.S.C. selected studies for inclusion in the meta-analysis; R.S.C., S.R., and M.K. extracted raw data from the studies; O.S. and G.A.-Z. performed the statistical analyses; and all authors interpreted the data, participated in the writing and review of the manuscript, and approved the final submitted version.
Conflict-of-interest disclosure: N.K. reports consultancy fees from Protagonist Therapeutics and PharmaEssentia. J.M.S. reports consultancy fees from AbbVie, MorphoSys, CTI Biopharma Corp, SDP Oncology, PharmaEssentia, Protagonist Therapeutics, and Sierra Oncology. The remaining authors declare no competing financial interests.
Correspondence: Ghaith Abu-Zeinah, Division of Hematology and Medical Oncology, Richard T. Silver Myeloproliferative Neoplasms Center, Weill Cornell Medicine, 1300 York Ave, Box 113, New York, NY 10065; email: gfa2001@med.cornell.edu.
References
Author notes
Data are available on request from the corresponding author, Ghaith Abu-Zeinah (gfa2001@med.cornell.edu).
The full-text version of this article contains a data supplement.