Key Points
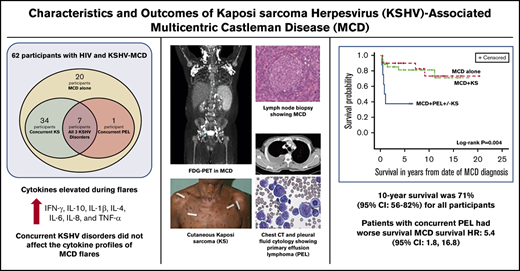
Among people living with HIV, KSHV-MCD is underdiagnosed but treatable if identified, leading to 10-year survival of 71% in this cohort.
KSHV-MCD can occur alone or with other KSHV-associated conditions, and the presence of concurrent PEL can impact survival.
Abstract
Kaposi sarcoma (KS)-associated herpesvirus (KSHV)–associated multicentric Castleman disease (MCD) is a relapsing and remitting systemic lymphoproliferative disorder characterized by severe inflammatory symptoms most common among people living with HIV (PLWH). Patients with KSHV-MCD may present with concurrent KSHV-associated diseases, such as KS and/or primary effusion lymphoma (PEL). We evaluated clinical and immunologic characteristics, the effects of concurrent KSHV malignancies, and treatments from the largest prospective natural history study of participants with KSHV-MCD within the United States. Treatment options administered at investigator discretion included high-dose zidovudine with valganciclovir (AZT/VGC), rituximab, or rituximab with liposomal doxorubicin (R-Dox) during KSHV-MCD flares. Survival analyses and prognostic factors were explored for all participants. Sixty-two participants with HIV were enrolled, including 20 with KSHV-MCD alone, 34 with KSHV-MCD and KS, 1 with KSHV-MCD and PEL, and 7 with all KSHV-associated diseases. Forty-four percent of KSHV-MCD diagnoses were made at our institution. Forty-four participants received rituximab-based therapies, 20 of whom had maintenance AZT/VGC or interferon. Participants receiving R-Dox and then maintenance AZT/VGC had the highest 5-year progression-free survival (89%). Cytokine profiles during KSHV-MCD flares did not differ by the presence of concurrent KSHV-associated diseases. The 10-year survival was 71% (95% confidence interval [CI], 56% to 82%) for all participants. A concurrent diagnosis of PEL negatively impacted survival (PEL hazard ratio, 5.4; 95% CI, 1.8 to 16.8). KSHV-MCD is an underdiagnosed condition among PLWH, including those with KS. KSHV-MCD has an excellent prognosis with appropriate treatment. Physicians should be alert for patients with multiple KSHV diseases, which impact optimal treatment and survival outcomes. This study was registered at www.clinicaltrials.gov as #NCT00099073.
Introduction
Kaposi sarcoma (KS)–associated herpesvirus (KSHV), also known as human herpesvirus 8, causes 3 principal conditions: KS, primary effusion lymphoma (PEL), and a plasmablastic form of multicentric Castleman disease (MCD).1-3 KSHV-MCD is a rare relapsing and remitting B-cell lymphoproliferative disorder that most often develops in people living with HIV.4,5 This condition is associated with elevated cytokine levels, including interleukin-6 (IL-6) and IL-10, and production of a KSHV-encoded homolog of IL-6 (viral IL-6 [vIL-6]).6,7 Patients usually present with lymphadenopathy and inflammatory symptoms, including fever and night sweats. Laboratory abnormalities include cytopenias, hypoalbuminemia, hyponatremia, and elevated C-reactive protein (CRP).7-10 Clinicians may incorrectly attribute signs and symptoms of KSHV-MCD to HIV infection or other infective processes. Diagnosis of KSHV-MCD requires pathological confirmation from an involved lymph node. KSHV-MCD can progress to multiorgan dysfunction and is lethal if untreated, with a median survival of 2 years. Though KSHV-MCD can occur alone, patients can present with concurrent KS or PEL or all 3 conditions (KS, KSHV-MCD, and PEL) together.11,12
The principal pathogenic cells in KSHV-MCD are KSHV-infected, λ-restricted plasmablasts, most commonly in the outer mantle zone or marginal zone of involved lymph nodes. A proportion of KSHV-infected cells express vIL-6 and sometimes express other KSHV lytic genes.4,13 KSHV-infected plasmablasts generally express OCT2, BLIMP1, and IRF4/MUM1 but lack PAX5, BCL6, and CD138.14,15 They are variably positive for CD20. Many symptoms and laboratory abnormalities associated with KSHV-MCD are caused by production of v-IL6 and increases in human IL-6 and IL-10.6,7,10
While there are no approved therapies for KSHV-MCD, a number of agents and regimens have now been recommended by the National Comprehensive Cancer Network Guidelines. KSHV-MCD flares can be treated with rituximab, an anti-CD20 monoclonal antibody, which leads to resolution of the signs and symptoms associated with an acute flares in the vast majority of patients.16-19 Furthermore, rituximab may be used for subsequent KSHV-MCD flares.20 However, rituximab is associated with worsening of KS in 35% to 67% of individuals with concurrent KSHV-MCD and KS.4 We developed a regimen combining rituximab and liposomal doxorubicin (R-Dox), a standard therapy for KS that also targets B cells, which led to responses in both KS and KSHV-MCD.8 Another treatment option we developed for KSHV-MCD is virus-activated cytotoxic therapy using high-dose zidovudine combined with valganciclovir (AZT/VGC). AZT and VGC are activated to toxic moieties by KSHV lytic genes (ORF21 and ORF36) and thus specifically target KSHV-MCD plasmablasts.21-23 Participants treated with oral AZT/VGC had a response rate of 86% with associated decreases in the KSHV viral load (VL), IL-6, IL-10, and CRP.24 Despite the treatment options available for KSHV-MCD, the risk of B-cell lymphoma, particularly PEL, remains high among patients with KSHV-MCD as compared with the general population.12 For patients with KSHV-MCD and PEL, available literature suggests that the simultaneous occurrence of PEL requires combination cytotoxic chemotherapy with curative intent.11 The cytokine profiles, outcomes, or prognostic implications of other concurrent KSHV-associated diseases in patients with KSHV-MCD are largely unknown and may be heterogenous.
Here, we present the characteristics, treatment, and survival outcomes from the largest series of participants with HIV infection and KSHV-MCD in the United States. We also compared cytokine and KSHV levels of participants with KSHV-MCD alone, those with concurrent KSHV-MCD and KS, and those with all 3 KSHV-associated diseases to better understand the pathogenesis of these conditions.
