Newer immune-based approaches based on recruitment and redirection of endogenous and/or synthetic immunity such as chimeric antigen receptor T cells or bispecific antibodies are transforming the clinical management of multiple myeloma (MM). Contributions of the immune system to the antitumor effects of myeloma therapies are also increasingly appreciated. Clinical malignancy in MM originates in the setting of systemic immune alterations that begin early in myelomagenesis and regional changes in immunity affected by spatial contexture. Preexisting and therapy-induced changes in immune cells correlate with outcomes in patients with MM including after immune therapies. Here, we discuss insights from and limitations of available data about immune status and outcomes after immune therapies in patients with MM. Preexisting variation in systemic and/or regional immunity is emerging as a major determinant of the efficacy of current immune therapies as well as vaccines. However, MM is a multifocal malignancy. As with solid tumors, integrating spatial aspects of the tumor and consideration of immune targets with the biology of immune cells may be critical to optimizing the application of immune therapy, including T-cell redirection, in MM. We propose 5 distinct spatial immune types of MM that may provide an initial framework for the optimal application of specific immune therapies in MM: immune depleted, immune permissive, immune excluded, immune suppressed, and immune resistant. Such considerations may also help optimize rational patient selection for emerging immune therapies to improve outcomes.
Unmet needs for MM immunotherapy
Over the past 2 decades, the outcome for patients with multiple myeloma (MM) has improved considerably; first, with the introduction of immune-modulatory drugs and proteasome inhibitors; and then with monoclonal antibodies targeting CD38.1 For example, the great majority of patients with newly diagnosed MM now experience tumor regression after modern induction regimens. More recently, T-cell redirection with chimeric antigen receptor (CAR) T cells and bispecific antibodies has also yielded high rates of tumor regression,2 leading to the regulatory approvals of therapy for patients with relapsed MM after ≥4 prior lines of therapy.3-6 These therapies also lead to impressive responses in earlier lines of therapy, prompting ongoing consideration of their application earlier in the course of the disease. In spite of these advances, most patients with MM eventually experience recurrent disease and eventually succumb to the underlying malignancy. Therefore, there remains an unmet need to improve current therapies to achieve durable unmaintained responses and possibly cures. In view of ongoing challenges with cost, access, toxicity, as well as variable durability of therapeutic benefit, it is desirable to better understand the mechanisms of resistance and optimize the application of immune therapy to maximize the potential to achieve cures. In addition, immune paresis, from both the underlying malignancy as well as the effects of therapy, is a major contributor to poor response to vaccines and ongoing risk of infections, which remain a major cause of mortality in patients with MM, even in the setting of remission.7 Although much progress in MM therapy has been achieved through the application of “next effective line of therapy,” the premise of this review is the unmet need to maximize the curative potential of first line of therapy.
Implications of preexisting immune types on immunotherapy: lessons from solid tumors
Over the past decade, immune therapy has been firmly established as 1 of the pillars of cancer therapy.8 Blockade of inhibitory immune checkpoints such as programmed death-1 (PD-1)/programmed death ligand-1 (PDL-1) led to durable remissions and cures in patients with some malignancies such as melanoma. Immune checkpoints blockade, by definition, depends on preexisting endogenous immunity.9 Therefore, the underlying immunogenicity of tumors reflected by the immune contexture such as the degree of T-cell infiltration (eg, hot tumors) or adaptive expression of PDL-1 has been correlated with responsiveness to these therapies. These concepts have led to biomarkers such as the expression of PD-L1 on tumor or immune cells, which serve as the basis of patient selection in some instances and have been incorporated into regulatory approval. It is also appreciated that such biomarkers are therapy specific and may only apply to the specific immune therapy in question. PD-1 blockade did not improve outcomes in randomized trials with unselected patients with MM,10 although some other checkpoints such as TIGIT and Lag-3 have been proposed in preclinical studies and show promise in early clinical trials.11,12 In contrast to solid tumors, strategies for T-cell redirection such as CAR T cells and bispecific antibodies have proven highly effective in MM.2 Therefore, we will focus on emerging data about how immune status might affect responsiveness to these therapies. As discussed further below, we suggest that although the overall strategies for immune therapy in MM differ considerably from that in solid tumors, the concept that preexisting immune status may affect responsiveness to emerging immune therapies may apply in MM as well.
