Key Points
Isolated and comutated DDX41 myeloid neoplasms have different characteristics.
DDX41-mutated AML has a relatively favorable outcome comparable to core binding factor AML.
Abstract
DDX41 mutations (germline and somatic) are associated with late onset myelodysplastic syndromes/acute myeloid leukemia (MDS/AML). Myeloid neoplasms (MN) with germline predisposition was identified as a distinct category in the 2016 WHO classification revision, including MN with germline DDX41 mutation. We retrospectively analyzed the molecular findings and clinical characteristics of thirty-three DDX41-mutated (mDDX41) patients at our institution. We identified 14 distinct pathogenic DDX41 variants in 32 patients and 8 DDX41 variants of unknown significance (VUS) in 9 patients. Five (16%) patients had a second DDX41 somatic mutation p.R525H and 13 (40%) had at least one additional oncogenic co-mutation in other genes. The median age at the time of diagnosis was 66 years, with male predominance (72%) and the majority of patients had normal cytogenetics (91%). Two-year overall survival (OS) was 86% and 6 (21%) MDS/AML patients with relatively preserved hematopoietic function were observed without further intervention. In comparison to AML patients with prognostically more favorable subtypes [t(8;21), n=27 and inv(16), n=40], mDDX41 patients in our cohort showed similarly favorable OS. Our study highlights that mDDX41-MN patients often have an indolent course and mDDX41-AML has comparable OS to favorable-risk AML.
Introduction
The DEAD-box helicase 41 (DDX41) gene, located on chromosome 5q35, is presumed to be a tumor suppressor gene, encoding a DEAD-box type RNA helicase. DDX41 is involved in pre-mRNA splicing, rRNA processing and innate immunity.1-3 Unlike other germline predisposition syndromes, which typically present at an earlier age, DDX41-associated germline cases are characterized by late-onset development of myeloid neoplasms (MNs), and may occur as sporadic germline events.1,2,4-12 DDX41 mutations account for 0.5% to 5% of adult myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) and typically present as high-risk disease, with male predominance and variable history of preceding cytopenias.1,2,13-16 Recent studies have reported that DDX41-related MN is associated with longer overall survival (OS) and response to lenalidomide.10,14,17,18 In this study, we describe the clinical and genetic features and survival outcomes of patients with mutated DDX41.
Methods
This is a single-institution study encompassing the Mayo Clinic Cancer Center sites (Rochester, Florida, and Arizona). After institutional review board approval, we retrospectively screened for mDDX41 cases from 4524 consecutive Mayo Clinic patient samples submitted for a 42-gene MN panel next-generation sequencing clinical assay in the Molecular Hematopathology Laboratory between July 2018 and December 2020. A chart review of mDDX41 cases between January 2009 and April 2021 was conducted. Of 1404 consecutive patients with the diagnosis of AML, 27 (1.9%) patients with t(8;21)(q22;q22.1); RUNX1-RUNX1T1 and 40 (2.8%) with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 were identified for survival comparison with mDDX41 AML. All statistical analyses were performed using JMP Pro 14.1.0 Software (supplemental Methods).