Methods
Study population
Protocol 04-C-0275 (NCT00099073) was initiated in the HIV/AIDS Malignancy Branch of the National Cancer Institute (NCI) in 2004 when there were no recommended therapies for KSHV-MCD management. This is natural history study of KSHV-MCD included prospective evaluation of several treatment options depending on the patient status during flares and observation if asymptomatic or during periods of remission. Entry on protocol required pathological confirmation of lymph node involvement by KSHV-MCD by the Laboratory of Pathology, NCI, applying the criteria defined by the World Health Organization classification and other standard references.25,26 Positivity for KSHV was confirmed by immunohistochemical staining for the latency-associated nuclear antigen (anti-ORF73 rat monoclonal antibody; Advanced Biotechnologies, Eldersburg, MD). KS was confirmed following biopsy of skin, lymph node, or visceral lesions. PEL was pathologically confirmed based on review of cytology from effusions or biopsy of extracavitary PEL.11 Ancillary studies, including flow cytometry or polymerase chain reaction, were employed to confirm monoclonality, as needed.
To understand the characteristics and impact of concurrent KSHV-associated diseases, participants were separated into 3 categories: those with KSHV-MCD alone (MCD alone), those with KSHV-MCD with biopsy-confirmed KS (MCD+KS), and those with PEL and KSHV-MCD with or without KS (MCD+PEL±KS).
In the case of 1 participant within the MCD+PEL±KS cohort, PEL was only identified at autopsy. This participant had progressive KSHV-MCD+KS, suffered from recurrent inflammatory disease manifestations despite KSHV-MCD treatment, and died within 1 year of diagnosis
Flare episodes were defined by the presence of ≥1 symptom and 1 laboratory abnormality attributed to KSHV-MCD, per previously defined criteria.8,24,27 Remission was defined as the time point when participants had met response criteria (Clinical Benefit Response Criteria in supplemental Table 1) from baseline measurements for ≥3 weeks. Participants were treated for ≥2 cycles beyond remission. Participants with MCD+PEL±KS had a fixed course of treatment of PEL. The KSHV-MCD clinical benefit criteria was used at the end of PEL therapy to evaluate KSHV-MCD response, and PEL response was evaluated separately using the Lugano classification criteria.28 When participants experienced KSHV-MCD remission, they entered into the observation phase and were reassessed every 3 to 6 months for 2 years and yearly thereafter. All but one of the patients were coenrolled on a companion protocol (01-C-0038; NCT00006518) that allowed for collection of tissue samples and additional samples not specified in 04-C-0275. Both protocols were approved by the NCI Institutional Review Board. All enrolled participants gave written informed consent in accordance with the Declaration of Helsinki.
Treatment
The protocol provided guidelines to select a specific regimen but allowed for a certain amount of flexibility to tailor the selection based on clinical assessment. Participants with asymptomatic disease were generally observed following study enrollment. Most symptomatic participants with or without KS were treated with R-Dox or high-dose AZT/VGC (regimens described in supplemental Table 2). Participants enrolled early in the study could receive optional consolidation therapy with maintenance interferon-α (IFN-α) or AZT/VGC following R-Dox. Participants with concurrent MCD+PEL±KS, and a few participants with particularly severe KSHV-MCD flares, were treated with dose-adjusted (DA)EPOCH-R (etoposide, prednisone, vincristine, doxorubicin, cyclophosphamide, and rituximab), a multiagent chemotherapy regimen often used for the treatment of HIV-associated non-Hodgkin lymphoma.29 (DA)EPOCH-R was administered alone or in combination with other agents in this study or on other ongoing protocols for 6 cycles. Participants were permitted to receive the same therapy for KSHV-MCD or a different treatment of subsequent flares. Participants received antiretroviral therapy and opportunistic infection prophylaxis in accordance with existing guidelines.30
Cytokines and KSHV VL
The KSHV VL per million peripheral blood mononuclear cells (PBMCs) was measured in DNA extracted from PBMCs by polymerase chain reaction using K6 primers.31 Measurement of serum levels of inflammatory cytokines (IFN-γ, IL-10, IL-12. IL-13, IL-1β, IL-2, IL-4, IL-6, IL-8, tumor necrosis factor-α [TNF-α]) was performed using the MSD 96-Well Multiarray Pro-inflammatory 10-plex assay (Meso Scale Discovery, Gaithersburg, MD). Cytokine levels were measured from samples obtained at the time of the first flare and remission on study entry and 4 weeks following treatment of MCD+PEL±KS participants.
Statistical considerations
Differences in baseline characteristics were evaluated using the Kruskal-Wallis test or Wilcoxon rank sum test for continuous variables and Mehta’s modification to the Fisher’s exact test for categorical variables. Analyses of the differences in cytokines between participants with MCD alone as compared with MCD+KS or MCD+PEL±KS were evaluated using the Wilcoxon rank sum test. Wilcoxon signed rank test was used to analyze the KSHV VL and cytokines from flare to remission/end of treatment. In view of the number of evaluations performed for cytokine analyses, a P value < .005 was considered statistically significant, and a P value between .005 and .05 was considered a strong trend. Univariate analyses of prognostic factors were studied using the Cox proportional hazard model with cytokines and other continuous variables dichotomized using the median values. Survival was evaluated by using the Kaplan-Meier method, and 2-sided log-rank tests were used to determine the statistical significance of differences between curves; where appropriate, Bonferroni correction was used to correct for multiple comparisons. As participants were able to move between therapy options, progression-free survival (PFS) was calculated from treatment initiation with a specific therapy to treat KSHV-MCD within this protocol (AZT/VGC, rituximab, or R-Dox [alone or with maintenance therapies]), until death or a subsequent MCD flare necessitating further treatment of participants with MCD alone or MCD+KS only irrespective of prior therapy. For overall survival, all-cause mortality was evaluated from diagnosis of MCD until death from any cause or censoring on 1 May 2020.
Results
Patient characteristics
Sixty-two participants (58 cisgender men and 4 cisgender women) with HIV and KSHV-MCD were enrolled between 2004 and 2019 (Table 1). The median age of all participants at the time of KSHV-MCD diagnosis was 44 years (range, 26-68 years). There were 20 participants with a diagnosis of MCD alone, 34 participants with MCD+KS, and 8 participants with MCD+PEL±KS (defined here as having PEL diagnosis within 12 months of MCD diagnosis). Among those with MCD+PEL±KS, 7 participants were diagnosed with all 3 conditions at the same time. Twenty participants (59%) with MCD+KS had T1 KS, whereas all participants with MCD+PEL±KS had stage T1 KS.