Systemic vs regional immune alterations in MM and MGUS
The concept that tumor cells from patients even in advanced MM remain sensitive to lysis by both innate and adaptive immune cells was demonstrated >25 years ago.13,14 All MM lesions are preceded by monoclonal gammopathy of undetermined significance (MGUS).15 Previous studies have documented the capacity of the immune system to specifically recognize these earliest lesions.16,17 However, tumor recognition of MGUS occurs in the backdrop of underlying systemic immune dysfunction, which originates early during myelomagenesis.18,19 Transition of MGUS to MM is associated with progressive attrition of TCF1+ T cells,20 previously shown to be capable of self-renewal and long-term persistence.18 Instead, MM bone marrow is characterized by an increase in more differentiated T cells, including granzyme B+ CD8+ T cells.18,19 In some patients, this differentiated T-cell compartment consists of large T-cell clones that correlate with poor outcome.21,22 In addition to adaptive immunity, MM bone marrow is also characterized by alterations in innate cells, including natural killer (NK) and natural killer T (NKT) cells, as well as myeloid and other regulatory cells with immune-suppressive features.18,23,24 Systemic alterations of immune cells in the tumor microenvironment in both MGUS and MM have been analyzed with newer single-cell technologies and linked to outcome.25-30 Changes in immune cells during early evolution to MM have been recently reviewed.31-33 Studies in preclinical models such as V-kappa myc mice have provided evidence for immune surveillance mediated by both T and NK cells.34 In this model, tumor immunity was enhanced by CD137 engagement34 and impaired by interleukin-18–mediated effects.35 In another model, regulatory T cells (Tregs) were implicated in suppressing tumor immunity.36 The concept of tumor-extrinsic control in MM immune surveillance is also supported by the finding of progressive growth of preneoplastic cells in humanized models.37 In addition to immune cells, stromal compartment is also altered in MM and exhibits an inflammatory phenotype.38 The application of single-cell genomics has also illustrated the transcriptional heterogeneity of T cells in the MM marrow microenvironment.19,25,26 It is important to note, however, that T cells isolated from marrow aspirates represent an admixture of several distinct populations including marrow resident39,40 and nonresident/in-transit cells, as well as contaminating T cells from blood.41 Several of these populations, except the truly marrow resident T cells, are likely shared with circulating T cells. Another limitation of many of the current studies in MM is that they lack insights into antigen specificity and functional aspects of T cells, particularly because only a proportion of T cells isolated from the bone marrow are expected to be tumor specific,13,42 and marrow aspirates may have varying degrees of hemodilution from blood.
A critical feature of malignant transition in MM is multifocal growth of tumors, accounting for the term “multiple” myeloma. Interestingly, this growth pattern is also observed in murine MM models, suggesting that it is an intrinsic feature of tumor biology during malignant transition.43,44 However, this feature creates the potential for distinct aspects of spatial interactions in the malignant phase, with emergence of regions of immune exclusion. Recent studies with both in vitro and in vivo models have shown that the entry of antigen-specific T cells into MM clusters depends in part on in situ stimulation by tumor-associated Clec9a+ dendritic cell (DCs).43 These insights support regional regulation of tumor-specific immunity, which as discussed below, may be critical for achieving durable responses after immunotherapies. The concept that regional alterations in immune responses may be important in the context of myeloma immunotherapy is also supported by the emerging evidence from clinical trials that patients with high disease burden and extramedullary disease may be at an increased risk of recurrence after current T-cell–based therapies, including bispecific antibodies.4-6 These considerations also urge the need to routinely include advanced imaging before initiation of these novel immune therapies.
