TO THE EDITOR:
Rearrangements in KMT2A (KMT2Ar) are associated with pediatric, adult, and therapy-induced acute leukemias. Infants with KMT2Ar acute lymphoblastic leukemia (ALL) have a poor prognosis, with an event-free-survival (EFS) of 33.6% to 36.9%.1-3 In the context of the remarkable improvements in the treatment of childhood ALL, the dismal outcome of infantile KMT2Ar ALL and the lack of any significant therapeutic progress for more than 2 decades are striking. The St. Jude Total Therapy 16 (Total 16) study (the most recently reported study of a program for childhood ALL that begun in 1962) yielded a 5-year EFS of 88.2%.4 Total 16 enrolled all subtypes of newly diagnosed pediatric patients with ALL, including infants as defined by less than 365 days of age. Intensity of treatment was adapted to presenting clinical and genomic features, and early treatment response as determined by minimal/measurable residual disease (MRD). KMT2Ar infants were treated on an intensified high-risk arm and received clofarabine in combination with cyclophosphamide and etoposide (CCE) on days 22 to 25 of Induction and during Reinduction I based on prior reports of the activity of clofarabine in the relapsed setting.5 Infants who lacked KMT2Ar and patients with KMT2Ar who were 1 year of age or older, received the same MRD-directed treatment plan given to all other patients enrolled on study. Here, we present the outcome of KMT2Ar infants with this treatment approach.
Between 29 October 2007 and 26 March 2017, 598 eligible patients younger than 19 years of age with newly diagnosed ALL were enrolled in the Total Therapy Study 16. The protocol was approved by the institutional review board, and written informed consent was obtained from the parents, guardians, or patients with assent from the patients as appropriate. Details of Total Therapy Study 16 treatment regimen have been reported.4 Duration of EFS was defined as the time from diagnosis until the date of failure (induction failure, relapse, death, or the development of a second malignancy). EFS and overall survival (OS) rates were estimated by the method of Kaplan and Meier and were compared with the log-rank test. Cumulative risk of major toxic effects of treatment in patients 1 year of age or older with high-risk ALL and infants with KMT2Ar ALL was estimated according to the method of Kalbleisch and Prentice and compared using Gray’s test. Outcome data updated on 1 May 2019 were used for all analyses.6,7 Median follow-up time for the patients alive at the time of analysis was 6.26 years (range, 1.15-11.43 years). All analyses were based on intent-to-treat and performed with SAS (version 9.4; SAS Institute, Cary, NC) and R version 3.3.0 (www.r-project.org). This clinical trial was registered at ClinicalTrials.gov as #NCT00549848.
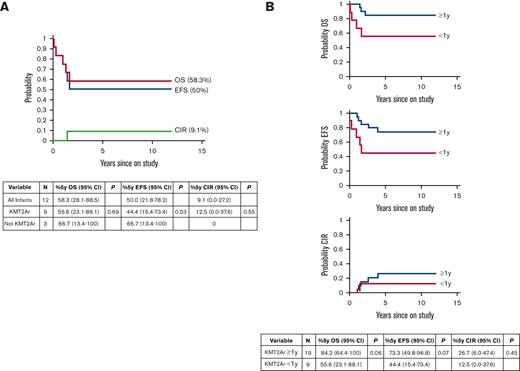
Twelve patients <1 year of age enrolled on Total 16 (Table 1). Nine patients had KMT2Ar and were assigned to the high-risk infant arm and were treated with a clofarabine-containing regimen. Two patients had favorable risk genomic features: ACIN-NUTM1 rearrangement or hyperdiploidy.8,9 One patient had a gamma delta (γδ) T-cell immunophenotype and was classified as high-risk based on a poor response to treatment as defined by detectable MRD at the end of induction (Table 1). Across the entire infant cohort, 5-year EFS and OS were 50% (95% CI, 21.8%-78.2%) and 58.3% (95% CI, 28.1%-88.5%), respectively (Figure 1A). Cumulative incidence of relapse was 9.1% (95% CI, 0%-27.2%) (Figure 1A). Both patients with favorable genomic features were cured with standard-risk treatment. Infants with KMT2Ar had a 5-year EFS of 44.4% (95% CI, 15.4%-73.4%) and OS of 55.6% (95% CI, 23.1%-88.1%). Statistical comparisons of outcomes between infants with or without KMT2Ar were not informative due to the small sample size.