Baseline characteristics of all patients and those with KSHV-MCD alone and concurrent KSHV-associated diseases
| Baseline characteristics . | All patients (N = 62) . | MCD alone (n = 20) . | MCD with concurrent KS (n = 34) . | MCD with concurrent PEL±KS (n = 8) . | P* . |
|---|---|---|---|---|---|
| Age at MCD diagnosis, median (IQR), y | 44 (38-54) | 46 (44-54) | 41 (46-48) | 46 (29-56) | .07 |
| Sex, n (%) | |||||
| Cisgender male | 58 (94) | 19 (95) | 31 (91) | 8 (100) | 1.00 |
| Cisgender female | 4 (6) | 1 (5) | 3 (9) | 0 | |
| Race, n (%) | .87 | ||||
| White | 31 (50) | 10 (50) | 16 (47) | 5 (62) | |
| Hispanic | 5 (8) | 2 (10) | 3 (9) | — | |
| Black | 16 (26) | 6 (30) | 7 (20) | 3 (38) | |
| African immigrant | 7 (11) | 2 (10) | 5 (15) | — | |
| Other | 3 (5) | — | 3 (9) | — | |
| ECOG performance status, n (%) | |||||
| 0-2 | 43 (69) | 17 (85) | 23 (68) | 3 (38) | .05 |
| ≥3 | 19 (31) | 3 (15) | 11 (32) | 5 (62) | |
| HIV characteristics at MCD diagnosis | |||||
| Time from HIV diagnosis to MCD diagnosis, median (IQR), y | 4.5 (0.7, 11.5) | 12.8 (3.9, 18.9) | 2.8 (0.4, 7) | 3.1 (0.3, 8.4) | .002 |
| CD4 T-cell count, median (IQR), cells/µL | 180 (100, 302) | 227 (114, 500) | 126 (88, 246) | 252 (180, 371) | .14 |
| HIV viral load at MCD diagnosis, median (IQR), copies/mL | <50 (<50, 2599) | <50 (<50, 563) | 376 (<50, 2599) | 3648 (<50, 30773) | .77 |
| On ART, n (%) | 60 (97) | 18 (90) | 34 (100) | 8 (100) | .20 |
| KSHV-MCD characteristics | |||||
| Rituximab therapy prior to enrollment, n (%) | 20 (32) | 11 (55) | 7 (20) | 2 (25) | .04 |
| Number of flares treated, median (range) | 2 (1-5) | 2 (1-5) | 2 (1-5) | 2 (1-4) | .17 |
| Diagnosis of MCD made at NCI, n (%) | 27 (44) | 7 (35) | 15 (44) | 5 (63) | .39 |
| KS characteristics | |||||
| Any KS, n (%) | 41 (66) | — | 34 (100) | 7 (88) | .19 |
| T1 stage KS, n (%) of all patients with KS | 27 (66) | — | 20 (59) | 7 (100) | .22 |
| Time from KS diagnosis to MCD, median (IQR), mo | 1.3 (0, 40) | — | 0.4 (0, 20) | 40 (0, 60) | .3 |
| Prior chemotherapy for KS, n (%) | 21 (34) | — | 16 (50) | 5 (50) | 1.00 |
| Number of number of prior KS therapies, median (range) | 1 (1-3) | — | 1 (0-3) | 1 (0-2) | 1.00 |
| Baseline characteristics . | All patients (N = 62) . | MCD alone (n = 20) . | MCD with concurrent KS (n = 34) . | MCD with concurrent PEL±KS (n = 8) . | P* . |
|---|---|---|---|---|---|
| Age at MCD diagnosis, median (IQR), y | 44 (38-54) | 46 (44-54) | 41 (46-48) | 46 (29-56) | .07 |
| Sex, n (%) | |||||
| Cisgender male | 58 (94) | 19 (95) | 31 (91) | 8 (100) | 1.00 |
| Cisgender female | 4 (6) | 1 (5) | 3 (9) | 0 | |
| Race, n (%) | .87 | ||||
| White | 31 (50) | 10 (50) | 16 (47) | 5 (62) | |
| Hispanic | 5 (8) | 2 (10) | 3 (9) | — | |
| Black | 16 (26) | 6 (30) | 7 (20) | 3 (38) | |
| African immigrant | 7 (11) | 2 (10) | 5 (15) | — | |
| Other | 3 (5) | — | 3 (9) | — | |
| ECOG performance status, n (%) | |||||
| 0-2 | 43 (69) | 17 (85) | 23 (68) | 3 (38) | .05 |
| ≥3 | 19 (31) | 3 (15) | 11 (32) | 5 (62) | |
| HIV characteristics at MCD diagnosis | |||||
| Time from HIV diagnosis to MCD diagnosis, median (IQR), y | 4.5 (0.7, 11.5) | 12.8 (3.9, 18.9) | 2.8 (0.4, 7) | 3.1 (0.3, 8.4) | .002 |
| CD4 T-cell count, median (IQR), cells/µL | 180 (100, 302) | 227 (114, 500) | 126 (88, 246) | 252 (180, 371) | .14 |
| HIV viral load at MCD diagnosis, median (IQR), copies/mL | <50 (<50, 2599) | <50 (<50, 563) | 376 (<50, 2599) | 3648 (<50, 30773) | .77 |
| On ART, n (%) | 60 (97) | 18 (90) | 34 (100) | 8 (100) | .20 |
| KSHV-MCD characteristics | |||||
| Rituximab therapy prior to enrollment, n (%) | 20 (32) | 11 (55) | 7 (20) | 2 (25) | .04 |
| Number of flares treated, median (range) | 2 (1-5) | 2 (1-5) | 2 (1-5) | 2 (1-4) | .17 |
| Diagnosis of MCD made at NCI, n (%) | 27 (44) | 7 (35) | 15 (44) | 5 (63) | .39 |
| KS characteristics | |||||
| Any KS, n (%) | 41 (66) | — | 34 (100) | 7 (88) | .19 |
| T1 stage KS, n (%) of all patients with KS | 27 (66) | — | 20 (59) | 7 (100) | .22 |
| Time from KS diagnosis to MCD, median (IQR), mo | 1.3 (0, 40) | — | 0.4 (0, 20) | 40 (0, 60) | .3 |
| Prior chemotherapy for KS, n (%) | 21 (34) | — | 16 (50) | 5 (50) | 1.00 |
| Number of number of prior KS therapies, median (range) | 1 (1-3) | — | 1 (0-3) | 1 (0-2) | 1.00 |
ART, antiretroviral therapy.
