Key Points
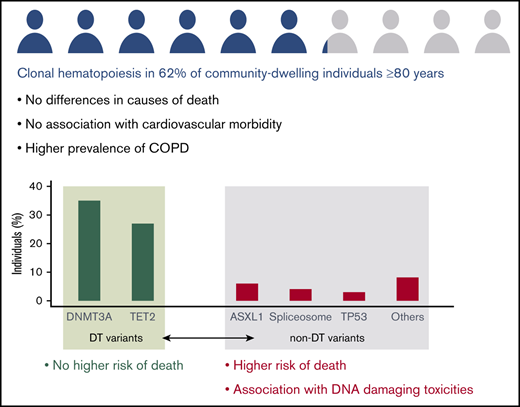
Clonal hematopoiesis is present in 62% of individuals ≥80 years and its prognostic implications are driver gene–specific.
ASXL1 and spliceosome variants are more frequent in individuals with estimated higher exposure to DNA damaging agents.
Abstract
Clonal hematopoiesis (CH), characterized by a fraction of peripheral blood cells carrying an acquired genetic variant, emerges with age. Although in general CH is associated with increased mortality and morbidity, no higher risk of death was observed for individuals ≥80 years. Here, we investigated CH in 621 individuals aged ≥80 years from the population-based LifeLines cohort. Sensitive error-corrected sequencing of 27 driver genes at a variant allele frequency ≥1% revealed CH in the majority (62%) of individuals, independent of gender. The observed mutational spectrum was dominated by DNMT3A and TET2 variants, which frequently (29%) displayed multiple mutations per gene. In line with previous results in individuals ≥80 years, the overall presence of CH did not associate with a higher risk of death (hazard ratio, 0.91; 95% confidence interval, 0.70-1.18; P = .48). Being able to assess the causes of death, we observed no difference between individuals with or without CH, except for deaths related to hematological malignancies. Interestingly, comparison of mutational spectra confined to DNMT3A and TET2 vs spectra containing other mutated genes, showed a higher risk of death when mutations other than DNMT3A or TET2 were present (hazard ratio, 1.48; 95% confidence interval, 1.06-2.08; P = .025). Surprisingly, no association of CH with cardiovascular morbidity was found, irrespective of clone size. Further, CH associated with chronic obstructive pulmonary disease. Data on estimated exposure to DNA damaging toxicities (ie, smoking, a history of cancer [as a proxy for previous genotoxic therapy], and job-related pesticide exposure) showed an association with spliceosome and ASXL1 variants, but not with DNMT3A and TET2 variants.
Introduction
During life, somatic mutations accumulate in hematopoietic stem and progenitor cells. A selective advantage of mutated hematopoietic stem and progenitor cells may result in detectable genetic mosaicism in peripheral blood; this is a phenomenon called clonal hematopoiesis (CH). The prevalence of CH increases strongly with age. Whole-exome sequencing has revealed CH to be virtually absent in individuals younger than 30 years of age, while being present in 20% to 30% of individuals aged 50 to 60 years.1,2 By using a more sensitive sequencing technique, small clones were shown to be almost ubiquitously present in (a small cohort of) middle-aged individuals.3 However, sensitive sequencing data in a large cohort of highly aged individuals are lacking.
CH has been shown to confer an increased risk of progression to hematological malignancies in the general population1,2,4 and to associate with peripheral blood cytopenia.5-8 Although the nonhematological implications are incompletely understood, CH has been associated with many prevalent age-related comorbidities, including cardiovascular diseases and inflammatory conditions.9-12 Potential risk factors for development of CH resemble those of myeloid malignancies, including age, a history of smoking, and exposure to chemo- or radiotherapy.13-16
In very aged individuals, the cumulative exposure to potential risk factors may render these individuals susceptible for development of CH. In addition, the potential adverse health effects of CH might contribute to the comorbid profile associated with high age. Surprisingly, CH did not associate with a higher risk of death in individuals ≥80 years of age,17 whereas inferior survival has been reported for younger individuals.1,2,18 The reasons behind this lack of prognostic impact in individuals ≥80 years remain unknown.
Here, we comprehensively investigated the clinical implications of CH in a large cohort of community-dwelling individuals ≥80 years from the prospective LifeLines study. Extensive data on health status, risk exposures, and cause of death allowed us to study the prevalence and outcomes of CH in more detail. Sensitive error-corrected sequencing of 27 genes allowed the investigation of hematopoietic clones at variant allele frequencies (VAF) ≥1%.