Clinical and biological features of infant patient with ALL on Total 16
| Case . | Risk status . | Age (y) . | WBC (×109/L) . | Sex . | Race . | Lineage . | Subtype . | CNS status . | Day 15 induction MRD∗ . | End of induction MRD∗ . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | High | 0.71 | 31.1 | F | White | B cell | KMT2A-MLLT10 | CNS-3 | 1.38% | Neg | Alive CR1 |
| 2 | Standard | 0.82 | 61.8 | M | Other | B cell | ACIN-NUTM1 | Traumatic no blasts | Neg | Neg | Alive CR1 |
| 3 | High | 0.16 | 54.4 | F | White | B cell | KMT2A-AFF1 | Traumatic with blasts | Neg | Neg | Expired TRM |
| 4 | High | 0.82 | 32.9 | M | White | B cell | KMT2A-MLLT10 | Traumatic no blasts | 13.7% | Neg | Alive CR1 |
| 5 | High | 0.44 | 52 | F | White | B cell | KMT2A-AFF1 | Traumatic with blasts | Neg | Neg | Alive CR1 |
| 6 | High | 0.71 | 450 | M | White | B cell | KMT2A-MLLT1 | CNS-2 | 0.012% | 0.07% | Alive CR1 |
| 7 | Standard | 0.79 | 11.2 | M | White | B cell | Hyperdiploid 51+ | CNS-3 | 0.006% | Neg | Alive CR1 |
| 8 | High | 0.52 | 411.9 | M | White | B cell | KMT2A-EPS15 | Traumatic with blasts | 1.274% | Neg | Expired TRM |
| 9 | High | 0.56 | 6.7 | F | Other | B cell | KMT2A-MLLT1 | CNS-2 | Neg | ND† | Expired TRM |
| 10 | High | 0.12 | 905.3 | F | White | B cell | KMT2A-MLLT1 | Traumatic with blasts | 2.911% | 0.011% | Expired TRM |
| 11 | High | 0.2 | 95.8 | F | Other | T cell | γδ T cell | Traumatic with blasts | 31.73% | 0.034% | Expired Relapse |
| 12 | High | 0.18 | 125.3 | M | White | B cell | KMT2A-AFF1 | CNS-2 | 2.88% | Neg | Alive CR2 |
| Case . | Risk status . | Age (y) . | WBC (×109/L) . | Sex . | Race . | Lineage . | Subtype . | CNS status . | Day 15 induction MRD∗ . | End of induction MRD∗ . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | High | 0.71 | 31.1 | F | White | B cell | KMT2A-MLLT10 | CNS-3 | 1.38% | Neg | Alive CR1 |
| 2 | Standard | 0.82 | 61.8 | M | Other | B cell | ACIN-NUTM1 | Traumatic no blasts | Neg | Neg | Alive CR1 |
| 3 | High | 0.16 | 54.4 | F | White | B cell | KMT2A-AFF1 | Traumatic with blasts | Neg | Neg | Expired TRM |
| 4 | High | 0.82 | 32.9 | M | White | B cell | KMT2A-MLLT10 | Traumatic no blasts | 13.7% | Neg | Alive CR1 |
| 5 | High | 0.44 | 52 | F | White | B cell | KMT2A-AFF1 | Traumatic with blasts | Neg | Neg | Alive CR1 |
| 6 | High | 0.71 | 450 | M | White | B cell | KMT2A-MLLT1 | CNS-2 | 0.012% | 0.07% | Alive CR1 |
| 7 | Standard | 0.79 | 11.2 | M | White | B cell | Hyperdiploid 51+ | CNS-3 | 0.006% | Neg | Alive CR1 |
| 8 | High | 0.52 | 411.9 | M | White | B cell | KMT2A-EPS15 | Traumatic with blasts | 1.274% | Neg | Expired TRM |
| 9 | High | 0.56 | 6.7 | F | Other | B cell | KMT2A-MLLT1 | CNS-2 | Neg | ND† | Expired TRM |
| 10 | High | 0.12 | 905.3 | F | White | B cell | KMT2A-MLLT1 | Traumatic with blasts | 2.911% | 0.011% | Expired TRM |
| 11 | High | 0.2 | 95.8 | F | Other | T cell | γδ T cell | Traumatic with blasts | 31.73% | 0.034% | Expired Relapse |
| 12 | High | 0.18 | 125.3 | M | White | B cell | KMT2A-AFF1 | CNS-2 | 2.88% | Neg | Alive CR2 |
WBC, white blood cell.