P value comparisons of 3 groups were determined by Mehta’s modification to Fisher’s exact test and by exact Kruskal-Wallis or exact Wilcoxon rank sum test as appropriate.
At study entry, the Eastern Cooperative Oncology Group (ECOG) performance status was 0-2 in 69% and ≥3 in 31% of all participants. With respect to HIV characteristics, the median time from HIV diagnosis to MCD diagnosis was 12.8 years for those with MCD alone, whereas among those with MCD+KS and MCD+PEL±KS, the time from HIV diagnosis to MCD was 2.8 years and 3.1 years, respectively (P = .002). All participants except 2 with MCD alone were on ART at the time of study entry. At MCD diagnosis the, median CD4 T-cell count among all participants was 180 cells/µL and the median HIV VL was <50 copies/mL. Both CD4 and HIV VL did not vary among the 3 patient groups.
Among all participants, 27 (44%) of all KSHV-MCD diagnoses had not been made on referral and were only made upon evaluation at our center. Those with KSHV-MCD alone were often referred for lymphadenopathy, pancytopenia, fevers, and/or night sweats; referrers frequently were considering the possibility of lymphoma. Nearly all participants with MCD+PEL±KS and MCD+KS presented with KS and/or effusions in addition to lymphadenopathy, fevers, and night sweats. In these cases, referrers were often concerned about progressive KS despite systemic KS treatment and well-controlled HIV. In 17 out of the 27 participants diagnosed with KSHV-MCD at our center (including 11 with concurrent KS), KSHV-MCD was diagnosed from a lymph node biopsy performed at our center. In the remaining 10 participants, review by NCI pathologists identified a diagnosis of KSHV-MCD on a biopsy performed elsewhere in which KSHV-MCD had not been diagnosed. Of the 35 participants with MCD diagnosed before referral, 20 (57% or 32% of all participants) had prior rituximab therapy at study entry, including 55% of those with MCD alone, 20% of those with MCD+KS, and 25% of those with MCD+PEL±KS (P = .04).
KSHV-MCD protocol treatments and PFS
Forty-four participants (71%) were administered ≥1 treatment for KSHV-MCD over the course of their enrollment in this protocol, and 8 additional participants with concurrent PEL received infusional (DA)EPOCH-R therapy either within this study or within another ongoing investigational study (NCT02911142). Two participants with severe KSHV-MCD and multiorgan dysfunction requiring admission to the intensive care unit were administered (DA)EPOCH-R. Among those with either MCD alone or concurrent KS, 17 participants were initially treated with AZT/VGC and 8 participants received rituximab alone (Table 2). Thirty-six participants received R-Dox chemotherapy; this was initial therapy in 61% of cases, and the majority of participants treated with this regimen had MCD+KS. Participants who initially received R-Dox alone or with maintenance therapy on study had a median hemoglobin 9.3 g/dL and median CRP 108 mg/L, and 27% of these patients had an ECOG 4 (supplemental Table 3), highlighting the severity of their illness. Twenty participants had maintenance therapy with either AZT/VGC or IFN-α following R-Dox (Table 2). The median duration of treatment leading to remission was 5 months for participants receiving AZT/VGC and 2 months for those receiving R-Dox alone. Those treated with R-Dox and IFN-α received a median of 7 months therapy (including a median of 4 months maintenance), and those treated with R-Dox received a median of 11 months therapy (including a median of 9 months maintenance).
Treatments received on study for KSHV-MCD alone or with concurrent KS, median duration of treatment, and 5-year PFS
| Treatment . | Number of patients receiving protocol treatment . | Median duration of treatment leading to KSHV-MCD remission, mo . | 5-y PFS, % (95% CI) . | P† . | |||
|---|---|---|---|---|---|---|---|
| All patients treated . | MCD alone . | MCD with concurrent KS . | As first regimen on protocol (%)* . | ||||
| AZT/VGC | 17 | 6 | 11 | 17 (100) | 5 | 26 (8, 49) | — |
| Rituximab alone | 8 | 6 | 2 | 5 (63) | 1 | 71 (26, 92) | .03 |
| Rituximab and liposomal doxorubicin | 36 | 7 | 29 | 22 (61) | 5 | 73 (53, 86) | — |
| No maintenance | 16 | 1 | 15 | 13 (81) | 2 | 62 (23, 85) | .02 |
| AZT/VGC maintenance | 10 | 5 | 5 | 7 (70) | 11 (9 mo of AZT/VGC in maintenance phase) | 87 (39, 98) | .0009 |
| IFN-α maintenance | 10 | 1 | 9 | 2 (20) | 7 (4 mo of IFN-α in maintenance phase) | 70 (33, 89) | .01 |
| Treatment . | Number of patients receiving protocol treatment . | Median duration of treatment leading to KSHV-MCD remission, mo . | 5-y PFS, % (95% CI) . | P† . | |||
|---|---|---|---|---|---|---|---|
| All patients treated . | MCD alone . | MCD with concurrent KS . | As first regimen on protocol (%)* . | ||||
| AZT/VGC | 17 | 6 | 11 | 17 (100) | 5 | 26 (8, 49) | — |
| Rituximab alone | 8 | 6 | 2 | 5 (63) | 1 | 71 (26, 92) | .03 |
| Rituximab and liposomal doxorubicin | 36 | 7 | 29 | 22 (61) | 5 | 73 (53, 86) | — |
| No maintenance | 16 | 1 | 15 | 13 (81) | 2 | 62 (23, 85) | .02 |
| AZT/VGC maintenance | 10 | 5 | 5 | 7 (70) | 11 (9 mo of AZT/VGC in maintenance phase) | 87 (39, 98) | .0009 |
| IFN-α maintenance | 10 | 1 | 9 | 2 (20) | 7 (4 mo of IFN-α in maintenance phase) | 70 (33, 89) | .01 |
Values shown are the number of patients treated as the first regimen on protocol; “%” indicates first regimen on the protocol among all patients treated with that regimen.