Methods
For this study, we used data from the prospective population-based LifeLines cohort. This multidisciplinary database includes 167 729 participants across 3 generations living in the northeast region of The Netherlands. A broad range of investigative procedures are performed at multiple time points with the aim of assessing the biomedical, sociodemographic, behavioral, physical, and psychological factors contributing to health and disease of the general population, with a special focus on multimorbidity and complex genetics.19 The LifeLines cohort has been shown to be representative of the general population in the northern part of The Netherlands.20 Here, we included all individuals ≥80 years of age. DNA samples from peripheral blood and extensive (bio)medical information were collected from all participants during the first study visit. Detailed information regarding the data and sample collection are provided in the supplemental Methods. The LifeLines study was performed in accordance with the Declaration of Helsinki and approved by the medical ethical committee of the University Medical Center Groningen.
Targeted error-corrected next-generation sequencing
DNA samples isolated from whole blood specimens were available for 624 of 661 individuals ≥80 years of age. Targeted next-generation sequencing for 27 myeloid and lymphoid malignancy-associated driver genes (supplemental Table 1) was performed using single-molecule molecular inversion probes on the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA). High-quality sequencing results were subsequently obtained for 621 individuals (supplemental Figure 1). Somatic variants were called based on a VAF ≥1% and ≥10 consensus variant reads. All variants were inspected and curated, excluding recurrent artifacts and polymorphisms. The mean number of aligned consensus reads was 9726, with a coverage >500× for 97.6% of all targeted regions (supplemental Figure 1). Details regarding panel design, library preparation, and data analyses were previously outlined.8
Selection of individuals with an elevated risk of exposure to DNA damaging toxicities
Availability of extensive health determinant data within the LifeLines cohort allowed us to classify individuals according to higher or lower risk of exposure to DNA damaging agents. Job-related exposure to pesticides (herbicides, fungicides, and insecticides) was estimated on the basis of the New Occupational Asthma-specific Job-Exposure Matrix (2016).21 A history of cancer, as a risk estimate for exposure to cytotoxic therapy and radiation, was based on self-report, with all skin cancers (apart from melanomas) excluded (supplemental Table 4). Smoking status of participants was categorized as current smoker, ex-smoker, or never smoker (supplemental Methods). An elevated risk of exposure to DNA damaging toxicities was defined as (1) medium or high job-related exposure risk to pesticides, (2) medical history of cancer, and/or (3) previous or current smoking.
Definitions of medical history
A history of myocardial infarction was defined on the basis of an electrocardiogram performed during the first study visit.22 A history of stroke or transient ischemic attack was based on a questionnaire and validated by use of anticoagulant medication (Anatomical Classification System [ATC] codes B01AC, B01AA, B01AE, or B01AF). Diabetes was defined as a self-reported history of diabetes, use of antidiabetic medication (ATC code A10), fasting blood glucose level ≥7 mmol/L, and/or random blood glucose level ≥11.1mmol/L. The number of current medications was used as a proxy for comorbidity, with polypharmacy defined as usage of ≥5 medications.23 Chronic obstructive pulmonary disease (COPD) was defined as self-reported COPD, emphysema, or chronic bronchitis in combination with the use of a bronchodilator (ATC code R03). Cognitive impairment was assessed by the Mini-Mental State Examination, with a score below the clinically validated cutoff <24 points defined as impaired cognitive function.24
Statistical analysis
Nonparametric data were graphically represented as median with interquartile range and statistically compared using Mann-Whitney U or Kruskal-Wallis test for 2-group or multiple-group comparison. Categorical data were presented as absolute numbers with percentages. Differences in proportions were assessed using χ2 or Fisher’s exact test, as appropriate. For the association between CH and medical history, odds ratios (ORs) with 95% confidence interval (CI) were reported from univariable logistic regression. Additional sensitivity analyses were performed by restricting the presence of CH to a VAF ≥2%, corresponding to the proposed definition of “clonal hematopoiesis of indeterminate potential.”25 For COPD, logistic regression was performed with age, sex, and smoking as covariables. Time to death was calculated from study inclusion until death from any cause, as derived from the Municipal Persons Records Database (last update June 2020). The Kaplan-Meier estimator was used for visual comparison of overall survival (OS). Hazard ratios (HRs) and 95% CI for risk of death were reported from Cox proportional hazard regression. Data on cause-specific mortality were obtained by linkage to the national death statistics registry (Statistics Netherlands; supplemental Methods). For cause-specific mortality, cumulative incidences were visualized. Multivariable regression analysis for cause-specific survival was performed using competing risk regression according to the method of Fine and Gray, with death from other causes considered a competing risk. In multivariable survival analyses, age and sex were included as covariables. Statistical analyses were performed using R version 3.5.2. Statistical tests were performed 2-sided and a P value < .05 was considered significant.
