Key Points
Given high relapse and CNS involvement of BPDCN, combination chemotherapy and prophylactic CNS-directed therapy are urgently needed.
HCVAD chemotherapy plays an important role in the treatment of BPDCN, even in the modern era of CD123-targeted therapy.
Abstract
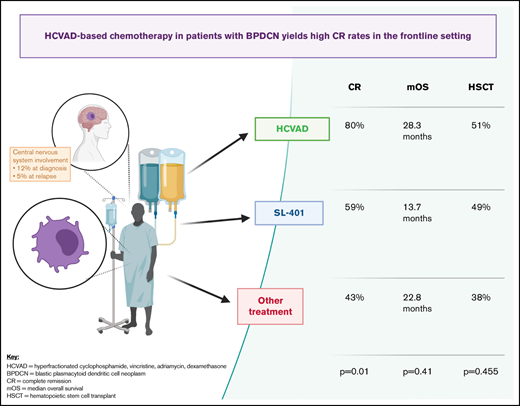
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a clinically aggressive blood cancer, often involving the skin, bone marrow, lymph nodes, and central nervous system (CNS) in 20% to 30% of patients. Despite significant progress in CD123- and BCL-2–targeted therapy, most patients are not cured without hematopoietic stem cell transplant (HSCT), and CNS relapses occur quite frequently. Combination approaches with targeted and chemotherapy agents plus incorporation of prophylactic CNS-directed therapy are urgently needed. In this setting, we sought to analyze outcomes using the cytotoxic chemotherapy backbone regimen hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone (HCVAD). We conducted a retrospective analysis of patients with BPDCN (n = 100), evaluating complete remission (CR) and median overall survival (OS) among 3 groups: those who received frontline HCVAD-based therapy (n = 35), SL-401 (n = 37), or other regimens (n = 28). HCVAD-based regimens yielded higher CR (80% vs 59% vs 43%; P = .01). There was no significant difference in OS (28.3 vs 13.7 vs 22.8 months; P = .41) or remission duration probability among treatment groups (38.6 vs not reached vs 10.2 months; P = .24). HSCT was performed in 51% vs 49% vs 38%, respectively (P = .455). These results suggest a continued important role for HCVAD-based chemotherapy in BPDCN, even in the modern targeted-therapy era, with high CR rates in the frontline setting. Further studies must establish the clinical activity, feasibility, and safety of doublet/triplet combinations of targeted therapies plus cytotoxic agents and the addition of CNS prophylaxis, with the ultimate goal of durable long-term remission for patients with BPDCN.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a clinically aggressive blood cancer with a unique clinical presentation often involving the skin, bone marrow, lymph nodes, and the central nervous system (CNS) in 20% to 30% of patients.1 This malignancy has a 3:1 male predominance, is more common in older patients, and may occur in isolation or in conjunction with other hematologic conditions, such as chronic myelomonocytic leukemia or myelodysplastic syndrome.2-4 BPDCN is generally characterized by CD123+, CD4+, CD56+ expression, as well as CD303,5 TCL-1, and TCF4,6 which has also led to treatment implications with the identification of novel therapeutic targets.7,8 Prior to the development of targeted agents against CD123, BPDCN was treated with local surgical or radiation therapy for cutaneous-limited disease9,10 or with multiagent cytotoxic chemotherapy regimens adopted from use in other hematologic malignancies, such as acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and lymphoma.11-13 Without subsequent hematopoietic stem cell transplant (HSCT), development of relapsed and refractory disease that is resistant to chemotherapy is high, with poor prognosis and a median survival <2 years.2
In recent years, the development of novel agents targeted against CD123 has changed the treatment landscape of BPDCN, yet relapses (systemic and CNS) still occur frequently in the setting of CD123-directed monotherapy.14 Targeted therapy with the BCL-2 inhibitor venetoclax, alone or in combination with chemotherapy, also demonstrated activity against BPDCN.15-17 However, given the lack of blood-brain barrier penetration of most available targeted agents, multiagent regimens with prophylactic CNS therapy administered with serial lumbar punctures with intrathecal chemotherapy, as used in the treatment of ALL, have also been used for BPDCN. We sought to analyze the outcomes associated with our most commonly administered cytotoxic chemotherapy backbone regimen for patients with BPDCN, hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone (HCVAD), plus alternating intrathecal methotrexate and cytarabine (ARA-C).18
Methods
We conducted a single-institution retrospective analysis of patients with BPDCN (n = 100) treated at The University of Texas MD Anderson Cancer Center from 1999 to 2020 by evaluating complete remission (CR) and overall survival (OS) of those who received frontline HCVAD-based vs non-HCVAD–based regimens. This retrospective analysis was approved by our local Institutional Review Board. Patients were included in this study if they had a confirmed case of BPDCN by expert pathology review at The University of Texas MD Anderson Cancer Center and were treated with systemic therapy. HCVAD-based regimens were administered to 35 patients and included HCVAD alone (n = 23), HCVAD + venetoclax (n = 4); mini-HCVAD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, and cytarabine at 0.5 g/m2 × 4 doses) + venetoclax, with or without enasidenib (n = 2); HCVAD + bortezomib (n = 2); modified HCVAD (n = 1), and HCVAD + SL-401 (tagraxofusp-erzs), with or without venetoclax (n = 3). Recorded data included patient demographics, cancer history, bone marrow involvement, serologic laboratory results, and specific disease mutations. Baseline patient demographic characteristics were summarized using mean (standard deviation) and median (minimum-maximum) for continuous variables and counts (percentage) for categorical variables. Time to complete response with first-line treatment (CR1) and OS (time to death or last follow-up) were calculated. Kaplan-Meier survival plots were generated, and a log-rank test (Mantel-Cox) pairwise analysis was used to compare OS and remission duration probability among group 1, group 2, and group 3. A P value ≤ .05 indicated statistical significance.