Immune contributions to myeloma therapy and outcomes
It is now appreciated that the immune system may contribute to the effects of several current MM therapies. Immune-modulatory drugs such as thalidomide, lenalidomide, pomalidomide, as well as newer drugs such as iberdomide lead to activation of T and NK cells in vivo through derepression of interleukin-2 transcription due to cereblon-mediated degradation of ikaros.45 Notably, effects of these drugs on T cells as well as NKT cells depend on signal 1 and T-cell receptor (TCR) engagement, again emphasizing the need to understand antigen-specific responses.46,47 Immune activation was linked to clinical responses to pomalidomide in early single-agent studies.48 Proteasome inhibitors may lead to immunogenic cell death in MM tumors, promoting the induction of tumor immunity via DCs,49 and signatures of immunogenic tumor cell death were correlated with outcome in patients with MM receiving triplet therapies.50 Both daratumumb and isatuximab are monoclonal antibodies that engage Fc-dependent mechanisms such as antibody-dependent cytotoxicity to mediate antitumor effects and are now integral to MM therapy.51 Belantamab has also been shown to induce immunogenic cell death.52 These data underscore the possibility that the immune system may play an important role in the antitumor effects of several MM therapies. This is also supported by studies correlating immune cell states with outcomes. As an example, differentiation states of T cells such as CD27+ T cells as well the presence of Tregs has been linked to outcome in large MM cohorts.21,30,53 An important message from these studies again is the high degree of variance in immune cell states and potential immune competence in MM cohorts. The impact of these differences in immune fitness and response to immune intervention was recently illustrated in studies documenting high variance in humoral and cellular immune response to severe acute respiratory syndrome coronavirus 2 vaccines in MM.54,55 Variance in immune status can not only affect response to therapy but also other outcomes including overall survival and risk of infections in patients with MM.
Immune correlates of response after T-cell redirection in MM
CAR T cells
Two CAR T-cell products targeting B-cell maturation antigen (BCMA), namely idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel), have now been approved for the therapy of relapsed MM after ≥4 lines of previous therapy.3,4 In spite of high rates of remission, including measurable residual disease (MRD) negativity, patients with MM remain at risk of ongoing relapse. Understanding the correlates of durable remissions after BCMA CAR T cells is an area of active research, and only some of the data sets have yet been fully published. Analysis of biospecimens from the first BCMA CAR T-cell clinical trial at the University of Pennsylvania illustrated a dynamic cross talk between CAR T cells, endogenous T cells, myeloid cells/DCs, and tumor cells that correlated with durable remissions.22 CAR T-cell infusion leads to expansion of endogenous T cells, predominantly in the TCF1+CD27+ T-cell compartment. Consistent with this, higher baseline TCR diversity was associated with longer progression-free survival (PFS). PFS was also correlated with properties of the myeloid/DC compartment, with the presence of Baff+ myeloid cells correlating with shorter PFS and DC-like populations with longer PFS.22 Early correlative analyses from patients treated with cilta-cel and ide-cel have also yielded similar findings. Among patients treated with ide-cel, increase in naïve and CD27+ early memory T cells correlated with longer PFS, whereas the presence of CD57+ senescent T cells correlated with shorter PFS.56 The expansion of CD8+ central memory T cells was correlated with longer PFS among patients treated with cilta-cel.57 Properties of the drug product, likely reflecting the immune state at the time of T-cell harvest,58 also correlate with outcome. For example, higher proportion of CD8+ stem cell features and lower proportion of CD4+ Treg-like cells in the product correlated with improved outcome.57 Effector functionality of the CAR T-cell product, as reflected in target-specific interferon gamma production, has also been shown to correlate with improved outcome in treated patients.56
TCEs
Initial studies with single-cell transcriptomics suggested that the presence of CXCR3+ effector CD8+ cells, but not other effector memory populations, correlated with response to bispecific T-cell engagers (TCEs).59 In contrast, the presence of TOX+ CD8+ cells was correlated with a lack of response. These elegant studies also described a correlation between early and sustained increases in clonality in CD8+ T cells after therapy and clinical response. Therefore, the capacity of the TCEs to engage and modify preexisting endogenous T cells may be critical for their antitumor activity. TCE-mediated expansion of T cells in ex vivo cultures was inhibited by the addition of anti-MHCI antibody.59 Correlative analyses on patients treated with BCMA bispecific teclistamab in the MajesTEC-1 