Results
High prevalence of CH at low VAF in individuals ≥80 years, dominated by DNMT3A and TET2 variants
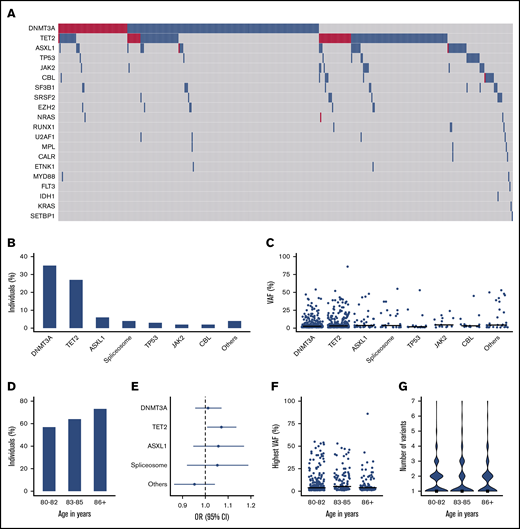
Within this community-based cohort of individuals aged ≥80 years, 382 of 621 (61.5%) evaluable participants carried CH at a VAF ≥1%. The mutational spectrum for the entire cohort is shown in Figure 1A. CH at older age was characterized by a predominance of DNMT3A and TET2 (DT) variants, with a higher prevalence of TET2 variants compared with younger cohorts (Figure 1B; supplemental Figures 2 and 3). A total of 219 (35%) individuals carried a mutated DNMT3A clone, of whom 27% carried multiple somatic variants in this gene. Mutated TET2 clones were detected in 166 (27%) individuals, of whom also a large proportion (24%) carried multiple variants in this gene. In contrast, ASXL1 (6%), spliceosome (4%) and TP53 (3%) variants were observed in a considerably lower proportion of individuals. Most variants were detected at low VAF (median, 4.2%; interquartile range, 2.1-13.0), with modest differences across all genes (Figure 1C). Total and differential blood counts for individuals with and without CH were comparable (supplemental Table 2). Upon classification of individuals ≥80 years into age groups, a continuing increase in the prevalence of CH with age was observed (P = .008; Figure 1D). This increasing prevalence was predominantly driven by mutated TET2 clones (OR, 1.07; 95% CI, 1.01-1.14; P = .025; Figure 1E). No significant changes in highest observed VAF per individual (P = .063; Figure 1F) or the number of somatic variants (P = .68; Figure 1G) were observed across the age groups. The most frequently detected single nucleotide variants were C>T substitutions (44.3%; supplemental Figure 2).
High prevalence of clonal hematopoiesis at low VAF in individuals ≥80 years, dominated by DNMT3A and TET2 variants. (A) Mutational landscape for somatic variants detected in this elderly cohort. Blue and red color indicate respectively 1 or ≥2 variants per gene. (B) Proportion of individuals carrying a somatic variant in recurrently (>10×) mutated genes. (C) VAFs for all detected variants in recurrently (>10×) mutated genes. (D) Bar plot showing the proportion of individuals with CH, stratified into 3 age groups. (E) Forest plot showing the odds ratio with 95% CI for the presence of most prevalent gene variants according to a 1-year increase in age. (F-G) Highest observed VAF (scatter plot) and number of somatic variants (violin plot) per individual categorized in 3 age groups. The horizontal line and rectangle represent median values for the respective group. The category of spliceosome variants includes SF3B1, SRSF2, and U2AF1.