Results
Patients are divided into 3 groups for comparison. Patients in group 1 received frontline HCVAD-based regimens (n = 35), patients in group 2 received frontline SL-401 (n = 37), and patients in group 3 received other treatment regimens (n = 28), as listed in detail in Table 1. The greatest percentage of male patients (94% vs 73% vs 75%; P = .045), youngest median age (61 vs 68 vs 65 years; P = .035), and greatest degree of lymph node involvement (40% vs 16% vs 14%; P = .022) were found in group 1 vs group 2 vs group 3, respectively. Patients in group 2 had the highest degree of skin involvement (92% in group 2 vs 77% in group 1 vs 64% in group 3; P = .024), whereas patients in group 3 had the highest percentage of RAS mutations (18% in group 3 vs 14% in group 1 vs 11% in group 2; P = .004). The overall rate of CNS involvement of BPDCN at diagnosis was 12% and was not different among treatment groups (20% in group 1 vs 3% in group 2 vs 14% in group 3; P = .07). Median follow-up during the study was 21.7 months (range, 1.3-71.2) among all patients. Within each group, the median follow-up was 16.2 months (range, 1.3-71.2) in group 1 vs 21.7 months (range, 4.3-69.0) in group 2 vs 25.7 months (range, 11.8-51.4) in group 3.
Baseline patient characteristics
| Variable . | Frontline HCVAD based, n = 35 . | Frontline SL-401, n = 37 . | Frontline other treatment, n = 28 . | P . |
|---|---|---|---|---|
| Sex | ||||
| Male | 33 (94) | 27 (73) | 21 (75) | .045 |
| Female | 2 (6) | 10 (27) | 7 (25) | |
| Age at Dx, median (range), y | 61 (20-86) | 68 (21-84) | 65 (14-86) | .035 |
| WBCs at Dx, median, (range), ×109/L | 6.4 (1.7-76.5) | 5.8 (1.9-56.8) | 6.0 (1.5-179) | .85 |
| Hgb at Dx, median (range), g/dL | 13.3 (6.8-17.0) | 13.1 (8.3-17) | 13.0 (7.7-17.1) | .088 |
| Plt at Dx, median (range), ×109/L | 141 (11-365) | 146 (39-407) | 136 (22-260) | .779 |
| LDH, U/L at Dx, median (range), (n = 30) | 508 (121-4108) | 505 (164-1800) | 523 (266-505) | .841 |
| BM Bl at Dx, median (range), % | 5 (0-95) | 14 (0-94 | 11 (0-86) | .788 |
| Involvement of BPDCN disease | ||||
| Bone marrow | 25 (71) | 26 (70) | 21 (75) | .911 |
| Skin | 27 (77) | 34 (92) | 18 (64) | .024 |
| Lymph node | 14 (40) | 6 (16) | 4 (14) | .022 |
| CNS | 7 (20) | 1 (3) | 4 (14) | .07 |
| Cytogenetics | ||||
| Complex cytogenetics | 10 (29) | 6 (16) | 4 (14) | .206 |
| Diploid cytogenetics | 17 (48) | 29 (78) | 17 (61) | |
| Mutations | ||||
| TET2 | 7/13 (54) | 28/35 (80) | 12/13 (92) | .054 |
| ASXL1 | 4/13 (31) | 12/35 (34) | 6/13 (46) | .677 |
| RAS | 2/14 (14) | 4/35 (11) | 9/50 (18) | .004 |
| Frontline therapy | ||||
| HCVAD | 32 (91) | |||
| HCVAD | 23 (66) | |||
| HCVAD + Ven | 4 (11) | |||
| Mini-HCVAD + Ven, with or without enasidenib | 2 (6) | |||
| HCVAD + bortezomib | 2 (6) | |||
| Modified HCVAD | 1 (3) | |||
| HCVAD + SL401, with or without Ven | 3 (9) | |||
| CHOP | 8 (29) | |||
| AML based | 6 (21) | |||
| Bortezomib based | 2 (7) | |||
| Hypomethylator based | 4 (14) | |||
| Other treatment regimens | 8 (29) | |||
| CR | 28 (80) | 22 (59) | 12 (43) | .01 |
| HSCT | 18 (51) | 17 (49) | 10 (36) | .455 |
| Hematopoietic stem cell transplant in CR1 | 15/28 (54) | 13/22 (59) | 4/12 (33) | .297 |
| OS, median, mo | 28.3 | 13.7 | 22.8 | .566 |
| CR1 duration, median, mo | 38.6 | 10.7 | 21.4 | .444 |
| Days to Rx1, median (range) | 27 (0-108) | 32 (0-112) | 28 (0-421) | .187 |
| Males | 27 (0-108) | 32 (11-84) | 39 (0-421) | .268 |
| Females | 46 (28-64) | 36 (0-112) | 30 (5-83) | .195 |
| Dx/Rx1 with skin only | 9 (26) | 15 (41) | 9 (32) | .406 |
| Days to Rx1, median (range) | 40 (8-95) | 36 (7-101) | 28 (0- 421) | .274 |
| Variable . | Frontline HCVAD based, n = 35 . | Frontline SL-401, n = 37 . | Frontline other treatment, n = 28 . | P . |
|---|---|---|---|---|
| Sex | ||||
| Male | 33 (94) | 27 (73) | 21 (75) | .045 |
| Female | 2 (6) | 10 (27) | 7 (25) | |
| Age at Dx, median (range), y | 61 (20-86) | 68 (21-84) | 65 (14-86) | .035 |