Results and discussion
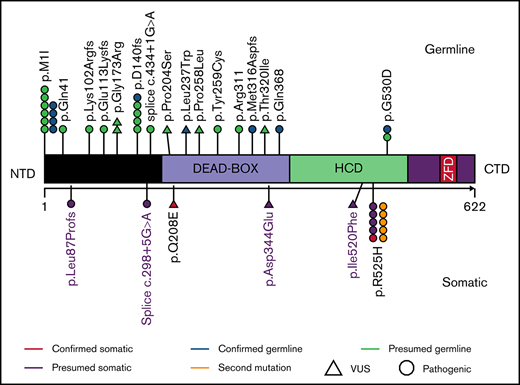
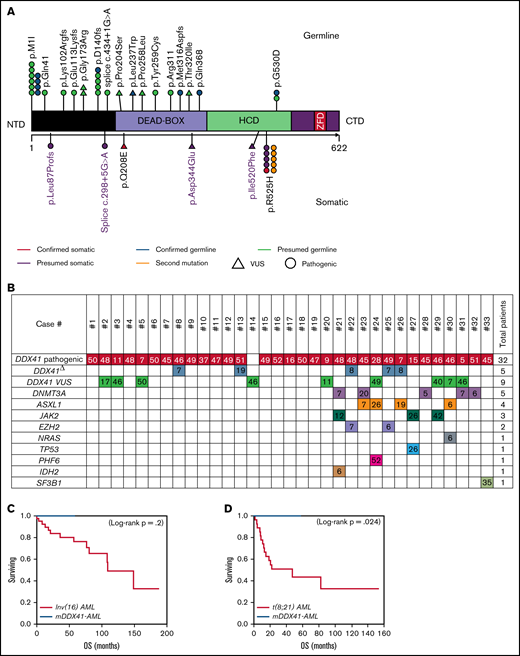
We identified 33 patients harboring DDX41 genetic alterations, of which 32 (97%) had at least 1 pathogenic DDX41 mutation and 1 (3%) had a DDX41 variant of uncertain significance (VUS) (proven to be a germline variant). Of the 32 patients harboring pathogenic mutations, 5 (16%) had a second DDX41 mutation (p.R525H), and 9 (27%) harbored a DDX41 VUS (Figure 1; supplemental Table 1). The germline origin of DDX41 variants was confirmed in 9 of 10 (90%) tested patients, among them at least 1 variant had variant allele frequency (VAF) ≥ 40%.
Characteristics of patients with DDX41 mutation. (A) Representation of DDX41 variants detected, positioned on the DDX41 protein and its functional domains. (B) Patterns of the mutations identified in the cohort of 33 patients with DDX41 mutation. The number reported in the box represents the VAF of each mutation. (C-D) Kaplan-Meier survival curves in 10 patients with mDDX41 AML compared with (C) 40 patients with inv 16 AML and (D) 27 patient with t(8;21) AML. CTD, C-terminal domain; ZFD, zinc finger domain; Δ, second mutation.
Characteristics of patients with DDX41 mutation. (A) Representation of DDX41 variants detected, positioned on the DDX41 protein and its functional domains. (B) Patterns of the mutations identified in the cohort of 33 patients with DDX41 mutation. The number reported in the box represents the VAF of each mutation. (C-D) Kaplan-Meier survival curves in 10 patients with mDDX41 AML compared with (C) 40 patients with inv 16 AML and (D) 27 patient with t(8;21) AML. CTD, C-terminal domain; ZFD, zinc finger domain; Δ, second mutation.
The median age at diagnosis was 66 years (range, 30-81 years), and 24 (72%) patients were men, consistent with late onset of mDDX41 MN and male predominance previously reported.8,14-16 ,19 All patients with AML were intermediate risk for the European LeukemiaNet (ELN), with a median marrow blast count of 29% (range, 20%-50%). The majority of MDSs were classified as excess blasts-2 (MDS-EB2; N = 13; 68%), similar to that previously reported in the literature.15,16,19 Eleven (58%) and 4 (21%) patients with MDS were classified as intermediate risk and high risk by the revised International Prognostic Scoring System (IPSS-R), respectively.