High prevalence of clonal hematopoiesis at low VAF in individuals ≥80 years, dominated by DNMT3A and TET2 variants. (A) Mutational landscape for somatic variants detected in this elderly cohort. Blue and red color indicate respectively 1 or ≥2 variants per gene. (B) Proportion of individuals carrying a somatic variant in recurrently (>10×) mutated genes. (C) VAFs for all detected variants in recurrently (>10×) mutated genes. (D) Bar plot showing the proportion of individuals with CH, stratified into 3 age groups. (E) Forest plot showing the odds ratio with 95% CI for the presence of most prevalent gene variants according to a 1-year increase in age. (F-G) Highest observed VAF (scatter plot) and number of somatic variants (violin plot) per individual categorized in 3 age groups. The horizontal line and rectangle represent median values for the respective group. The category of spliceosome variants includes SF3B1, SRSF2, and U2AF1.
Sex and exposure to DNA damaging toxicities as risk factor for CH at high age
Besides age, other risk factors may be associated with the occurrence of driver gene mutations or their expansion. Here, we investigated the effect of gender and a higher risk of exposure to DNA damaging toxicities, including smoking, a history of cancer (as a proxy for previous genotoxic therapy), and job-related pesticide exposure, on CH.
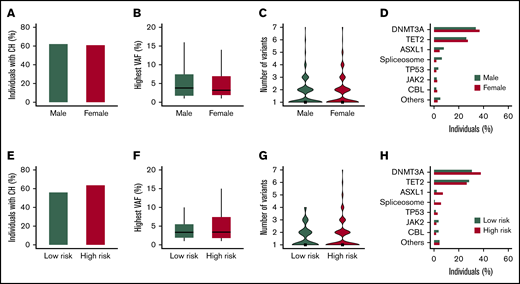
Male sex is a risk factor and associates with worse outcomes in multiple myeloid malignancies.26 In this cohort of individuals ≥80 years of age, no differences in the prevalence of CH (P = .82; Figure 2A), VAF (P = .28; Figure 2B), or number of somatic variants (P = .60; Figure 2C) were observed between male and female participants. Overall, the mutational spectrum was comparable, apart from a higher proportion of males carrying spliceosome variants (P = .008; Figure 2D).
Sex and exposure to DNA damaging toxicities as a risk factor for clonal hematopoiesis at high age. (A-D) The mutational spectrum displayed according to sex. (E-H) The mutational spectrum according to the risk of exposure to DNA damaging toxicities. (A,E) The proportion of individuals with CH. (B,F) The highest observed VAF for individuals with CH. Boxplots represent the median and 25th to 75th percentile, with whiskers extending to the highest and lowest 5th percentile. (C,G) Violin plots show the number of somatic variants for individuals with CH, with rectangles indicating the median. (D,H) The proportion of individuals carrying a recurrent (>10×) gene mutation. The category of spliceosome variants includes SF3B1, SRSF2, and U2AF1.
Sex and exposure to DNA damaging toxicities as a risk factor for clonal hematopoiesis at high age. (A-D) The mutational spectrum displayed according to sex. (E-H) The mutational spectrum according to the risk of exposure to DNA damaging toxicities. (A,E) The proportion of individuals with CH. (B,F) The highest observed VAF for individuals with CH. Boxplots represent the median and 25th to 75th percentile, with whiskers extending to the highest and lowest 5th percentile. (C,G) Violin plots show the number of somatic variants for individuals with CH, with rectangles indicating the median. (D,H) The proportion of individuals carrying a recurrent (>10×) gene mutation. The category of spliceosome variants includes SF3B1, SRSF2, and U2AF1.
An elevated risk of exposure to DNA damaging toxicities (for n = 404) was not significantly associated with differences in the prevalence of CH (64% vs 56%; P = .14; Figure 2E), VAF (P = .40; Figure 2F), or number of somatic variants (P = .12; Figure 2G). However, investigation of individual driver genes revealed a higher prevalence of spliceosome (6% vs 1%; P = .014) and ASLX1 (7% vs 2%; P = .035) variants, a finding that was consistently observed for all 3 investigated potential DNA damaging exposures. In general, an elevated risk of exposure to DNA damaging toxicities was not associated with DNMT3A (38% vs 31%; P = .15) and TET2 (26% vs 28%; P = .74) variants, except from a higher prevalence of DNMT3A in current smokers (P = .042) (Figure 2H; supplemental Figure 4).