P values are log-rank tests of PFS comparing AZT/VGC with other KSHV-MCD regimens used that are adjusted for multiple comparisons (Bonferroni test). Patients who received (DA)EPOCH±R (8 patients) are not included in this table.
Those who received AZT/VGC had a 26% 5-year PFS (95% confidence interval [CI], 8% to 49%) (Table 2). Participants who received R-Dox without maintenance had a 62% 5-year PFS (95% CI, 23% to 85%), while participants who received R-Dox and maintenance AZT/VGC had a 5-year PFS of 87% (95% CI, 39% to 98%; supplemental Figure 1). Comparing the other KSHV-MCD regimens to AZT/VGC and adjusting for multiple comparisons, rituximab alone and R-Dox with or without maintenance had higher 5-year PFS (Table 2). Of the 17 participants initially treated with AZT/VGC, 4 participants required no further treatment of KSHV-MCD, and 13 participants were subsequently treated with rituximab alone (2 participants) or R-Dox with maintenance regimens for a subsequent flare.
Following rituximab-based treatment on the study, 28 of 44 participants experienced no further KSHV-MCD flare. Four participants had 1 subsequent flare, and 1 patient had 2 flares; all of these resolved with repeated treatment with R-Dox. Twelve participants with MCD+KS treated with rituximab alone or R-Dox resulting in KSHV-MCD remission received additional treatment of KS with liposomal doxorubicin, paclitaxel, or experimental protocols within our group due to stable or progressive KS. Overall, 7 participants with MCD+KS had KS progression during or soon after therapy for MCD. One participant had progressive KS during R-Dox; in another case, KS progression was seen during AZT/VGC maintenance; and in the remaining 5 participants, KS progression was noted within 6 weeks of rituximab cessation.
Laboratory, cytokine, and KSHV-VL profiles in KSHV-MCD and concurrent KSHV-associated conditions
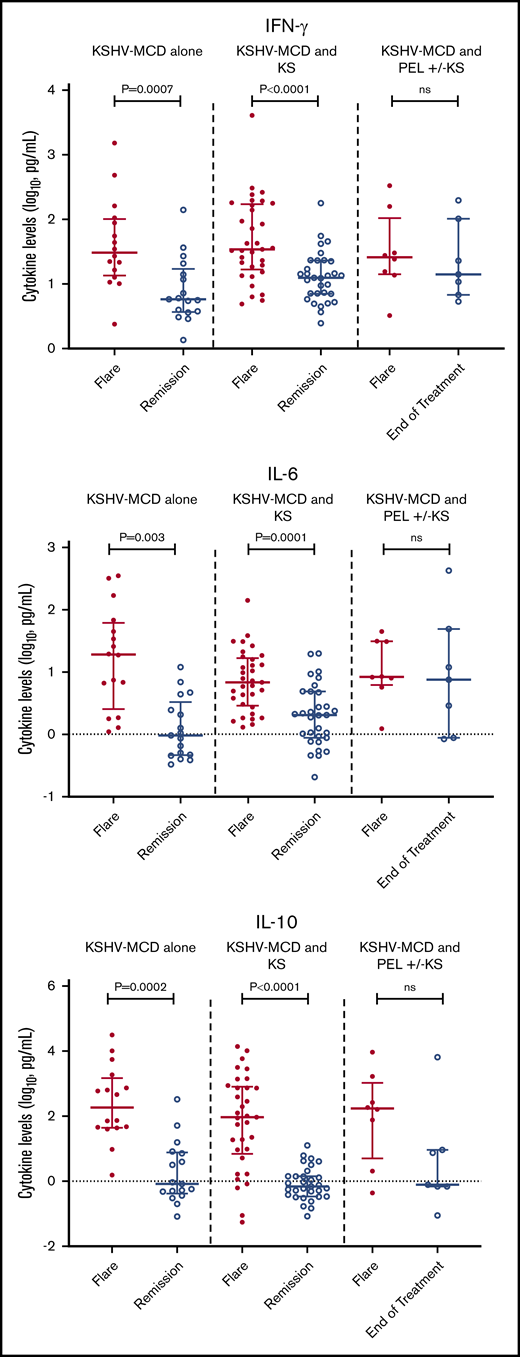
Laboratory findings that are characteristic of MCD flares, including anemia, thrombocytopenia, hypoalbuminemia, and elevated CRP, were seen among all participants (Table 3). Seven cytokines (IFN-γ, IL-10, IL-1β, IL-4, IL-6, IL-8, and TNF-α) were elevated among all participants with MCD with or without concurrent KSHV-associated diseases during flares when compared with median normal serum values.32 There were no statistically significant differences in any of the cytokine or laboratory values during flares between MCD alone as compared with MCD+KS or MCD+PEL±KS (Table 3). Among all participants, 6 cytokines (IFN-γ, IL-10, IL-13, IL-4, IL-6, and TNF-α) decreased significantly from flare to remission/end of treatment (supplemental Table 4). Interestingly, while IL-13 levels were within normal range during flares, levels decreased from flare to remission. Among the cytokines most associated with KSHV-MCD pathogenesis, there was a significant decrease from flare to remission in IL-10, IL-6, and IFN-γ for participants with MCD+KS or MCD alone. However, these changes were not seen among those with MCD+PEL±KS at the end of treatment (Figure 1).