trial suggested that a higher proportion of naïve T cells correlated with improved response, whereas the presence of Tregs and PD1+ Tim3+ T cells correlated with a lack of response.60 In another analysis of patients with MM treated with teclistamab, a higher proportion of effector memory T cells and lower proportion of Tregs correlated with improved response to therapy.61 Together, these studies suggest that the clinical response to TCEs may be affected by the preexisting properties of T cells. Some of the features relating to tumor burden including advanced stage, presence of extramedullary disease, as well as excess soluble ligand (such as soluble BCMA), potentially providing a “sink effect,” may also affect outcomes in patients treated with TCEs.5,6 Further studies are needed to better understand the mechanisms by which TCEs redirect and sustain anti-MM immunity. Improved understanding of this biology may also allow for strategies to reduce adverse events including the risk of infections after TCE therapy.62
Limitations of current studies
Most of the current studies evaluating the correlates of response after T-cell redirection in MM have been based on methods such as mass/flow cytometry and single-cell transcriptomics from blood or bone marrow aspirates. In some studies, T cells from relatively small numbers of patients were pooled for comparisons, which may not meet the assumptions of the statistical tests used. Analyses of functional aspects of immune cells in these studies are limited, and assumptions based on T-cell phenotypes in other models or tissues may not apply to redirected T cells. Importantly, spatial analyses of immune cells including redirected T cells (eg, CAR T cells or T cells bound to bispecific antibody) would be critical to understand the mechanisms underlying T-cell redirection. Considering the emerging data that CAR T-cell therapy can lead to alterations in endogenous T cells,22 the capacity for long-term disease control or cures after these therapies may also depend on the induction of tumor-specific immune responses.
Vaccines, the next frontier?
Vaccines represent 1 of the greatest triumphs of modern medicine, but their application in cancer including MM remains yet unrealized. As discussed above, the appreciation that preexisting durable tumor control may depend on preexisting endogenous immunity has revitalized interest in vaccine-based approaches to boost endogenous responses. Initial studies have demonstrated the feasibility of boosting antigen-specific and tumor-specific T-cell responses in vivo. These studies have targeted clonal immunoglobulin-associated idiotype, shared tumor antigens, plasma cell antigens, or fusions of DCs with whole tumor cells.63-66 DCs have also been used to boost innate immunity, such as NKT cells, in patients with MM.67 Recent advances in understanding the mechanisms of T-cell infiltration also suggest that it may not be sufficient to simply elicit T-cell responses via vaccines, and it may be critical to actively drive vaccine-elicited T cells into tumors.68 Nonetheless, the stage is now set for future combinations of vaccines with strategies addressing immune-suppressive factors or with immune redirection.
Integrating spatial biology of MM into immune types
The importance of spatial biology has long been appreciated as being critical for the diagnosis and management of lymphoid tumors such as non-Hodgkin lymphoma. Recent studies have illustrated the spatial heterogeneity of MM, both in terms of tumor genetics and changes in the immune microenvironment.43,69,70 As discussed earlier, understanding spatial immune contexture has proven clinically useful for the application of immune checkpoint blockade in solid tumors.71 The principle of immune redirection therapy, by definition, relies on redirection and, hence, altering the spatial dynamics of immune cells. Therefore, as these therapies gain prominence in MM therapeutics, we suggest that it would be critical for future studies to account for spatial immunobiology of MM to better interpret the results and improve outcomes associated with such immune therapies. The methods for spatial analysis of tumor tissues as well as downstream analysis are rapidly evolving.72,73 These advances include methods with greater depth and resolution, as well as advances in machine learning and the application of artificial intelligence tools.73 Although the application of some of these methods on human bone marrow biopsies remains an area of active research, we emphasize the need to use whole-slide–based approaches to study the spatial aspects of myeloma. This is because of previous studies with MM biopsies showing that immunologically distinct lesions (eg, T-cell infiltrated as well as T-cell poor) can coexist in the same biopsy.43 Integrating imaging-directed biopsies into clinical care may further improve our understanding of spatial heterogeneity in myeloma. Future clinical trials in MM should also try to harmonize the initial processing of biopsies and prevent harsh decalcification methods that may affect downstream application of emerging spatial methods.