Association of CH with common age-related comorbidities
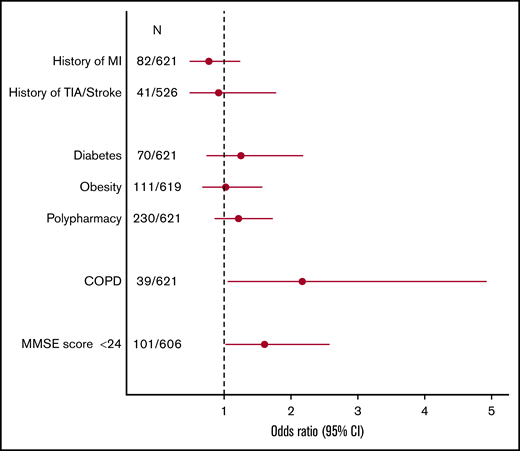
We next evaluated the association of CH with frequent comorbid conditions at older age. In this cohort, we could not ascertain an association between CH and a history of myocardial infarction (OR, 0.77; 95% CI, 0.48-1.24; P = .28; n = 82), history of transient ischemic attack or stroke (OR, 0.92; 95% CI, 0.48-1.78; P = .79; n = 41), or diabetes (OR, 1.32; 95% CI, 0.79-2.27; P = .31; n = 70; Figure 3). The absence of an association with cardiovascular history was confirmed when restricting to individuals with CH ≥2% VAF (supplemental Figure 5). Obesity (OR, 1.02; 95% CI, 0.67-1.57; P = .91; n = 111) and polypharmacy (OR, 1.18; 95% CI, 0.84-1.65; P = .35; n = 230), as a general marker for comorbidity, also did not associate with CH. In contrast, an association of CH and a history of COPD was found (OR, 2.18; 95% CI, 1.06-4.95; P = .046; n = 39). This finding was confirmed in a multivariable model with inclusion of age, sex, and smoking status = .038, supplemental Table 3)(OR, 2.26; 95% CI, 1.09-5.18; P = .038; supplemental Table 3). In addition, comparable effect estimates were observed for those with a VAF ≥2%. In the presence of CH, a significantly higher proportion of individuals scored below the clinical cutoff of 24 for the Mini-Mental State Examination, a widely used screening test for cognitive function (OR, 1.60; 95% CI, 1.02-2.58; P = .045; n = 101). However, this effect was not substantiated when restricting the analyses to individuals with a VAF ≥2% (supplemental Figure 5).
Comorbid profile of clonal hematopoiesis in highly aged individuals. Forest plot showing odds ratios for the association between the presence of CH and prevalent age-related comorbidities, derived from univariable logistic regression. Circles indicate the odds ratio, with horizontal lines corresponding to the 95% CI. Polypharmacy was defined as ≥5 medications. Obesity was defined as body mass index ≥30 kg/m2. MI, myocardial infarction; MMSE, Mini-Mental State Examination; N, number of individuals with respective medical history and total number of evaluable individuals; TIA, transient ischemic attack.
Comorbid profile of clonal hematopoiesis in highly aged individuals. Forest plot showing odds ratios for the association between the presence of CH and prevalent age-related comorbidities, derived from univariable logistic regression. Circles indicate the odds ratio, with horizontal lines corresponding to the 95% CI. Polypharmacy was defined as ≥5 medications. Obesity was defined as body mass index ≥30 kg/m2. MI, myocardial infarction; MMSE, Mini-Mental State Examination; N, number of individuals with respective medical history and total number of evaluable individuals; TIA, transient ischemic attack.