Laboratory findings and cytokines of interest between diseases during first KSHV-MCD flare on protocol
| . | Normal range or normal median (range)* . | All patients . | MCD alone . | MCD with concurrent KS . | P† . | MCD with concurrent PEL ± KS . | P† . |
|---|---|---|---|---|---|---|---|
| Flare laboratory findings | |||||||
| Hemoglobin, g/dL | 13.7-17.5 | 9.5 (8.0, 11.5) | 10.3 (8, 11.3) | 9.5 (7.9, 11.5) | .83 | 9.2 (8.4, 11.5) | .97 |
| Platelets, ×103/µL | 161-347 | 117 (59, 253) | 90 (37, 344) | 118 (70 184) | .93 | 195 (55, 347) | .94 |
| Albumin, g/dL | 3.5-5.2 | 2.7 (2.2, 3.3) | 2.7 (2.1, 3.4) | 2.9 (2.4, 3.4) | .67 | 2.5 (1.5, 3.0) | .24 |
| CRP, mg/L | 0-4.99 | 88.1 (19.3, 144) | 97 (27.1, 195) | 101.2 (14.5, 144) | .56 | 70.6 (50, 88.1) | .38 |
| Flare, median (IQR) | |||||||
| IFN-γ, pg/mL | 3.77 (0.64-14.4) | 32.9 (15.6, 139.6) | 30.9 (14.6, 97) | 34.3 (18.4, 165) | .74 | 26.0 (14.6, 94.3) | .79 |
| IL-10, pg/mL | 0.2 (0.06-3.08) | 100.8 (18.7, 730) | 265.6 (44.4, 1290) | 92.6 (9.2, 730) | .36 | 171.6 (39, 958.4) | .79 |
| IL-12, pg/mL | 0.29 (0.26-0.38) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.5) | 0.1 (0.1, 0.3) | .42 | 0.2 (0.1, 0.3) | .46 |
| IL-13, pg/mL | 1.65 (0.6-2.78) | 0.5 (0.1, 0.9) | 0.6 (0.1, 1) | 0.5 (0.1, 1) | .79 | 0.5 (0.1, 0.8) | .53 |
| IL-1β, pg/mL | 0.16 (0.11-24.3) | 0.3 (0.2, 0.6) | 0.3 (0.1, 0.6) | 0.3 (0.2, 0.6) | .88 | 0.4 (0.2, 0.6) | .88 |
| IL-2, pg/mL | 0.52 (0.22-2.68) | 0.6 (0.4, 0.9) | 0.6 (0.4, 1) | 0.4 (0.3, 0.9) | .68 | 0.8 (0.4, 1.2) | .42 |
| IL-4, pg/mL | Not detectable | 0.08 (0.06, 0.14) | 0.1 (0.06, 0.20) | 0.08 (0.05, 0.1) | .26 | 0.07 (0.06, 0.1) | .32 |
| IL-6, pg/mL | 0.47 (0.16-27.2) | 8 (3.8, 25.8) | 19 (4.2, 56.3) | 6.8 (3.0, 15.9) | .12 | 8.3 (6.8, 31.0) | .74 |
| IL-8, pg/mL | 0.47 (0.16-27.2) | 63.1 (19.3, 107.4) | 86.1 (18.7, 157.9) | 50 (19.3, 91.3) | .32 | 78.5 (28.8, 117.1) | .65 |
| TNF-α, pg/mL | 0.36 (0.10-1.75) | 9 (6.5, 12.6) | 9.4 (6.2, 12.4) | 8.7 (7.1, 13.1) | .89 | 8.4 (6.5, 16.4) | .79 |
| KSHV-VL, log10 copies/106 PBMCs | NA | 3.8 (3.0, 5.1) | 3.9 (3.0, 5.2) | 3.9 (3.2, 5) | .78 | 3.7 (1.1, 4.2) | .65 |
| . | Normal range or normal median (range)* . | All patients . | MCD alone . | MCD with concurrent KS . | P† . | MCD with concurrent PEL ± KS . | P† . |
|---|---|---|---|---|---|---|---|
| Flare laboratory findings | |||||||
| Hemoglobin, g/dL | 13.7-17.5 | 9.5 (8.0, 11.5) | 10.3 (8, 11.3) | 9.5 (7.9, 11.5) | .83 | 9.2 (8.4, 11.5) | .97 |
| Platelets, ×103/µL | 161-347 | 117 (59, 253) | 90 (37, 344) | 118 (70 184) | .93 | 195 (55, 347) | .94 |
| Albumin, g/dL | 3.5-5.2 | 2.7 (2.2, 3.3) | 2.7 (2.1, 3.4) | 2.9 (2.4, 3.4) | .67 | 2.5 (1.5, 3.0) | .24 |
| CRP, mg/L | 0-4.99 | 88.1 (19.3, 144) | 97 (27.1, 195) | 101.2 (14.5, 144) | .56 | 70.6 (50, 88.1) | .38 |
| Flare, median (IQR) | |||||||
| IFN-γ, pg/mL | 3.77 (0.64-14.4) | 32.9 (15.6, 139.6) | 30.9 (14.6, 97) | 34.3 (18.4, 165) | .74 | 26.0 (14.6, 94.3) | .79 |
| IL-10, pg/mL | 0.2 (0.06-3.08) | 100.8 (18.7, 730) | 265.6 (44.4, 1290) | 92.6 (9.2, 730) | .36 | 171.6 (39, 958.4) | .79 |
| IL-12, pg/mL | 0.29 (0.26-0.38) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.5) | 0.1 (0.1, 0.3) | .42 | 0.2 (0.1, 0.3) | .46 |
| IL-13, pg/mL | 1.65 (0.6-2.78) | 0.5 (0.1, 0.9) | 0.6 (0.1, 1) | 0.5 (0.1, 1) | .79 | 0.5 (0.1, 0.8) | .53 |
| IL-1β, pg/mL | 0.16 (0.11-24.3) | 0.3 (0.2, 0.6) | 0.3 (0.1, 0.6) | 0.3 (0.2, 0.6) | .88 | 0.4 (0.2, 0.6) | .88 |
| IL-2, pg/mL | 0.52 (0.22-2.68) | 0.6 (0.4, 0.9) | 0.6 (0.4, 1) | 0.4 (0.3, 0.9) | .68 | 0.8 (0.4, 1.2) | .42 |
| IL-4, pg/mL | Not detectable | 0.08 (0.06, 0.14) | 0.1 (0.06, 0.20) | 0.08 (0.05, 0.1) | .26 | 0.07 (0.06, 0.1) | .32 |
| IL-6, pg/mL | 0.47 (0.16-27.2) | 8 (3.8, 25.8) | 19 (4.2, 56.3) | 6.8 (3.0, 15.9) | .12 | 8.3 (6.8, 31.0) | .74 |
| IL-8, pg/mL | 0.47 (0.16-27.2) | 63.1 (19.3, 107.4) | 86.1 (18.7, 157.9) | 50 (19.3, 91.3) | .32 | 78.5 (28.8, 117.1) | .65 |
| TNF-α, pg/mL | 0.36 (0.10-1.75) | 9 (6.5, 12.6) | 9.4 (6.2, 12.4) | 8.7 (7.1, 13.1) | .89 | 8.4 (6.5, 16.4) | .79 |
| KSHV-VL, log10 copies/106 PBMCs | NA | 3.8 (3.0, 5.1) | 3.9 (3.0, 5.2) | 3.9 (3.2, 5) | .78 | 3.7 (1.1, 4.2) | .65 |
NA, not available.