Twenty (60%) patients had an isolated DDX41 mutation, whereas 13 (40%) had at least 1 additional comutation in other genes. Isolated mDDX41 cases showed male predominance relative to their comutated counterparts (85% vs 54%; P = .05; Table 1). The median number of comutations in the 13 cases was 1 (range,1-3), and the most common comutations occurred in DNMT3A (N = 5, 38%), ASXL1 (N = 4, 30%), JAK2 (N = 3, 23%), and EZH2 (N = 2, 15%; Figure 1; supplemental Tables 1 and 2). The incidence of TP53 mutation was infrequent (3%), comparable to what was reported by Sébert et al15 (6%) but lower than that reported by Quesada et al16 (32%). Similarly, we observed a low incidence of splicing factor comutations (3%), in keeping with the report from Sébert et al.10,16 Our cohort had fewer comutations (median of 0; range, 0-3) than what reported by recent studies, and interestingly, the comutation median VAF observed here was low 7% (range, 5%-52%).15,16
Characteristics and hematologic features of patients with isolated and comutated DDX41 mutation
| Variable . | Isolated . | Comutated . | P . |
|---|---|---|---|
| No. of patients, (%) | 20 (60) | 13 (40) | |
| Age, y, median (range), at diagnosis | 65 (30-81) | 66 (50-76) | .767 |
| Sex (male), n (%) | 17 (85) | 7 (54) | .0496* |
| Hemoglobin, g/dL, median (range) | 11.2 (7.5-15.6) | 10.05 (6.6-14) | .1988 |
| Leukocytes, 109/L, median (range) | 2.15 (1-4.4) | 2.4 (1.6-8.5) | .1239 |
| Thrombocytes, 109/L, median (range) | 87 (28-241) | 94 (63-571) | .1443 |
| ANC, median (range) | 0.925 (0.16-3.73) | 1.005 (0.65-4.78) | .2058 |
| MCV median (range) | 104.3 (85.2-114.8) | 105.6 (90-115) | .7151 |
| RDW, median (range) | 14.2 (12.4-23.4) | 15.05 (12.5-21.3) | .2824 |
| BM blasts, median (range) | 13 (1-45) | 12 (0-50) | .6056 |
| BM blasts (AML only), median (range) | 34 (20-45) | 25.5 (21-50) | .91 |
| Number of comutations, median (range) | 0 | 1 (1-3) | |
| DDX41 VAF % | 48 (7-52) | 45 (5-51) | .1656 |
| DDX41 mutations > 1 | 2 (10) | 3 (23) | .306 |
| Pathogenic mutation type | |||
| Missense | 5 (26) | 3 (23) | .8354 |
| Nonsense | 1 (5) | 2 (15) | .3347 |
| Frameshift | 4 (21) | 5 (38) | .2820 |
| Splice site mutation | 0 | 2 (15) | .0774 |
| Start-loss variant | 9 (47) | 1 (7) | .0174* |
| Diagnosis | |||
| MDS | 13 (65) | 6 (46) | .2845 |
| AML | 6 (30) | 4 (30) | .96 |
| MPN | 0 | 2 (15) | |
| Carrier | 1 (5) | 0 | |
| CCUS | 0 | 1 (7) | |
| Abnormal cytogenetics | 0 | 3 (25) | .0188* |
| Family history | |||
| Solid or hematologic malignancies | 11 (61) | 12 (92) | .0501 |
| Solid tumors | 6 (33) | 10 (77) | .0166* |
| Hematologic malignancies | 8 (44) | 4 (30) | .4405 |
| Gastrointestinal malignancies | 3 (15) | 3 (23) | .6040 |
| Genitourinary malignancies | 2 (10) | 4 (30) | .1496 |
| Lung cancer | 1 (5) | 3 (23) | .1345 |
| Breast cancer | 2 (10) | 2 (15) | .6832 |
| Any personal history | |||
| Hematologic malignancies | 0 | 1 (7) | .2078 |
| Solid tumors | 1 (5) | 3 (23) | .1200 |
| DDX41 VUS | |||
| Yes | 5 (25) | 4 (30) | .7161 |
| No | 15 (75) | 9 (70) |
| Variable . | Isolated . | Comutated . | P . |
|---|---|---|---|
| No. of patients, (%) | 20 (60) | 13 (40) | |
| Age, y, median (range), at diagnosis | 65 (30-81) | 66 (50-76) | .767 |
| Sex (male), n (%) | 17 (85) | 7 (54) | .0496* |
| Hemoglobin, g/dL, median (range) | 11.2 (7.5-15.6) | 10.05 (6.6-14) | .1988 |