Incident hematological malignancies and all-cause and cause-specific survival according to the presence of CH
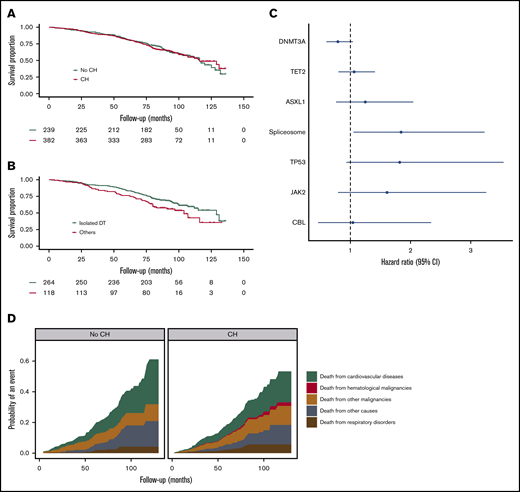
During a median follow-up of 6.9 years, 241 deaths (39%) occurred in this cohort of individuals aged ≥80 years. Overall, CH did not confer a higher risk of death (Figure 4A). This finding was confirmed in a multivariable model with age and sex as covariables (HR, 0.91; 95% CI, 0.70-1.18; P = .48). We subsequently compared OS rates for individuals with mutational spectra confined to the frequently observed TET2 and DNMT3A (isolated DT) variants vs other variants. A significantly higher risk of death was observed for individuals carrying mutational spectra other than isolated DT variants (Figure 4B). This finding was confirmed in multivariable age- and sex-corrected analysis (HR, 1.48; 95% CI, 1.06-2.08; P = .025). Although limited by low numbers, we evaluated the survival impact of individual genes. In univariable analysis, spliceosome variants (HR, 1.84; 95% CI, 1.05-3.23; P = .033; Figure 4C) were associated with a higher risk of death. Comparable risk estimates were observed for clones with mutated TP53 and JAK2, although not reaching statistical significance. Individuals with a VAF ≥5% or individuals with multiple mutated genes were not at higher risk of death (supplemental Figure 6). During follow-up, a total of 6 individuals developed a pathology-proven hematological malignancy, of whom all had CH at baseline, with a mutational spectrum enriched for larger clones and multiple somatic variants (supplemental Table 4). Death from hematological malignancies was exclusively observed in the presence of CH at baseline. The cumulative incidences of death from other causes were comparable for individuals with and without CH, with no statistical differences in probabilities of death from cardiovascular disease (P = .29), solid malignancies (P = .66), or respiratory disorders (P = .43) when evaluated in a multivariable competing risk regression model (Figure 4D; supplemental Table 5).
Survival implications of clonal hematopoiesis at high age. (A) Kaplan-Meier curve for overall survival (OS), according to the presence of CH. (B) Kaplan-Meier curve for OS stratified according to mutational spectrum: individuals carrying variants exclusively in DNMT3A and/or TET2 (isolated DT) vs all other individuals with CH (others). (C) Forest plot for risk of death, according to the presence of recurrent (>10×) gene mutations. Circles indicate hazard ratios, with lines extending to the 95% CI. (D) Probability of cause-specific death for individuals with and without CH. Results are based on calculations by the authors using nonpublic microdata from Statistics Netherlands. The category of spliceosome variants includes SF3B1, SRSF2, and U2AF1.
Survival implications of clonal hematopoiesis at high age. (A) Kaplan-Meier curve for overall survival (OS), according to the presence of CH. (B) Kaplan-Meier curve for OS stratified according to mutational spectrum: individuals carrying variants exclusively in DNMT3A and/or TET2 (isolated DT) vs all other individuals with CH (others). (C) Forest plot for risk of death, according to the presence of recurrent (>10×) gene mutations. Circles indicate hazard ratios, with lines extending to the 95% CI. (D) Probability of cause-specific death for individuals with and without CH. Results are based on calculations by the authors using nonpublic microdata from Statistics Netherlands. The category of spliceosome variants includes SF3B1, SRSF2, and U2AF1.
Discussion
Using large-scale sensitive error-corrected sequencing at a sensitivity of 1% VAF, we show CH to be present in 62% of individuals ≥80 years of age. This confirms, as has been suggested with highly sensitive sequencing in healthy 50- to 60-year-old participants in the Nurses' Health Study,3 that small-sized clones are present in the majority of individuals at high age. Further, in agreement with previous work,17 TET2 and DNMT3A variants dominated the observed high prevalence of CH. Interestingly, a substantial proportion of individuals ≥80 years of age carried multiple DNMT3A and TET2 variants.
Although many studies have demonstrated decreased OS in individuals with CH,1,2,18 CH does not confer a higher risk of death in individuals ≥80 years, thereby confirming previous findings by van den Akker et al17 in another cohort of individuals ≥80 years. The survival impact of CH may be masked by the high overall mortality rate of highly aged individuals. Alternatively, survivorship bias leading to a selection of individuals with low-risk clones may provide an explanation for the absent survival impact. In line with this hypothesis, the high prevalence of DT clones in this elderly cohort may indicate a (beneficial) mechanism by which these clones compensate for a functional decline of aging stem cells to maintain normal hematological output.27,28 Indeed, when comparing the survival impact of mutational spectra confined to TET2 and DNMT3A vs mutational spectra containing other mutated genes, absence of survival impact for DT clones was confirmed. However, our data show that CH at older age is not completely harmless because a higher risk of death was observed in individuals with mutations other than TET2 or DNMT3A. By linkage to national registries, we were able to evaluate causes of death. This revealed no differences in causes of death between individuals with or without CH, except for deaths related to hematological malignancies, which occurred exclusively in the presence of CH.