| WBCs at Dx, median, (range), ×109/L | 6.4 (1.7-76.5) | 5.8 (1.9-56.8) | 6.0 (1.5-179) | .85 |
| Hgb at Dx, median (range), g/dL | 13.3 (6.8-17.0) | 13.1 (8.3-17) | 13.0 (7.7-17.1) | .088 |
| Plt at Dx, median (range), ×109/L | 141 (11-365) | 146 (39-407) | 136 (22-260) | .779 |
| LDH, U/L at Dx, median (range), (n = 30) | 508 (121-4108) | 505 (164-1800) | 523 (266-505) | .841 |
| BM Bl at Dx, median (range), % | 5 (0-95) | 14 (0-94 | 11 (0-86) | .788 |
| Involvement of BPDCN disease | ||||
| Bone marrow | 25 (71) | 26 (70) | 21 (75) | .911 |
| Skin | 27 (77) | 34 (92) | 18 (64) | .024 |
| Lymph node | 14 (40) | 6 (16) | 4 (14) | .022 |
| CNS | 7 (20) | 1 (3) | 4 (14) | .07 |
| Cytogenetics | ||||
| Complex cytogenetics | 10 (29) | 6 (16) | 4 (14) | .206 |
| Diploid cytogenetics | 17 (48) | 29 (78) | 17 (61) | |
| Mutations | ||||
| TET2 | 7/13 (54) | 28/35 (80) | 12/13 (92) | .054 |
| ASXL1 | 4/13 (31) | 12/35 (34) | 6/13 (46) | .677 |
| RAS | 2/14 (14) | 4/35 (11) | 9/50 (18) | .004 |
| Frontline therapy | ||||
| HCVAD | 32 (91) | |||
| HCVAD | 23 (66) | |||
| HCVAD + Ven | 4 (11) | |||
| Mini-HCVAD + Ven, with or without enasidenib | 2 (6) | |||
| HCVAD + bortezomib | 2 (6) | |||
| Modified HCVAD | 1 (3) | |||
| HCVAD + SL401, with or without Ven | 3 (9) | |||
| CHOP | 8 (29) | |||
| AML based | 6 (21) | |||
| Bortezomib based | 2 (7) | |||
| Hypomethylator based | 4 (14) | |||
| Other treatment regimens | 8 (29) | |||
| CR | 28 (80) | 22 (59) | 12 (43) | .01 |
| HSCT | 18 (51) | 17 (49) | 10 (36) | .455 |
| Hematopoietic stem cell transplant in CR1 | 15/28 (54) | 13/22 (59) | 4/12 (33) | .297 |
| OS, median, mo | 28.3 | 13.7 | 22.8 | .566 |
| CR1 duration, median, mo | 38.6 | 10.7 | 21.4 | .444 |
| Days to Rx1, median (range) | 27 (0-108) | 32 (0-112) | 28 (0-421) | .187 |
| Males | 27 (0-108) | 32 (11-84) | 39 (0-421) | .268 |
| Females | 46 (28-64) | 36 (0-112) | 30 (5-83) | .195 |
| Dx/Rx1 with skin only | 9 (26) | 15 (41) | 9 (32) | .406 |
| Days to Rx1, median (range) | 40 (8-95) | 36 (7-101) | 28 (0- 421) | .274 |
Unless otherwise noted, data are n (%).
BM Bl, bone marrow blast; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; Dx, diagnosis; Hgb, hemoglobin; Plt, platelet; LDH, lactate dehydrogenase; Rx1, first treatment; Ven, venetoclax; WBC, white blood cell.
P values in bold denote statistical significance.
Twenty-three patients received frontline HCVAD alone. The median number of treatment cycles to response was 1 (range, 1-3), and the median number of total treatment cycles was 4 (range, 1-8). Reasons for treatment discontinuation were myelosuppression (n = 3), HSCT (n = 3), relapse (n = 2), no response (n = 2), radiation therapy (n = 1), and completion of 8 full cycles of therapy (n = 1). Dose adjustments for age or performance status for HCVAD are included in supplemental Table 1. Overall, treatment was well tolerated among all groups. One early death was reported in a patient treated with HCVAD who developed renal failure, and 1 early death was reported in a patient treated with SL-401 who developed capillary leak syndrome, tumor lysis syndrome, and renal failure.
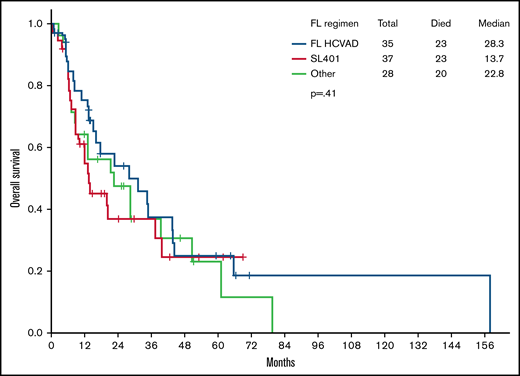
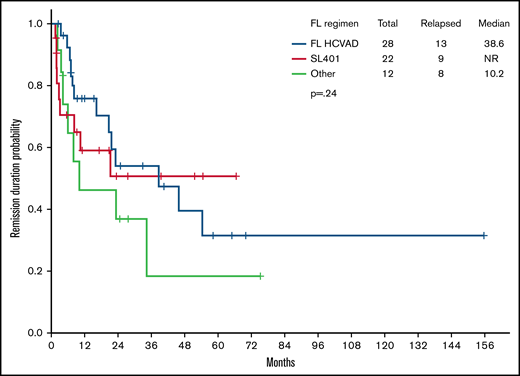
Importantly, frontline HCVAD-based regimens yielded higher CR rates (80% in HCVAD-based vs 59% in SL-401 vs 43% in others; P = .01). There was no statistically significant difference in OS (median OS, 28.3 vs 13.7 vs 22.8 months; P = .41) (Figure 1) or remission duration probability (38.6 vs NR vs 10.2 months; P = .24) (Figure 2) among treatment groups. Pairwise comparisons between cohorts did not reveal any significant differences in median OS: HCVAD vs other treatment (P = .434), HCVAD vs SL-401 (P = .329), and SL-401 vs other treatment (P = .797). HSCT was performed in 51% of those receiving frontline HCVAD-based treatment vs 49% of those receiving frontline SL-401 vs 38% of those receiving other treatment regimens (P = .455).