| Leukocytes, 109/L, median (range) | 2.15 (1-4.4) | 2.4 (1.6-8.5) | .1239 |
| Thrombocytes, 109/L, median (range) | 87 (28-241) | 94 (63-571) | .1443 |
| ANC, median (range) | 0.925 (0.16-3.73) | 1.005 (0.65-4.78) | .2058 |
| MCV median (range) | 104.3 (85.2-114.8) | 105.6 (90-115) | .7151 |
| RDW, median (range) | 14.2 (12.4-23.4) | 15.05 (12.5-21.3) | .2824 |
| BM blasts, median (range) | 13 (1-45) | 12 (0-50) | .6056 |
| BM blasts (AML only), median (range) | 34 (20-45) | 25.5 (21-50) | .91 |
| Number of comutations, median (range) | 0 | 1 (1-3) | |
| DDX41 VAF % | 48 (7-52) | 45 (5-51) | .1656 |
| DDX41 mutations > 1 | 2 (10) | 3 (23) | .306 |
| Pathogenic mutation type | |||
| Missense | 5 (26) | 3 (23) | .8354 |
| Nonsense | 1 (5) | 2 (15) | .3347 |
| Frameshift | 4 (21) | 5 (38) | .2820 |
| Splice site mutation | 0 | 2 (15) | .0774 |
| Start-loss variant | 9 (47) | 1 (7) | .0174* |
| Diagnosis | |||
| MDS | 13 (65) | 6 (46) | .2845 |
| AML | 6 (30) | 4 (30) | .96 |
| MPN | 0 | 2 (15) | |
| Carrier | 1 (5) | 0 | |
| CCUS | 0 | 1 (7) | |
| Abnormal cytogenetics | 0 | 3 (25) | .0188* |
| Family history | |||
| Solid or hematologic malignancies | 11 (61) | 12 (92) | .0501 |
| Solid tumors | 6 (33) | 10 (77) | .0166* |
| Hematologic malignancies | 8 (44) | 4 (30) | .4405 |
| Gastrointestinal malignancies | 3 (15) | 3 (23) | .6040 |
| Genitourinary malignancies | 2 (10) | 4 (30) | .1496 |
| Lung cancer | 1 (5) | 3 (23) | .1345 |
| Breast cancer | 2 (10) | 2 (15) | .6832 |
| Any personal history | |||
| Hematologic malignancies | 0 | 1 (7) | .2078 |
| Solid tumors | 1 (5) | 3 (23) | .1200 |
| DDX41 VUS | |||
| Yes | 5 (25) | 4 (30) | .7161 |
| No | 15 (75) | 9 (70) |
ANC, absolute neutrophil count; BM, bone marrow; CCUS, clonal cytopenia of undetermined significance; MCV, mean corpuscular volume; MPN, myeloproliferative neoplasms; RDW, red cell distribution width.
Statistically significant.
The most common pathogenic mutation type was the initiation codon substitution (start-loss) variant p.M1I (N = 10, 31%; Table 1; supplemental Table 1), previously described as the second most common germline DDX41 variant in Whites and the most common in Swedish population.8,14,15 Among isolated cases, 47% had p.M1I, whereas only 8% of comutated cases harbored p.M1I (P = .02). Twenty-one (65.6%) DDX41 mutations clustered in the N-terminal domain (NTD), 4 (12.5%) in the DEAD-box domain, and 7 (22%) in the helicase-C domain (HCD). Mutations located in the HCD were more likely to have a concomitant DDX41 VUS compared with NTD mutations (N = 6, 86% vs N = 2, 10%; P = .0001).
Sixteen patients (52%) had a family history (FH) of solid tumors and 12 (39%) had a FH of hematologic malignancies. Comutated cases were more likely to have FH of solid tumors (77% vs 33%; P = .02; Table 1).20 However, this difference was not significant for hematologic or subgroups of solid malignancies. None of the HCD-mutated cases had FH of solid tumors, in comparison with 70% seen in NTD-mutated cases (P = .001), supporting the reported prevalence of germline mutations in the NTD.8
Cytogenetic results were available in 32 cases, and 29 (91%) showed a normal karyotype. Karyotypic abnormalities were thus infrequent (N = 3, 9%), consistent with previous reports.15,16,19 Interestingly, all 3 cases with karyotypic abnormalities were comutated cases (P = .02).