Extensive data on medical history were available for the majority of included individuals. Interestingly, although this was previously shown for younger individuals,2,9 there was no relation between CH and cardiovascular comorbidities in this elderly cohort, even when restricting to larger clone size. In contrast, we confirmed the previously reported association of CH with COPD in individuals ≥80 years, after correction for smoking status.
The cumulative exposure of very aged individuals to potential DNA damaging agents warranted the exploration of these agents as a risk factor for CH in this unique cohort. Collectively, a higher risk of exposure to DNA damaging toxicities, including smoking, a history of cancer, and estimated pesticide exposure, associated with a mutational spectrum biased toward spliceosome and ASXL1 variants. This is in line with previous studies showing ASXL1 mutations to be highly prevalent in current smokers.13,16 Apart from a higher prevalence of DNMT3A variants in current smokers, DT variants were not found to be enriched in individuals with a higher risk of previous exposure to DNA damaging toxicities. This suggests that exposures may differentially affect the emergence of specific CH-associated gene mutations, which deserves validation in a larger cohort.
In conclusion, using sensitive error-corrected sequencing, CH was present in 62% of individuals ≥60 years. The spectrum of CH was dominated by small-sized DNMT3A and TET2 clones. This confirms the widely supported hypothesis of a nearly ubiquitous presence of small-sized clones in aged individuals. When comparing the prognostic impact of different mutational spectra, a higher risk of death was observed for individuals with mutations other than TET2 or DNMT3A. In addition, our data provide an indication for differential comorbid conditions associated with CH at high age. Analysis of potential underlying etiologies revealed higher estimated exposure to DNA damaging toxicities for mutational spectra that include spliceosome and ASXL1 variants, whereas no such association was detected for mutations in DNMT3A and TET2.
The manuscript is based on data from the LifeLines Cohort Study. LifeLines adheres to standards for data availability. The data catalog of LifeLines is publicly accessible at www.lifelines.nl. All international researchers can obtain data at the LifeLines research office (research@lifelines.nl), for which a fee is required. Results from cause of death analyses are based on calculations by the authors using nonpublic microdata from Statistics Netherlands. Under certain conditions, these microdata are accessible for statistical and scientific research. For further information, contact microdata@cbs.nl.
Acknowledgments
The authors thank all participants of the LifeLines cohort study, everyone contributing to the study setup and design, PALGA (Dutch Pathology Registry) and Statistics Netherlands. The authors also thank the Genome Technology Center, Radboud University Medical Center, for performing NovaSeq sequencing.
The LifeLines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport; the Dutch Ministry of Economic Affairs; the University Medical Center Groningen; University Groningen; and the Northern Provinces of The Netherlands. This work is part of the MDS-RIGHT project, which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 634789—“Providing the right care to the right patient with MyeloDysplastic Syndrome at the right time.” This work was further supported by a grant from the Dutch Cancer Foundation (KWF10813). The funder of this study had no role in study design, collection, analysis, and interpretation of data, and writing or approval of the manuscript.
Authorship
Contribution: G.H. and J.H.J. were principal investigators and involved in all aspects of the study, including design, collection, and interpretation of the data; A.O.d.G. and I.A.v.Z. contributed to study design; A.O.d.G. supervised next-generation sequencing analyses; J.B.S. and I.A.v.Z. performed statistical analyses and wrote the first version of the manuscript; all authors contributed to analysis and interpretation of the data; and the final manuscript was read and approved by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joop H. Jansen, Department of Laboratory Medicine, Laboratory of Hematology, Radboud University Medical Center, Internal postal code 475, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: Joop.Jansen@radboudumc.nl; and Gerwin Huls, Department of Hematology, University Medical Center Groningen, Internal postal code DA21, PO Box 30001, 9700 RB Groningen, The Netherlands; e-mail: g.huls@umcg.nl.
References
Author notes
I.A.v.Z., J.B.S., A.O.d.G., J.H.J., and G.H. contributed equally to this study.
The full-text version of this article contains a data supplement.