OS. There was no difference in median OS between those who received frontline (FL) HCVAD and those who did not (28.3 months with FL HCVAD vs 13.7 months with SL-401 vs 22.8 months with other treatment regimens; P = .41).
OS. There was no difference in median OS between those who received frontline (FL) HCVAD and those who did not (28.3 months with FL HCVAD vs 13.7 months with SL-401 vs 22.8 months with other treatment regimens; P = .41).
Remission duration probability. There was no difference in median remission duration probability between those who received frontline (FL) HCVAD and those who did not (38.6 months with FL HCVAD vs NR with SL-401 vs 10.2 months with other treatment regimens; P = .24).
Remission duration probability. There was no difference in median remission duration probability between those who received frontline (FL) HCVAD and those who did not (38.6 months with FL HCVAD vs NR with SL-401 vs 10.2 months with other treatment regimens; P = .24).
Further analysis of patients who received HCVAD alone (n = 23) vs those who received SL-401 (n = 37) yielded a median OS of 31.4 vs 13.7 months (P = .258) and a median duration of CR1 of 23.2 months vs not reached (NR) (P = .888). Subset survival analysis comparing only patients aged 60 to 75 years in each group yielded a median OS of 22.9 months in HCVAD-based regimens vs 20.2 months in SL-401 vs 8.6 months in other regimens, respectively (P = .630) (supplemental Figure 1) and a median duration of CR1 of 21.6 months vs NR vs NR (P = .731) (supplemental Figure 2). Directly comparing these age-specific cohort outcomes in patients who received HCVAD vs SL-401 did not reveal any difference in median OS (22.9 vs 20.2 months; P = .828) or median duration of CR1 (21.6 months vs NR; P = .805).
Characteristics of responders and nonresponders in each treatment group are shown in Table 2. Among patients treated with frontline HCVAD, compared with nonresponders (n = 7), responders (n = 28) were more frequently male (100% vs 71%; P = .04), had a lower white blood cell count at diagnosis (6.0 × 109/L vs 26.2 × 109/L; P = .03), and were more frequently bridged to HSCT (61% vs 14%, P = .03), with HSCT performed in CR1 (54% vs 0%). When analyzing patients treated with frontline SL-401, compared with nonresponders (n = 15), responders (n = 22) had a higher platelet count at diagnosis (177 × 109/L vs 117 × 109/L; P = .02) and were more frequently bridged to HSCT (64% vs 20%; P = .01), with HSCT performed in CR1 (59% vs 0%). Finally, for patients treated with other frontline regimens, compared with nonresponders (n = 16), responders (n = 12) had similar baseline characteristics but were more frequently bridged to HSCT (58% vs 19%; P = .001), with HSCT performed in CR1 (33% vs 0%). In patients who responded to treatment, the incidence of CNS relapse in the entire cohort (n = 62) was 5%; it was 4% in those who received frontline HCVAD vs 0% in those who received frontline SL-401 vs 17% in those who received other frontline treatment (P = .09).
Characteristics of responders vs nonresponders by treatment group
| Variable . | HCVAD, N = 35 . | SL401, N = 37 . | Other, N = 28 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Responders, n = 28 . | Nonresponders, n = 7 . | P . | Responders, n = 22 . | Nonresponders, n = 15 . | P . | Responders, n = 12 . | Nonresponders n=16 . | P . | |
| Sex | |||||||||
| Male | 28 (100) | 5 (71) | .004 | 15 (68) | 12 (80) | .43 | 9 (75) | 12 (75) | 1 |
| Female | 0 | 2 (29) | 7 (32) | 3 (20) | 3 (25) | 4 (25) | |||
| Age at Dx, median (range), y | 56 (20-77) | 67 (56-86) | .07 | 68 (22-84) | 74 (21-82) | .57 | 58 (14-77) | 70 (32-86) | .06 |
| WBC at Dx, median (range), ×109/L | 6 (1.7-24.3) | 26.2 (1.9-76.5) | .03 | 5.9 (1.9-10.2) | 4.9 (2-56.8) | .57 | 6.9 (1.5 −179) | 5.4 (1.7 −14.2) | .52 |
| Hgb at Dx, median (range), g/dL | 13.3 (6.8-17) | 12 (7.2-15.9) | .39 | 13.7 (8.3-17) | 12.6 (9.4-15.5) | .51 | 11.6 (8-15.7) | 11.6 (7.7 −17.1) | .88 |
| Plt at Dx, median (range), ×109/L | 140 (18-325) | 142 (11-365) | .45 | 177 (61-396) | 117 (39-407) | .02 | 111 (22-260) | 157 (53-273) | .26 |
| LDH, U/L at Dx, median (range), n = 30 | 508 (121-799) | 712 (191- 4108) | .57 | 533 (164-811) | 516 (164-1800) | .72 | NA | 386 (266-505) | |
| BM Bl at Dx, median (range), % | 4 (0-95) | 18 (1.2-54) | .65 | 3 (0-94) | 26 (1-92) | .15 | 23 (0-86) | 13.5 (0-77) | .91 |