Overall, 2 (7%) patients died after a median follow-up of 20 months. Six (20%) patients (5 MDS and 1 AML) were observed because of stable blood indices with a median follow-up of 6.6 months (range, 1.5-32.6 months). Twenty-three (80%) patients received treatment with median time from diagnosis to treatment initiation of 0.7 months (range, 0-92 months). Overall response rate in patients with MDS/AML was 77%, and median time to response was 3.2 months (range, 0.9-20.5 months; supplemental Table 3). Fifteen (68%) patients achieved complete remission (CR), 2 (9%) patients had hematologic improvement, and 3 (23%) patients did not respond. Patients with AML had 100% CR when treated with induction chemotherapy or hypomethylating agents (HMA) plus venetoclax regimen, and median time to CR was 1 month (range, 0.86-4.1 months). Of the 9 (39%) patients who received second-line therapy, 6 (75%) achieved CR. Four (21%) of 19 patients with MDS progressed into AML with a median time to progression of 16 months (range, 1.3-27.6 months), and the 2-year progression-free survival rate for patients with MDS was 62%.
In mDDX41 MDS/AML, the median OS was not reached, and the 2-year OS was 86% (95% confidence interval: 57%-97%). There was no statistically significant difference in OS between 2-year OS for isolated and comutated (P = .99) responders and nonresponders (90% vs 50%; P = .38) and treatment compared with the 2-year OS for the observation group (83% vs 100%; P = .52; supplemental Figure 1). Twelve (41%) patients with MDS/AML received hematopoietic stem cell transplantation (HSCT), and there was no difference in 2-year OS between patients who received HSCT vs no HSCT (87% vs 86%; P = 1.0; supplemental Figure 1).
All patients with mDDX41 AML were alive at the end of follow-up without reaching median OS. Comparing the OS of mDDX41 AML to the prognostically favorable group of core-binding factor AML, a statistically significant superior outcome was observed in mDDX41-AML compared with AML with t(8;21) (2-year OS, 100% vs 51%; P = .024; Figure 1D). In comparison with AML with inv(16), mDDX41 AML showed a trend to better OS; however, statistical significance was not achieved (2-year OS, 100% vs 84%; P = .2), supporting at least noninferior clinical outcome (Figure 1C).
Our study reaffirms some previous observations and demonstrates several novel findings in patients with MN and mDDX41. Isolated DDX41 mutations were associated with male predominance (85%), the start-loss p.M1I mutation (47%), normal cytogenetics (100%), and less frequent association with a FH of solid tumors (33%) compared with their comutated counterpart. Patients with mDDX41 MN have a low incidence of TP53 and splicing factor gene comutations. Despite the categorization of all patients with mDDX41 AML intermediate risk for ELN, we found they fit favorable-risk AML in our study cohort.21-23 Finally, some mDDX41 MN cases can be observed for a long time if they have preserved hematopoiesis, and therapeutic intervention could be delayed. We acknowledge that our study is limited by the retrospective nature and small sample size.
Authorship
Contribution: A.N., A.A.-K., H.B.A., and D.V. designed the study, interpreted the data, and wrote the manuscript; A.N. collected the data and conducted the statistical analysis; M.V.S., J.M.F., T.B., A.T., M.R.L., M.M.P., N.G, A.A.M., L.S., H.B.A., and A.A.-K., cared for the patients and provided patient information; R.H., P.N., D.J., and D.V. performed the next-generation sequencing; P.G. performed cytogenetic analysis; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hassan Alkhateeb, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester MN 55905; e-mail: alkhateeb.hassan@mayo.edu.
References
Author notes
H.B.A. and A.N. contributed equally to this work.
Gene panel sequencing data are available by request to the corresponding author at alkhateeb.hassan@mayo.edu.
The full-text version of this article contains a data supplement.