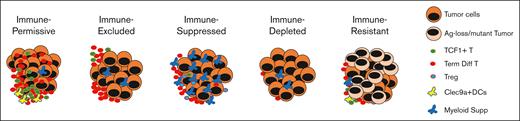
Below, we propose an initial framework for 5 major spatial immune types, based on the analysis of MM tumors, as well as insights emerging from solid tumors.43,74,75 As has been observed in solid tumors, >1 type may coexist in an individual patient, and in that setting, the higher-risk lesion may affect clinical behavior or resistance to therapy. The proposal builds on some key findings from recent studies in MM including the detection of areas of immune exclusion, the role of DCs in T-cell entry in model systems, the correlation between the proximity of DCs and T cells and outcome, and the impact of antigen loss on the efficacy of T-cell redirection.43,76 The proposed major immune types, as discussed below, are immune depleted (ID), immune permissive (IP), immune excluded (IE), immune suppressed (IS), and immune resistant (IR). The biologically defining features of these types are noted in Figure 1 and potential clinical implications in Table 1. We anticipate that the application of newer artificial intelligence and machine learning tools may further refine these categories for clinical application.
Proposed spatial immune types of myeloma. Development of myeloma is characterized by the clustered growth of tumor cells that creates distinct spatial immune types as shown. These immune types may affect the optimal application of immunotherapy in MM. Ag, antigen; supp, suppressors.
Proposed spatial immune types of myeloma. Development of myeloma is characterized by the clustered growth of tumor cells that creates distinct spatial immune types as shown. These immune types may affect the optimal application of immunotherapy in MM. Ag, antigen; supp, suppressors.
Potential clinical implications of MM immune types for the application of immune therapy
| . | IP . | IE . | IS . | ID . | IR . |
|---|---|---|---|---|---|
| Proposed defining feature(s) | T-cell hot spots with infiltration and Clec9a DCs, lack of terminal differentiation in T-cell clones | T cells at tumor margins without infiltration and lack of Clec9a DCs | Inhibitory myeloid infiltration, immune-suppressive cells, and T-cell exhaustion | Systemic and regional lymphoid depletion | Loss of T-cell redirection target and resistance to immune recognition |
| Clinical aspects | Expected favorable course earlier in disease evolution | Biology possibly similar to extramedullary plasmacytomas | Potentially diverse mechanisms | Lymphopenia, ? with impaired hematopoiesis, extreme age, frailty, and prior extensive chemotherapy | Target-specific loss or mutations in targets for T-cell redirection |
| Response to T-cell redirection | Yes, durable responses | Yes, but may not be durable | Yes, but not durable | Unlikely but high risk of CAR T-cell manufacturing failure | No, but resistance may be limited to specific therapies |
| Possible solutions/therapeutic goals | Target early eradication of residual disease | Enhance T-cell entry and DC or NK recruitment | Combinations to overcome suppression and optimal combinations may be pathway/mechanism specific | Direct tumor targeting and restore lympho-hematopoiesis | Alternate targets or combinatorial targeting and alternate immune cells (eg, NK/NKT) |
| . | IP . | IE . | IS . | ID . | IR . |
|---|---|---|---|---|---|
| Proposed defining feature(s) | T-cell hot spots with infiltration and Clec9a DCs, lack of terminal differentiation in T-cell clones | T cells at tumor margins without infiltration and lack of Clec9a DCs | Inhibitory myeloid infiltration, immune-suppressive cells, and T-cell exhaustion | Systemic and regional lymphoid depletion | Loss of T-cell redirection target and resistance to immune recognition |
| Clinical aspects | Expected favorable course earlier in disease evolution | Biology possibly similar to extramedullary plasmacytomas | Potentially diverse mechanisms | Lymphopenia, ? with impaired hematopoiesis, extreme age, frailty, and prior extensive chemotherapy | Target-specific loss or mutations in targets for T-cell redirection |