| Involvement of BPDCN disease | |||||||||
| Bone marrow | 21 (75) | 4 (57) | .35 | 15 (68) | 11 (73) | .74 | 11 (92) | 10 (63) | .08 |
| Skin | 20 (71) | 7 (100) | .11 | 20 (91) | 14 (93) | .79 | 6 (50) | 12 (75) | .17 |
| Lymph node | 12 (43) | 2 (29) | .49 | 5 (23) | 1 (7) | .19 | 1 (8) | 3 (19) | .44 |
| Cytogenetics | |||||||||
| Complex cytogenetics | 7 (25) | 3 (43) | .71 | 4 (18) | 2 (13) | .61 | 2 (17) | 2 (13) | .92 |
| Diploid cytogenetics | 13 (46) | 4 (57) | 16 (27) | 13 (87) | 8 (67) | 9 (56) | |||
| Mutations | |||||||||
| TET2 | 5/10 (50) | 2/3 (67) | .61 | 17/21 (81) | 11/14 (79) | .86 | 6/7 (86) | 6/6 (100) | .34 |
| ASXL1 | 4/10 (40) | 0/3 (0) | .19 | 7/21 (33) | 5/14 (36) | .88 | 2/7 (29) | 4/6 (67) | .17 |
| RAS | 1/11 (9) | 1/3 (33) | .23 | 1/21 (5) | 3/14 (21) | .13 | 2/7 (29) | 3/8 (38) | .71 |
| CR, n | 28 | 0 | 22 | 0 | 12 | 0 | |||
| HSCT | 17 (61) | 1 (14) | .03 | 14 (64) | 3 (20) | .01 | 7 (58) | 3 (19) | .001 |
| HSCT in CR1 | 15 (54) | 0 | .01 | 13 (59) | 0 | 0 | 4 (33) | 0 | .01 |
| Days to Rx1, median (range) | 27 (0-108) | 28 (15-95) | 44 (0-84) | 56 (10-112) | 7 (0-421) | 50 (3-337) | |||
| Male | 27 (0-108) | 27 (15-95) | 40 (0-57) | 59 (12-112) | 7 (0-421) | 56 (3-337) | |||
| Female | NA | 46 (28-64) | 49 (11-84) | 22 (10-66) | 6 (5-83) | 22 (19-26) | |||
| Dx/Rx1 with skin only | 6 (21) | 3 (43) | 9 (41) | 6 (40) | 2 (17) | 7 (44) | |||
| Days to Rx1, median (range) | 36 (8-83) | 53 (26-95) | 48 (7-84) | 68 (18-101) | 50 (5-94) | 63 (20-337) | |||
| Variable . | HCVAD, N = 35 . | SL401, N = 37 . | Other, N = 28 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Responders, n = 28 . | Nonresponders, n = 7 . | P . | Responders, n = 22 . | Nonresponders, n = 15 . | P . | Responders, n = 12 . | Nonresponders n=16 . | P . | |
| Sex | |||||||||
| Male | 28 (100) | 5 (71) | .004 | 15 (68) | 12 (80) | .43 | 9 (75) | 12 (75) | 1 |
| Female | 0 | 2 (29) | 7 (32) | 3 (20) | 3 (25) | 4 (25) | |||
| Age at Dx, median (range), y | 56 (20-77) | 67 (56-86) | .07 | 68 (22-84) | 74 (21-82) | .57 | 58 (14-77) | 70 (32-86) | .06 |
| WBC at Dx, median (range), ×109/L | 6 (1.7-24.3) | 26.2 (1.9-76.5) | .03 | 5.9 (1.9-10.2) | 4.9 (2-56.8) | .57 | 6.9 (1.5 −179) | 5.4 (1.7 −14.2) | .52 |
| Hgb at Dx, median (range), g/dL | 13.3 (6.8-17) | 12 (7.2-15.9) | .39 | 13.7 (8.3-17) | 12.6 (9.4-15.5) | .51 | 11.6 (8-15.7) | 11.6 (7.7 −17.1) | .88 |
| Plt at Dx, median (range), ×109/L | 140 (18-325) | 142 (11-365) | .45 | 177 (61-396) | 117 (39-407) | .02 | 111 (22-260) | 157 (53-273) | .26 |
| LDH, U/L at Dx, median (range), n = 30 | 508 (121-799) | 712 (191- 4108) | .57 | 533 (164-811) | 516 (164-1800) | .72 | NA | 386 (266-505) | |
| BM Bl at Dx, median (range), % | 4 (0-95) | 18 (1.2-54) | .65 | 3 (0-94) | 26 (1-92) | .15 | 23 (0-86) | 13.5 (0-77) | .91 |
| Involvement of BPDCN disease | |||||||||
| Bone marrow | 21 (75) | 4 (57) | .35 | 15 (68) | 11 (73) | .74 | 11 (92) | 10 (63) | .08 |
| Skin | 20 (71) | 7 (100) | .11 | 20 (91) | 14 (93) | .79 | 6 (50) | 12 (75) | .17 |
| Lymph node | 12 (43) | 2 (29) | .49 | 5 (23) | 1 (7) | .19 | 1 (8) | 3 (19) | .44 |
| Cytogenetics | |||||||||
| Complex cytogenetics | 7 (25) | 3 (43) | .71 | 4 (18) | 2 (13) | .61 | 2 (17) | 2 (13) | .92 |
| Diploid cytogenetics | 13 (46) | 4 (57) | 16 (27) | 13 (87) | 8 (67) | 9 (56) | |||
| Mutations | |||||||||
| TET2 | 5/10 (50) | 2/3 (67) | .61 | 17/21 (81) | 11/14 (79) | .86 | 6/7 (86) | 6/6 (100) | .34 |
| ASXL1 | 4/10 (40) | 0/3 (0) | .19 | 7/21 (33) | 5/14 (36) | .88 | 2/7 (29) | 4/6 (67) | .17 |
| RAS | 1/11 (9) | 1/3 (33) | .23 | 1/21 (5) | 3/14 (21) | .13 | 2/7 (29) | 3/8 (38) | .71 |
| CR, n | 28 | 0 | 22 | 0 | 12 | 0 | |||
| HSCT | 17 (61) | 1 (14) | .03 | 14 (64) | 3 (20) | .01 | 7 (58) | 3 (19) | .001 |
| HSCT in CR1 | 15 (54) | 0 | .01 | 13 (59) | 0 | 0 | 4 (33) | 0 | .01 |
| Days to Rx1, median (range) | 27 (0-108) | 28 (15-95) | 44 (0-84) | 56 (10-112) | 7 (0-421) | 50 (3-337) | |||
| Male | 27 (0-108) | 27 (15-95) | 40 (0-57) | 59 (12-112) | 7 (0-421) | 56 (3-337) | |||
| Female | NA | 46 (28-64) | 49 (11-84) | 22 (10-66) | 6 (5-83) | 22 (19-26) | |||
| Dx/Rx1 with skin only | 6 (21) | 3 (43) | 9 (41) | 6 (40) | 2 (17) | 7 (44) | |||
| Days to Rx1, median (range) | 36 (8-83) | 53 (26-95) | 48 (7-84) | 68 (18-101) | 50 (5-94) | 63 (20-337) | |||
Unless otherwise noted, data are n (%).