MRD, minimal residual disease as determined by flow cytometry.
ND, not determined, patient expired before CCE and did not have an end of induction marrow.
Outcomes ofpatients with KMT2Arenrolled in St. Jude Total Therapy 16 Study. (A) Treatment outcome for all patients <1 year of age on study (N = 12). See Table 1 for clinical and biological features. 5-year OS, 5-year EFS, and 5-year cumulative incidence of relapse are shown. Ninety-five percent of confidence intervals are indicated in the table. There was no statistically significant difference in outcomes when comparing infants with KMT2Ar and without KMT2Ar. (B) Treatment outcome for all patients with KMT2Ar on study (N = 19 KMT2Ar ≥ 1 year of age and N = 9 KMT2Ar <1 year of age). Ninety-five percent of confidence intervals are indicated in the table. There was a trend toward inferior OS and EFS in infants with KMT2Ar which did not reach statistical significance. There is no difference in CIR for infants with KMT2Ar and older patients.
Outcomes ofpatients with KMT2Arenrolled in St. Jude Total Therapy 16 Study. (A) Treatment outcome for all patients <1 year of age on study (N = 12). See Table 1 for clinical and biological features. 5-year OS, 5-year EFS, and 5-year cumulative incidence of relapse are shown. Ninety-five percent of confidence intervals are indicated in the table. There was no statistically significant difference in outcomes when comparing infants with KMT2Ar and without KMT2Ar. (B) Treatment outcome for all patients with KMT2Ar on study (N = 19 KMT2Ar ≥ 1 year of age and N = 9 KMT2Ar <1 year of age). Ninety-five percent of confidence intervals are indicated in the table. There was a trend toward inferior OS and EFS in infants with KMT2Ar which did not reach statistical significance. There is no difference in CIR for infants with KMT2Ar and older patients.
A total of 28 patients with KMT2Ar were enrolled on Total 16 across all age groups; 19 patients ≥1 year of age received standard-risk therapy, and 9 patients <1 year of age received high-risk therapy on the infant treatment arm with CCE. The probabilities of 5-year EFS and OS in KMT2Ar patients ≥1 year of age and those <1 year of age were 73.3% (95% CI, 49.8%-96.8%) vs 44.4% (95% CI, 15.4%-73.4%) (P = .07) and 84.2% (95% CI, 64.4%-100%) vs 55.6% (95% CI, 23.1%-88.1%) (P = .06), respectively (Figure 1B). Six of the 9 KMT2Ar infants were MRD-positive (0.012%-13.7%; median, 2.13%) on day 15 of induction before CCE, 4 of whom became MRD-negative (<0.01%) and 2 of whom had a reduction in MRD from 0.012% to 0.07% and 2.911% to 0.011% after CCE (Table 1). The trend toward superior outcomes in older patients with KMT2Ar that has been previously reported was not due to a lower incidence of relapse in this series because the 5-year cumulative incidence of relapse was 26.7% (95% CI, 6%-47.4%) in patients >1 year of age and 12.5% (95% CI, 0%-37.6%) in those <1 year of age (P = .45) (Figure 1B).10
Five KMT2Ar infants are alive (4 in CR1, 1 in CR2), while 4 died in CR1 (supplemental Table 1). Three deaths were secondary to infection, including a multidrug resistant soft tissue bacterial infection during remission induction (days 1-21), a respiratory syncytial virus pneumonia during Reinduction II, and a chronic parainfluenza 3 infection during continuation weeks 70 to 101 which led to chronic pneumonitis and interstitial fibrosis, respectively. The fourth patient developed grade 5 pulmonary hypertension following induction, a complication potentially compounded by a presenting white blood cell count of 905 × 109/L and pulmonary leukostasis. A comparison of 3-year cumulative risk of major toxic effects of treatment revealed that high-risk infants had a trend toward a lower incidence of asparaginase allergic reactions, osteonecrosis, hyperglycemia, and pancreatitis when compared with high-risk patients who were ≥1 year of age which did not reach statistical significance; in contrast, the incidence of thrombosis, hepatic toxicity, and seizures, was similar in high-risk patients regardless of age (supplemental Table 2). High-risk infants had a higher risk of fever and neutropenia (P = .003) and severe infection (P < .003) compared with their older high-risk counterparts.