| Response to T-cell redirection | Yes, durable responses | Yes, but may not be durable | Yes, but not durable | Unlikely but high risk of CAR T-cell manufacturing failure | No, but resistance may be limited to specific therapies |
| Possible solutions/therapeutic goals | Target early eradication of residual disease | Enhance T-cell entry and DC or NK recruitment | Combinations to overcome suppression and optimal combinations may be pathway/mechanism specific | Direct tumor targeting and restore lympho-hematopoiesis | Alternate targets or combinatorial targeting and alternate immune cells (eg, NK/NKT) |
IP
T cells in these lesions typically lack terminally differentiated CD8+ T-cell clones21 and are instead enriched for TCF1+ cells with greater proliferative potential. T cells are enriched in hot spots proximate with Clec9a+ conventional type 1 DCs.43 T cells readily infiltrate these tumors. As such, these patients are likely excellent candidates for T-cell redirection and may derive prolonged and potentially curative benefit from immune therapies. Early eradication of residual disease may be critical to achieving cures in this group.77 Tumors earlier in the course of evolution to MM may also fall into this group18 and may provide the rationale for the consideration of T-cell redirection in earlier stages of MM development, if safety could be ensured.
IE
Although T cells abound in these lesions, they do not efficiently enter tumors, presumably due to a lack of effective antigen presentation via local DCs.43 Although T-cell redirection may theoretically overcome this limitation, durability of responses may be compromised if durable responses depend on both redirected and endogenous T cells. Combination approaches to enhance T-cell infiltration, such as recruitment of DCs may particularly benefit this subset. The biology of such lesions also resembles those with extramedullary disease and may contribute to increased risk of recurrence.
IS
T cells in these patients can theoretically be suppressed by several distinct immune-suppressive mechanisms including immune suppression by myeloid cells or Tregs. Expression of inhibitory T-cell checkpoints on these T cells may also represent a pathway-specific target to improve the efficacy of T-cell redirection. Chronic and frequent dosing of TCE may itself promote T-cell exhaustion and paradoxically create adaptive resistance.
ID
The presence of systemic lymphopenia in these patients is a challenge to the efficacy of T-cell redirection, particularly with bipecifics. The mechanisms underlying lymphopenia may be diverse and related both to malignancy as well as therapy (eg, prior chemotherapy). These patients, and particularly those with lymphopenia, may also be at an increased risk of CAR T-cell manufacturing failures.78
IR
Although several of the mechanisms noted earlier (eg, Tregs, T-cell exhaustion, and myeloid cells) may, in principle, contribute to immune resistance, we restrict this category to primary resistance of tumors mediated by the inability to bind tumor-targeting moiety (such as by genetic loss79 or mutation of target76,80) in the case of T-cell redirection or loss of TCR recognition (such as by major histocompatibility complex loss)81 in the setting of endogenous immunity. Because the binding epitopes for each of the bispecific antibodies may differ, this mechanism is expected to be both target (eg, BCMA or G protein–coupled receptor, class C, group 5, member D [GPRC5D]) and agent specific and can, in principle, only be overcome by switching targets or agents. A small proportion of immune therapy–naïve patients with MM may carry monoallelic copy number losses in T-cell redirection targets such as BCMA. However, BCMA antigen loss has been described in up to 40% of relapsing cases after TCE therapy.76,79,80,82,83 Although the presence of antigen loss/mutation can be detected genetically, recent data suggest that it may be more practical to test tumor binding of the bispecific antibody ex vivo, if available for diagnostic testing.76 The potential for the emergence of IR subclones is a strong argument for multiepitope targeting in initial therapy, both for TCE and CAR T cells in the future.