For cytokines, median and normal range are from 27 normal serum human samples tested in the proinflammatory panel 1 (human) kit.32
P values are for comparisons with MCD alone.
Difference between flare and remission in IFN-γ, IL-6, and IL-10 in patients with MCD alone, MCD with KS, or MCD and PEL with or without KS.
Difference between flare and remission in IFN-γ, IL-6, and IL-10 in patients with MCD alone, MCD with KS, or MCD and PEL with or without KS.
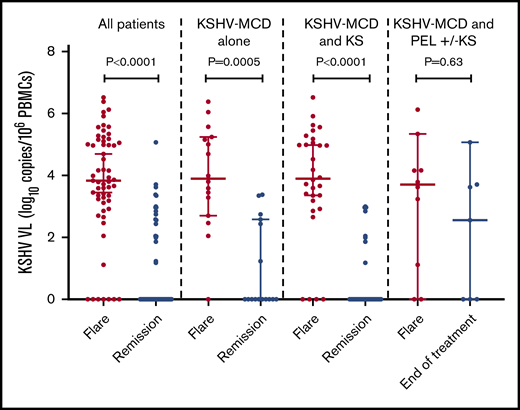
The median circulating KSHV VL among all participants during flares was 3.8 log10 copies/106 PBMCs (interquartile range [IQR], 3.0; 5.1 log10 copies/106 PBMCs), which decreased significantly following remission (P < .0001; supplemental Table 4; Figure 2). There was no difference in the KSHV-VL levels during flares between participants with KSHV-MCD alone compared with participants with either MCD+KS or MCD+PEL±KS (Table 3). KSHV-VL levels decreased substantially from flare to remission for participants with MCD alone (P = .0005) and MCD+KS (P < .0001), but not from flare to end of treatment of those with MCD+PEL±KS (Figure 2).
KSHV-VL differences in the first flare and remission or end of treatment (patients with concurrent PEL) in all patients, patients with MCD alone, and patients with MCD and KS. Among patients with MCD and PEL with or without KS, this was the difference between flare to the end of multiagent chemotherapy for PEL.
KSHV-VL differences in the first flare and remission or end of treatment (patients with concurrent PEL) in all patients, patients with MCD alone, and patients with MCD and KS. Among patients with MCD and PEL with or without KS, this was the difference between flare to the end of multiagent chemotherapy for PEL.
Survival outcomes and prognostic factors
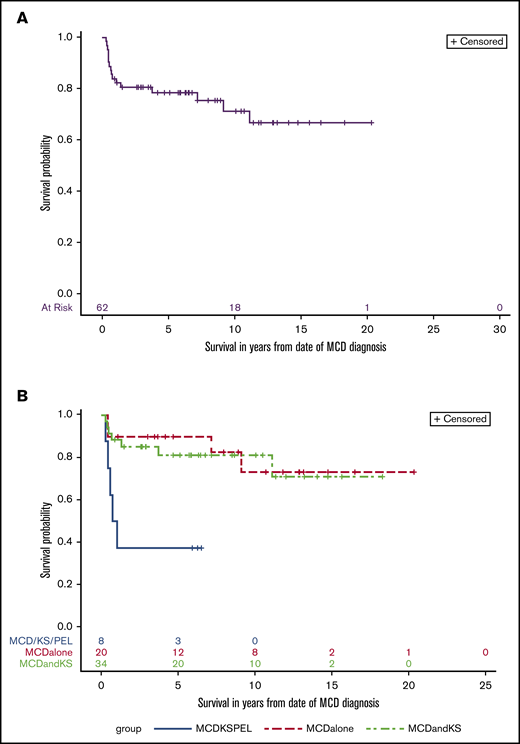
The median follow-up time for all participants on this study was 5.9 years, and over the course of this study, 16 participants died. The 10-year overall survival for all participants was 71% (95% CI, 56% to 82%; Figure 3A). There were statistically significant differences in the comparisons among the 3 subgroups with respect to overall survival (log-rank P = .0041; Figure 3B). A concurrent diagnosis of PEL negatively impacted survival (hazard ratio [HR], 5.4; 95% CI, 1.8 to 16.8; P = .003); participants with MCD+PEL±KS had a 5-year overall survival of 38% (95% CI, 9% to 67%), and 10-year survival was not reached. Among those with MCD alone, 5-year overall survival was 90% (95% CI, 66 to 97), and the 10-year survival was 73% (95% CI, 42% to 90%); for those with MCD+KS, the 10-year survival was 81% (95% CI, 63% to 91%). In the follow-up period, 2 participants with MCD+KS (both initially treated with R-Dox) were diagnosed with PEL 6 and 12 years after their MCD diagnosis. Both participants are alive more than 2 years after treatment using (DA)EPOCH-R with lenalidomide (NCT02911142).
Overall survival in KSHV-MCD. Kaplan-Meier curve of all patients (A) and by group (B) log-rank test (P = .004).
Overall survival in KSHV-MCD. Kaplan-Meier curve of all patients (A) and by group (B) log-rank test (P = .004).
In addition to concurrent diagnosis of PEL, concurrent stage T1 KS (HR, 3.0; 95% CI, 1.1 to 8.4; P = .035) also impacted overall survival (Table 4). However, those with T1 KS without PEL did not have increased mortality (HR, 1.5; 95% CI, 0.6 to 4.3; P = .4), suggesting that most of the effect of T1 KS on survival was due to its association with PEL. None of the inflammatory cytokine levels measured during flares were prognostic overall, nor was KSHV-VL.