BM Bl, bone marrow blast; Dx, diagnosis; Hgb, hemoglobin; LDH, lactate dehydrogenase; NA, not applicable; Plt, platelet; Rx1, first treatment; WBC, white blood cell.
P values in bold denote statistical significance.
Discussion
Although classified as a myeloid malignancy, BPDCN has long demonstrated features overlapping with ALL at the clinical and translational levels. Immunohistochemical analysis has identified various molecular mutations with therapeutic implications in BPDCN. Deletions in tumor suppressor genes (CDKN1B, ETV6, HNRNPK, RB1, SFRP4), mutated myeloid genes (SXL1, ZRSR2, TET2), dysregulated transcription factor SOX4, and overexpression of BCL-2 and CCND1 have pathogenic implications in BPDCN.19,20 Recurrent abnormalities in transcription factor regulator IKZF1, leading to aberrancy in cell-to-cell adhesion, is directly implicated in the pathogenesis of BCR-ABL1+ pre-B-cell ALL and BPDCN.19 Further, roughly 1 in 10 patients with BPDCN can exhibit the 8q24/MYC gene arrangement, which may play a role in pathogenesis and frequently responds favorably to ALL-type chemotherapy regimens, such as HCVAD.21
Cytotoxic chemotherapy, traditionally used in BPDCN prior to the advent of novel targeted agents, has been able to successfully induce CR, generally followed by allogeneic or autologous HSCT in appropriate patients.12,22-26 Multiagent chemotherapy, with the addition of high-dose corticosteroids, has continued to demonstrate benefit in the treatment of patients with BPDCN over time.11,12,27 Historical cytotoxic chemotherapy regimens adopted from AML, ALL, and lymphoma for use in BPDCN include combinations of anthracyclines, alkylating agents, vinca alkaloids, antimetabolites, platinum-based agents, topoisomerase inhibitors, and high-dose corticosteroids.28-30
Although singlet therapy successfully induces remission in a number of patients, prognosis and OS with monotherapy approaches generally remain poor, and relapse is high, often within skin, CNS, or bone marrow.2,29,31-33 Once relapsed, BPDCN usually becomes highly resistant to chemotherapy regimens34 and therefore becomes unable to proceed with attempt at cure with chemotherapy reinduction and transition to secondary HSCT.35 The ubiquitous overexpression of CD123 on BPDCN cells led to the concept that targeting this surface receptor would improve outcomes for patients with BPDCN.36-39 The treatment landscape for BPDCN changed after the development of SL-401 for targeting CD123. Since its approval by the US Food and Drug Administration in December of 2018, SL-401 has been used for frontline therapy, alone or in combination with other chemotherapeutic or targeted agents, in clinical trials.40 Other successful CD123-targeted agents include the antibody-drug conjugate IMGN 632 (US Food and Drug Administration Breakthrough Designation for relapsed/refractory BPDCN; October, 2020),41 CD123 chimeric antigen receptor T cells,42 and others, including the BCL-2 inhibitor venetoclax.43
The use of HCVAD was shown to have significant efficacy in ALL prior to its use in BPDCN. In ALL, induction HCVAD therapy plus intrathecal prophylaxis results in a 91% CR rate, with a 5-year OS of 39% and a 5-year CR rate of 38%, with low CNS relapse.18 The combination regimen adapted from ALL for use in BPDCN consists of HCVAD during cycles 1, 3, 5, and 7 (hyperfractionated cyclophosphamide [300 mg/m2, IV, over 3 hours every 12 hours on days 1-3], vincristine [2 mg, IV, on days 4 and 11], adriamycin [50 mg/m2, IV, over 24 hours on day 4), and dexamethasone [40 mg IV/oral on days 1-4 and days 11-14]) alternating during cycles 2, 4, 6, and 8 with methotrexate (200 mg/m2, IV, over 2 hours, followed by 800 mg/m2, IV, over 22 hours) and ARA-C (3 g/m2, IV, over 2 hours, every 12 hours on days 2-3) plus CNS prophylaxis with intrathecal methotrexate (12 mg, day 2) and intrathecal ARA-C (100 mg, day 8), which allows for an intensive induction therapy regimen with synergistic antileukemic effects in patients with BPDCN. Over time, HCVAD regimens have been adapted to include numerous schemas, doses, and schedules. Patients treated with HCVAD frequently become neutropenic and must be monitored closely for the development of infections requiring prompt initiation of antibiotics and treatment of sepsis, as well as frequent laboratory monitoring and transfusion of blood products, as needed, during therapy.
Most ALL regimens have consisted of a vinca alkaloid and glucocorticoids.18 Corticosteroids, such as dexamethasone, have been shown to suppress cellular DNA synthesis and cause lysis of leukemic blast cells in acute leukemia.44 The adjunctive use of corticosteroids also demonstrated substantial disease control in BPDCN.45 In older patients who may not be able to tolerate intensive chemotherapy regimens, single-agent vinca alkaloid plus dexamethasone was reported to lead to CR and durable clinical response with minimal toxicity.46 The use of dexamethasone has systemic activity and CNS penetration in BPDCN and is an integral component of HCVAD; therefore, it remains an important part of treatment regimens in BPDCN.