Integrating genetic and immune types
It is well recognized that tumors coevolve with changes in the immune system. Early studies of T-cell redirection in MM already illustrate a dynamic cross talk between tumors and redirected and endogenous T cells.22 At present, MM is classified based on cytogenetic changes in tumor cells, both for genetic subtypes as well as assessment of disease risk.1 The latter, along with eligibility for stem cell transplant, forms the basis of current therapy algorithms. However, as immune-based approaches enter the front line, it is likely that these algorithms will need to be revisited, and risk features may depend on specific therapies. Genomic changes in tumor cells are known to affect many aspects of response to immune therapies.84 These include features such as antigen loss/mutations, defects in antigen presentation, susceptibility to immune-mediated cell death, expression of immune-suppressive factors, capacity for excluding immune cells, expression and immunogenicity of neoantigens, and plasticity and heterogeneity of tumors, to name a few. Some potential examples of genomic alterations with implications for immune microenvironment include alterations in Wnt signaling/dickkopf-1,18 c-myc, or NF-κB signaling. Because definitive studies in this regard will need adequate power, we urge the community to include spatial immunology together with genetic analyses and imaging in current clinical trials, to allow for the integration of genetic and immune types in the future.
Potential implications of immune types on patient selection and therapeutic approaches
The primary impetus behind the immune types proposed above is that they may provide a framework for the optimal application of immune therapies in the future. An early decision point may be the identification of IR or ID phenotypes with direct implications for immune redirection. These patients are poor candidates for target-specific TCEs, but those with IR phenotype may benefit from TCEs against alternate targets (eg, GPRC5D in case of BCMA loss). Lesions with high risk of antigen loss (eg, monoallelic loss) are also prime candidates for multitargeted therapies.85 Discovery of newer targets for CAR T cells targeting MM tumor cells and its precursor lesions remains a major unmet need and will be critical to overcome tumor heterogeneity and improve outcomes in IR lesions. ID lesions may initially require strategies that mediate immunogenic cell death as well improvement of cytopenias. IE lesions may benefit from combinations with strategies such as immunomodulating drugs48 that engage innate cells to recruit DCs,86 vaccines,87 or approaches to induce immunogenic cell death.49,50,88 IS lesions may, in addition, require strategies targeting suppressive elements such as targeting immune checkpoints89 or immune-suppressive cells.90 Finally, IP lesions may be the most amenable to durable remissions with time-limited therapies and avoiding adverse effects of prolonged TCEs.
Although the use of immune therapy and particularly T-cell redirection in MM in the immediate future is likely to be determined largely by global/regional access, cost, and regulatory approvals, we posit that maximizing the curative potential of these highly effective therapies may be key to optimizing clinical benefit in the long term. Factors that may affect these choices may include host and tumor genetics, tumor heterogeneity, as well as immune contexture. In this regard, it may become essential to address the heterogeneity of tumors at the outset and better understand spatial immune types in an individual patient to maximize immune-mediated tumor lysis and early eradication of residual disease.91 Achieving this goal will require systematic integration of genomic analysis of tumors, imaging, immune monitoring, spatial biology, and emerging artificial intelligence and machine learning tools into the next phase of MM clinical research and eventually clinical care.
Acknowledgment
M.V.D. is supported in part by funds from Leukemia and Lymphoma Society Specialized Center of Research, the National Institutes of Health (CA197603 and CA260563), and the International Myeloma Society, and Paula and Rodger Riney Foundation.
Authorship
Contribution: M.V.D. wrote the manuscript.
Conflict-of-interest disclosure: M.V.D. has served on the advisory boards for Bristol Myers Squibb, Lava Therapeutics, Janssen, and Sanofi.
Correspondence: Madhav V. Dhodapkar, Winship Cancer Institute, 1365 Clifton Rd, Atlanta, GA 30332; email: madhav.v.dhodapkar@emory.edu.
References
Author notes
Data are available on request from the corresponding author, Madhav V. Dhodapkar (madhav.v.dhodapkar@emory.edu).