To further study the contribution of clofarabine to severe infections, we compared the incidence in infants with KMT2Ar and in high-risk patients ≥1 year of age who received 1 or more clofarabine-containing Reintensification chemotherapy cycles before hematopoietic stem cell transplant in first remission (CR1). There was a significantly higher frequency of infections in infants, suggesting that age contributed to this treatment-related toxicity (mean number of episodes, 2.39 vs 1, P < .001, Poisson regression modeling).
Our data support the activity of clofarabine in reducing MRD in newly diagnosed infants with KMT2Ar ALL, with MRD-positive rates during remission induction of 66.7% before CCE and 25% after CCE consistent with prior reports (Table 1).11 Consistent with end of induction MRD responses, cumulative incidence of relapse was low at 12.5% in this small cohort of patients, however 3 of the 4 deaths due to toxicity in first remission occurred less than 1 year from diagnosis when the majority of infants relapsed (Figure 1B). Overall outcomes were not improved due to the high incidence of treatment mortality, which was primarily a result of grade 5 infections (3 of 4 treatment-related mortality events in patients with KMT2Ar, supplemental Table 1), consistent with prior reports.12 Infectious events occurred either before exposure of CCE (case 9), or during continuation between 7 and 53 weeks after both CCE courses had been completed (case 3, 8) suggesting prolonged immunosuppression. An analysis of severe infections across all patients receiving clofarabine on Total 16 suggests that in addition to the high-intensity chemotherapy exposure, age <1 year contributes to an increased risk of this complication with the caveat that the timing of clofarabine exposure differed in these 2 groups.13 The immune system in infants has been well studied; children are born with an immature innate and adaptive immune system that matures and acquires memory over time.14 The concurrent development of leukemia during the first year of life likely blunts development of the immune response as the marrow gets replaced with malignant cells, potentially resulting in an increased susceptibility to infections compared with older patients. Of the 4 grade 5 infections, 2 were the result of viral infections (respiratory syncytial virus and parainfluenza 3) for which there are limited treatment options. Viral complications also remain a significant cause of morbidity and mortality in the stem cell transplant setting, highlighting the need for antiviral therapies.15
In conclusion, although our findings do not support the use of clofarabine in the upfront setting, the reduction in MRD seen in our study and others suggests that it may be considered as a salvage option if other immunotherapeutic approaches have failed or are not feasible (eg, CD19 negative relapse), with the caveat that toxicity may ultimately preclude transplant.
Acknowledgments: The authors thank the St. Jude Clinical Trials Office.
The work was supported by National Cancer Institute grants CA021765 (all authors), CA36401 (C.-H.P.), P50-GM115279 (C.-H.P.), and the American Lebanese Syrian Associated Charities.
Contribution: S.J. and C.H.P. contributed in conceptioning and designing; D.P., C.P., T.A.G., and S.J. analyzed and interpreted the data; T.A.G. was responsible for the first draft; and all authors contributed to the writing and approval of the manuscript, and collected and assembled the data.
Conflict-of-interest disclosure: E.C.S. reports stock and other ownership interests in Cogent, Nkarta, and Medisix; consulting or advisory role with Medisix and Nkarta; patents, royalties, and other intellectual property from Juno, Bristol-Myers Squibb, Nkarta, and Medisix; and patent applications on leukemia immunophenotyping and MRD monitoring. D.C. reports stock and other ownership interests in Cogent, Nkarta, and Medisix; consulting or advisory role with Medisix and Nkarta; patents, royalties, and other intellectual property from Juno, Bristol-Myers Squibb, Nkarta, and Medisix; and patent applications on leukemia immunophenotyping and MRD monitoring. H.D.S. reports consulting or advisory role with Servier. H.I. reports research funding from Servier; consulting or advisory role with Amgen and Jazz Pharmaceuticals. T.A.G. reports stock and other ownership interests in Bristol-Myers Squibb. C.H.P. reports consulting or advisory role with Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Correspondence: Tanja A. Gruber, 1000 Welch Rd, Suite 300, Palo Alto, CA 94304; e-mail: tagruber@stanford.edu.
References
Author notes
Data are available on request from the corresponding author, Tanja A. Gruber (tagruber@stanford.edu).
The full-text version of this article contains a data supplement.