Anthracyclines were first added to adult ALL regimens in the CALGB 7612 study. Daunorubicin was added to a regimen containing vincristine, prednisone, and l-asparaginase; it achieved improved CR rates and median duration of remission in patients compared with those who did not receive anthracyclines.47 This was followed by successful reciprocation with doxorubicin/adriamycin, the anthracycline used in the HCVAD backbone.48 Finally, the addition of cyclophosphamide into the treatment of ALL has been suggested to achieve a more rapid and durable CR and has been incorporated into treatment regimens for the past 2 decades with success.
Despite the successful use of targeted agents, alone or in combination on a clinical trial, relapsed and refractory disease remains a treatment challenge. Furthermore, it is unclear to what extent CD123-targeted agents cross the blood-brain barrier and are able to effectively treat BPDCN with CNS involvement. Research has found a high rate of relapse into the CNS, as well as CNS infiltration by neoplastic cells, in up to 30% of patients with BPDCN.49 We recently reported that the incidence of cerebrospinal fluid involvement was 22% in patients with BPDCN, of which 57% were discovered in the frontline setting; we hypothesize that perhaps more patients with BPDCN develop occult CNS involvement throughout their disease course.1 In this regard, the HCVAD regimen already includes 2 lumbar punctures per cycle for the first 4 cycles for a total of 8 in patients with ALL and, therefore, represents a potential direct application for BPDCN. In this cohort, we noted an overall incidence of CNS involvement in 12% of patients at the time of diagnosis, as well as relapsed BPDCN involvement in the CNS in 5% of patients who had previously responded to frontline therapy regimens.
Although CD123-targeted therapy has improved the treatment landscape for patients with BPDCN, there is room for improvement in selecting the optimal frontline therapy. Historical cytotoxic chemotherapy regimens have had various degrees of efficacy over time. In a recent analysis of patients with BPDCN treated with systemic therapy, there was a higher CR rate and a trend toward improved OS and progression-free survival in those treated with HCVAD compared with cyclophosphamide, doxorubicin, vincristine, and prednisone–based chemotherapy regimens or SL-401.50 However, the prior analysis also noted that, in patients who made it to allogeneic HSCT, there were similar post-HSCT outcomes among all treatment groups.50
The current study shows that utilization of combination cytotoxic therapy with HCVAD resulted in a significantly higher CR rate compared with frontline CD123-targeted monotherapy with SL-401. Our cohort of patients was quite heterogeneous; a notable difference is that patients who received frontline HCVAD were an average of 7 years younger than patients who received frontline SL-401. Further, there were several treatment combinations in the frontline HCVAD group, and 23 of the 35 patients received HCVAD alone. Subgroup analysis showed that age adjustment and analysis of HCVAD alone did not change the finding that no statistically significant difference was found between treatment groups in terms of OS. Interestingly, despite the significant improvement in CR rate with HCVAD, there was no difference in OS among groups. This may be due to the heterogenous group of patients with varying degrees of disease involvement and of subsequent lines of therapy. Among responders in each of the frontline treatment groups, a common significant variable was the ability to undergo HSCT, especially in CR1, which, regardless of frontline treatment regimen selected, remains a very important part of a curative approach in BPDCN, especially for younger/fit patients.51 Despite no improvement in median OS compared with other treatment regimens, the ability to yield a higher CR rate with low rates of adverse events suggests that HCVAD retains an important role in the frontline treatment of BPDCN. As above, HCVAD represents the optimal combination of vinca alkaloid, glucocorticoid, anthracycline, and combination chemotherapy, in addition to intrathecal chemotherapy, as an intensive and efficacious frontline regimen for BPDCN.
Although this is a large data set, limitations of this analysis include its single-institution retrospective nature. Another limitation is that many patients with BPDCN are older and frail and may not be able to tolerate the intensive HCVAD chemotherapy regimen. In this setting of older patients, we have successfully modified the treatment to mini-HCVD (with elimination of anthracycline, 50% reduction in doses, further age-adjusted doses), as used as salvage therapy in relapsed/refractory ALL.52 This, along with the success and tolerability of corticosteroids as above,44,45 allows for alterations in the backbone of HCVAD-based treatment regimens to fit each patient based on his/her individual needs.
Conclusions
Despite significant progress in CD123- and BCL-2-targeted monotherapy approaches in BPDCN, most patients are not cured outside of HSCT, and CNS relapses are now emerging commonly in the modern targeted therapy era of BPDCN. Combination approaches with both targeted and cytotoxic chemotherapy incorporating prophylactic CNS-directed therapy are urgently needed in this disease. These results establish high rates of CR for patients treated with frontline HCVAD and confirm a baseline role, even in the modern targeted-therapy era, for cytotoxic chemotherapy regimens in the treatment of BPDCN; in particular, confirming a role for HCVAD-based chemotherapy in BPDCN. Further studies are ongoing to establish the clinical feasibility, safety, and activity of doublet and triplet combinations of targeted therapies with cytotoxic agents with the goal of durable long-term remissions. Our group is actively investigating triplet combination with HCVAD as a comprehensive combination therapy protocol for BPDCN that includes the 3 most active regimens, CD123, BCL-2, and ALL-based cytotoxic approach with SL-401/venetoclax/HCVAD, in addition to intrathecal chemotherapy for CNS prophylaxis (www.clinicaltrials.gov, #NCT04216524).
Authorship
Contribution: N.P., N.R.W., and H.K. wrote the manuscript; and all authors designed the study, analyzed data, and approved the final version of the manuscript.
