TO THE EDITOR:
Acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) is a high-risk AML subtype with a reported frequency of 24% to 35% of all AMLs.1-3 AMLs that develop after prior therapy (therapy-related myeloid neoplasms), with recurrent genetic abnormalities and those with NPM1 or biallelic CEBPA mutations, are excluded regardless of morphologic dysplasia.4,5 AML-MRC diagnosis is straightforward when there is an antecedent history of myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN) or when dysplasia (>50%) is present in at least 2 cell lineages. However, when these conditions are not met, the diagnosis depends on World Health Organization–defined MDS-associated cytogenetic abnormalities. In general, the complexity of modern hematopathology diagnoses requires the timely incorporation of cytogenetics/fluorescence in situ hybridization (FISH) and/or molecular findings.6
AML-MRC, similar to therapy-related AML, is associated with a poor prognosis2 and lower response rates using conventional chemotherapy.7-9 Based on better survival data, the US Food and Drug Administration approved CPX-351 (Vyxeos, Jazz Pharmaceuticals), a fixed-dose liposomal formulation of daunorubicin and cytarabine for the treatment of AML-MRC and therapy-related MRC.7 In addition, alternative treatments including lower intensity therapies (hypomethylating agents with or without venetoclax, or low-dose cytarabine plus either glasdegib or venetoclax) might be better options in older patients.2 Early diagnosis of AML-MRC is crucial to make use of these newer therapies. Although the history of MDS or MDS/MPN is usually available upfront, enough maturing non-blast hematopoietic cells might not be available for the assessment of dysplasia. Regardless, for the latter, AML-MRC designation is contingent on excluding a CEBPA or NPM1 mutation. In addition, metaphase analysis and FISH results are generally not available at the time of initial diagnosis of AML; the turnaround time (TAT) of conventional chromosomal analysis ranges from 7 to 21 days.10 Our aim was to identify the percentage of cases that qualified for a diagnosis of AML-MRC solely based on MDS-associated cytogenetic abnormalities and thus would have benefited from upfront CPX-351 induction chemotherapy or other alternative therapies.
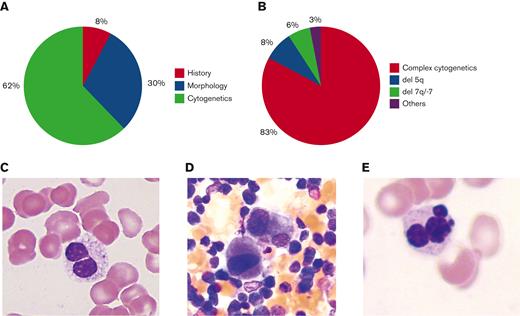
We identified 64 AML-MRC cases with archived bone marrow samples (Henry Ford Health System) over a period of 15 years. Of the 64 patients, a history of MDS or MDS/MPN was present in 5 patients (8%) (Figure 1A), and only 19 patients (30%) had more than 50% dysplasia in ≥2 lineages (Figure 1A,C-E). Remaining either had no dysplasia, dysplasia <50%, and/or dysplasia in only 1 lineage or lacked sufficient differentiated cells to assess dysplasia. The most frequently reported dysplastic cell line was granulocytic (45%), followed by megakaryocytic (38%) and erythroid (16%). A striking 62% (40/64) of cases required MDS-associated cytogenetic abnormalities for AML-MRC diagnosis (Figure 1A). Of AML-MRC cases, 83% (53/64) had complex cytogenetics as defined by ≥3 unrelated abnormalities, 8% (5/64) had del(5q), 6% (4/64) had −7 or del(7q), and 3% (2/64) had other MDS-associated abnormalities (Figure 1B). The study was approved by the Henry Ford Hospital Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
AML-MRC diagnosis. (A) Percentage of cases diagnosed based on morphology, history, or MDS-associated cytogenetics. (B) Percentage distribution of MDS-associated cytogenetic abnormalities. (C) Pseudo-Pelger-Huet cell. (D) Dysplastic monolobated megakaryocytes and megakaryocytes with separated nuclear lobes. (E) Dysplastic multinucleated erythroid cell. Original magnification ×1000 for panels C-E.
AML-MRC diagnosis. (A) Percentage of cases diagnosed based on morphology, history, or MDS-associated cytogenetics. (B) Percentage distribution of MDS-associated cytogenetic abnormalities. (C) Pseudo-Pelger-Huet cell. (D) Dysplastic monolobated megakaryocytes and megakaryocytes with separated nuclear lobes. (E) Dysplastic multinucleated erythroid cell. Original magnification ×1000 for panels C-E.