Conflict-of-interest disclosure: N.P. has served as a consultant for Cellectis, Plexxikon, Aptitude Health, Roche Diagnostics, Novartis Pharmaceuticals, Bristol Myers Squibb Co., Springer Science + Business Media, ImmunoGen, Inc, Incyte, DAVA Oncology, MustangBio, AbbVie Pharmaceuticals, Celgene Corporation, Stemline Therapeutics, Inc., Protagonist Therapeutics, Inc., ClearView Healthcare Partners, Blueprint Medicines, Pacylex Pharmaceuticals. He has received research funding from Samus, LFB Biotechnologies, Novarttic Pharmaceuticals, CareDx, Inc, Daiichi Sankyo, Inc., Sager Strong Foundation, Affymetrix, and Stemline Therapeutics, Inc. He has served membership on an entity's card of Directors or advisory committees; ASCO Leukemia Advisory Panel, HemOnc Times/Oncology Times, Dan's House of Hope, and Stemline Therapeutics, Inc. K.S. has served on the board of directors or an advisory committee for Daiichi-Sankyo and Pfizer and has acted as a consultant for and received research funding from Novartis. J.D.K. has received research funding from Kiromic, Angle, and Stemline Therapeutics. N.J. has received research funding from Pharmacyclics, AbbVie, Servier, ADC Therapeutics, Cellectis, Adaptive Biotechnologies, Genentech, AstraZeneca, Bristol Myers Squibb, Pfizer, Fate Therapeutics, Aprea Therapeutics, Precision Biosciences, and Incyte and has received honoraria from AbbVie, Servier, ADC Therapeutics, Cellectis, Adaptive Biotechnologies, Genentech, AstraZeneca, Bristol Myers Squibb, TG Therapeutics, Janssen, BeiGene, and Precision Biosciences. G.B. has acted as a consultant for Novartis, GlaxoSmithKline, and Protagonist; has served on the board of directors or an advisory committee for Novartis, Takeda, and argenx; and has received research funding from Ryvu Therapeutics and Astex. F.R. has received honoraria from Xencor, AstraZeneca, Syros Pharmaceuticals, Bristol Myers Squibb, AbbVie, Celgene, Novartis, Amgen, Jazz, Astex, Taiho, and Agios; has received research funding from Xencor, Syros Pharmaceuticals, Bristol Myers Squibb, AbbVie, Celgene, Prelude, Amgen, Jazz, Astex, Taiho, and Agios; has acted as a consultant for Syros Pharmaceuticals; and has served on the board of directors or advisory committee for Bristol Myers Squibb, and Celgene. N.D. has served as a consultant and received research funding from Pfizer, Gilead Sciences, Inc., Sevier, Genentech, Astellas, AbbVie, Trovagene, Amgen, Trillium, ImmunoGen, Bristol Myers Squibb, and Daiichi Sankyo. He has also received research funding from Hanmi Pharmaceutical, FATE Therapeutics, Novimmune, Glycomimetics, Karyopharm, and Newave. He has consulted for Novartis, DAVA Oncology (Arog), Celgene, Syndax, Shattuck Labs, Agios, Kite Pharmaceuticals, SOBI, STAR Therapeutics, and Jazz Pharmaceuticals, where he is also a Data Monitoring Committee member. T.K. has served as a consultant for Sanofi-Aventis, Jazz, Novartis, Liberum, Agios Pharmaceuticals, Daiichi Sankyo, AbbVie, Genentech, and Pfizer. He has received grant/research support from Bristol Myers Squibb, AbbVie, Amgen, Genentech, has collaborated with PulmoTech, AstraZeneca, Cellinkos, Ascentage Pharma, GenFleet Therapeutics, Astellas, and has served on the Speakers Bureau at Cure. C.D. has acted as a consultant for Agios/Servier and AbbVie; has received honoraria from Agios/Servier, Novartis, Takeda, Immune-Onc Therapeutics, Bristol Myers Squibb, Forma, Foghorn, and Celgene, a Bristol Myers Squibb company; has received research funding from Agios/Servier, AbbVie, Immune-Onc Therapeutics, Bristol Myers Squibb, Forma, Foghorn, and Celgene, a Bristol Myers Squibb company; holds stock options in Notable Labs; and has served on the board of directors or an advisory committee for Notable Labs and GlaxoSmithKline. E.J. has received research funding from Amgen, AbbVie, Spectrum, Bristol Myers Squibb, Takeda, Pfizer, Adaptive, and Genentech. M.Q. has served on the advisory board for Bristol Myers Squibb and Oncopeptides, and has received research funding from Bioline, Neximmune, Angiocrine, Amgen, and Janssen. M.K. has received grant support and research funding from Sanofi, AstraZeneca, Ascentage, Agios, Ablynx, Calithera, Forty Seven, Rafael Pharmaceuticals, Stemline therapeutics, Cellectis, and KisoJi Biotechnology. She has consulted and received honoraria and grant support/research funding from Genentech, F. Hoffmann-La Roche, and AbbVie. She has received patents and royalties, intellectual property rights, and research funding from Novartis and Eli Lilli. She also holds stock options, patents and royalties, and intellectual property rights in Reata Pharmaceuticals. H.K. has received research funding from Immunogen, Ascentage, Bristol Myers Squibb, Amgen, Daiichi-Sankyo, AbbVie, Pfizer, Jazz, and Novartis and has received honoraria from Astra Zeneca, Amgen, NOVA Research, KAHR Medical Ltd, AbbVie, Pfizer, Ipsen Pharmaceuticals, Astellas Health, Aptitude Health, Novartis, Precision Biosciences, and Taiho Pharmaceutical Canada. The remaining authors declare no competing financial interests.
Correspondence: Naveen Pemmaraju, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: npemmaraju@mdanderson.org.
References
Author notes
Requests for data sharing may be submitted to Naveen Pemmaraju (npemmaraju@mdanderson.org).
The full-text version of this article contains a data supplement.