Following these findings, a pilot (18 months) preliminary cytogenetics/metaphase read protocol was instituted for 24- or 48-hour cultures with the goal of early reporting of MDS-associated and recurrent AML cytogenetics. This initial pilot included 1096 cases; 62 were AML cases and 17 were AML-MRC cases (with 10 of 17 cases [59%] requiring cytogenetics for AML-MRC diagnosis). The average TAT was 2.4 and 9.12 days for the preliminary and final cytogenetic reports, respectively. Based on preliminary cytogenetics, 5 patients were treated with azacytidine with or without venetoclax, and 1 patient was treated with Vidaza (azacytidine); the rest received standard chemotherapy or hospice. For most cases (97.3%), the findings in the final report were identical to the preliminary findings. Importantly, none of the abnormal preliminary findings had to be rectified on final reporting. Only 29 cases (2.6%) had a discrepancy between a normal preliminary karyotype vs an abnormal final karyotype; these were all non-AML cases. Based on these promising data, this process was incorporated into the routine workflow. All cases with a myeloid indication that require a preliminary report have a 24- and 48-hour traditional cytogenetics culture established. Cultures are manually harvested; cell suspensions are dropped on slides and stained using automated instrumentation. Subsequently, the slides from the 24-hour cultures are scanned using an automated metaphase scanning system and distributed to technologists who perform a 5-cell metaphase analysis, the results of which are given to the cytogeneticist. We follow the College of American Pathologists and American College of Medical Genetics guidelines to define a clone, that is, the presence of at least 2 cells containing the same extra chromosome(s) or structural chromosome abnormality or by the presence of at least 3 cells that have lost the same chromosome. The cytogeneticist interprets the findings and sends the preliminary report via secure email to the hematology-oncology and hematopathology team. To facilitate this change, the cytogenetics laboratory began using the automated metaphase scanning system 3 times per day to ensure that slides needed for a preliminary analysis could be prioritized. Automation of the slide preparation and staining allowed these cases to be more efficiently scanned, and this reduced slide preparation time from 6 minutes to 30 seconds per slide. Currently, every bone marrow sample that comes through the laboratory receives a preliminary report based on the earliest diagnostically relevant culture (24- to 48-hour cultures for myeloid and 72-hour cultures for B- and T-cell–stimulated cultures).
To conclude, 62% of our cases needed cytogenetic studies to render a diagnosis of AML-MRC. In the absence of an AML-MRC diagnosis, patients are put on the generic AML induction chemotherapy (7+3) and cannot be typically switched to CPX-351 later because of toxicity issues.7 Therefore, it is crucial to have a preliminary cytogenetic result within 2 to 3 days of an AML diagnosis to be able to accurately diagnose and treat most patients with AML-MRC in a timely manner. We demonstrate that a feasible option for generating rapid karyotype data is a preliminary conventional cytogenetics read on 24- and 48-hour cultures. There are alternative promising assays available for generating karyotypic data, for example, chromosomal microarray or next-generation cytogenetics, including the use of whole-genome sequencing or optical genome mapping, but these might not be able to provide information within 2 to 3 days of morphologic diagnosis of AML.11-13 The MDS FISH panel used by most institutions typically includes EGR1 (5/5q−), D7S486 (7/7q−), CEP8 (+8), and D20S108 (20q). Although most abnormalities would have been identified by our FISH assay, 30% of cases had cytogenetic abnormalities that would have been missed on a routine MDS panel. In addition, FISH for AML and not MDS is typically ordered for AML; the AML FISH panel varies between institutions and may or may not include probes relevant for AML-MRC diagnosis. An extended rapid FISH panel might serve the same purpose as preliminary conventional cytogenetics; however, it might come with additional logistical issues and costs. Considering that up to one-third of AMLs are AML-MRCs, a timely diagnosis is crucial to ensure appropriate therapy for this relatively common AML subtype as well as for other AML subtypes. The recent incorporation of gemtuzumab as front-line induction therapy for CD33+ AMLs, especially core binding factor AMLs, that is, [t(8;21)(q22;q22) or inv(16) (p13q22)/t(16;16)] also reinforces the need for rapid cytogenetics.14,15 This can be achieved via a streamlined workflow in the cytogenetics laboratory and timely communication with the clinical and hematopathology teams. In conclusion, regardless of the technology, which might vary between institutions and between resource-rich and resource-poor countries, the recognition of a need to improve the karyotype TAT is of great importance.
Acknowledgments: The authors thank Tracy George, Robert Hasserjian, and Daniel Arber for their expert comments during the preparation of this manuscript.
Contribution: A.V., M.P.M., K.G., and K.V.I. performed the research and data analysis; P.K. provided the clinical data and patient details; B.M.S. provided the cytogenetic raw data; A.V., B.M.S., and M.P.M. wrote the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madhu P. Menon, ARUP Laboratories, Department of Pathology, University of Utah School of Medicine, MS115-G04, 500 Chipeta Way, Salt Lake City, UT 84108-1221;.
References
Author notes
Data are available on request from the corresponding author, Madhu P. Menon (madhu.menon@aruplab.com).