Univariate Cox proportional hazard model for overall survival
| Variable . | HR (95% CI) . | P . |
|---|---|---|
| Age (above vs below median) | 1.8 (0.7-5.0) | .25 |
| CD4 count (<100 vs ≥100) | 0.5 (0.2-1.4) | .18 |
| HIV VL (<100 vs ≥100) | 1.3 (0.3-5.3) | .70 |
| Concurrent KS | 1.8 (0.6-5.7) | .30 |
| Concurrent T1 KS | 3.0 (1.1-8.4) | .035 |
| T1 KS without PEL | 1.5 (0.6-4.3) | .40 |
| Concurrent PEL | 5.4 (1.8-16.8) | .003 |
| KSHV VL, log10 copies/106 PBMCs (above vs below median) | 1.1 (0.4-3.2) | .85 |
| Cytokines (above vs below median), pg/mL | ||
| IFN-γ | 0.7 (0.3-1.9) | .50 |
| IL-10 | 0.8 (0.3-2.1) | .60 |
| IL-12 | 1.1 (0.4-3.0) | .86 |
| IL-13 | 1.0 (0.4-2.9) | .93 |
| IL-1β | 0.7 (0.3-2.0) | .52 |
| IL-2 | 1.5 (0.5-4.3) | .41 |
| IL-4 | 1.4 (0.5-4.0) | .51 |
| IL-6 | 2.1 (0.7-6.2) | .17 |
| IL-8 | 1.1 (0.4-3.1) | .85 |
| TNF-α | 0.9 (0.3-2.5) | .83 |
| Variable . | HR (95% CI) . | P . |
|---|---|---|
| Age (above vs below median) | 1.8 (0.7-5.0) | .25 |
| CD4 count (<100 vs ≥100) | 0.5 (0.2-1.4) | .18 |
| HIV VL (<100 vs ≥100) | 1.3 (0.3-5.3) | .70 |
| Concurrent KS | 1.8 (0.6-5.7) | .30 |
| Concurrent T1 KS | 3.0 (1.1-8.4) | .035 |
| T1 KS without PEL | 1.5 (0.6-4.3) | .40 |
| Concurrent PEL | 5.4 (1.8-16.8) | .003 |
| KSHV VL, log10 copies/106 PBMCs (above vs below median) | 1.1 (0.4-3.2) | .85 |
| Cytokines (above vs below median), pg/mL | ||
| IFN-γ | 0.7 (0.3-1.9) | .50 |
| IL-10 | 0.8 (0.3-2.1) | .60 |
| IL-12 | 1.1 (0.4-3.0) | .86 |
| IL-13 | 1.0 (0.4-2.9) | .93 |
| IL-1β | 0.7 (0.3-2.0) | .52 |
| IL-2 | 1.5 (0.5-4.3) | .41 |
| IL-4 | 1.4 (0.5-4.0) | .51 |
| IL-6 | 2.1 (0.7-6.2) | .17 |
| IL-8 | 1.1 (0.4-3.1) | .85 |
| TNF-α | 0.9 (0.3-2.5) | .83 |
Of the 16 participants who died, 11 died of their KSHV-associated diseases (supplemental Table 5). Among participants with MCD+KS, 2 participants died of pulmonary KS despite combination of rituximab and chemotherapy for KS, and 2 died of multiorgan failure. All 5 deaths in participants with PEL were from refractory disease. Four participants (2 with MCD alone and 2 with MCD+KS) died >5 years after their treatment from other causes; 1 patient died of pancreatic cancer, 1 patient died of a hyperglycemic state due to uncontrolled diabetes mellitus, and 2 participants died at home of unclear causes; an autopsy could not be performed in these cases.
Discussion
This long-term study of a US-based cohort of 62 participants KSHV-MCD provides important observations about the pathogenesis and natural history of KSHV-MCD, the cytokine characteristics of participants with KSHV-MCD and other KSHV-associated diseases, and long-term responses to various therapies. The long-term outcomes were generally favorable, with an overall survival of 71% at 10 years for all participants. Deaths in this cohort were most commonly due to a treatment-refractory KSHV-associated malignancy. In these analyses, a concurrent diagnosis of PEL was associated with increased mortality; increased mortality in participants with T1 KS was driven by its association with PEL. Oksenhendler et al estimated that the 2-year probability of developing non-Hodgkin lymphoma among individuals with MCD was 24%.12 Among participants with MCD alone or MCD+KS, only 2 of the 54 participants developed PEL 6 and 12 years after their initial MCD diagnosis, suggesting lower rates of PEL onset in this cohort. These 2 individuals and the majority of participants on this study received rituximab alone or in combination with chemotherapy, which is hypothesized to decrease the risk of lymphoma onset in this population.12,20 In general, KSHV-MCD outcomes in this study are similar to retrospective analyses from European centers.16,20 The 5-year survival among participants with KSHV-MCD and a diagnosis of PEL was 38%, which is better than earlier reports in the literature.33,34
Comparison between KSHV-MCD therapies demonstrated that participants treated with R-Dox and maintenance AZT/VGC had the highest 5-year PFS and that participants with R-Dox, with or without maintenance had better PFS than AZT/VGC given as initial therapy. A limitation of this comparison is that these therapies were not randomized and many had received prior therapy. However, the participants treated with R-Dox tended to be sicker than those treated with AZT/VGC. The overall benefit of maintenance after rituximab therapy with AZT/VGC is not clear at this point; while this led to higher 5-year PFS, this option needs to be weighed against the potential toxicities associated with AZT/VGC and the high salvage rates following retreatment with rituximab alone.20
Compared with normal values, 7 cytokines (IFN-γ, IL-10, IL-1β, IL-4, IL-6, IL-8, and TNF-α) were increased during flares in all participants with KSHV-MCD. Elevated KSHV-VL levels, TNF-α, IL-10, IL-6, and v-IL6 were observed in an earlier study of 21 participants within this KSHV-MCD cohort.7 In addition to KSHV-VL, IFN-γ, TNF-α, IL-10, and IL-6, we also noted statistically significant decreases in IL-4 and IL-13 from flares to remission. These cytokines implicate the potential role of the JAK-STAT pathway in KSHV-MCD,35-37 which is activated by vIL-6.38 We did not identify distinct cytokine or KSHV-VL differences between different groups during flares that would aid diagnosis of concurrent KSHV diseases. Having >1 KSHV-associated disease did not significantly alter the flare cytokine profiles. These similarities contribute to making the diagnosis of these disorders challenging and further reinforce the view that these conditions are part of a spectrum of KSHV-associated diseases with similar cytokine dysregulation.
This study also highlights the importance of considering a diagnosis of KSHV-MCD in people living with HIV in populations with a high KSHV seroprevalence or among patients with HIV-associated KS who have unexplained inflammatory syndromes. Forty-four percent of all KSHV-MCD diagnoses were made at our center; the majority were referred for worsening or persistent KS. Despite well-controlled HIV and higher CD4 counts, these individuals had more than one KSHV-associated disorder. From an HIV perspective, >95% of the participants were on ART at the time of an MCD diagnosis and were virologically suppressed, with a CD4 count >100. In the present study, 66% of KSHV-MCD participants had KS, which is consistent with previous studies showing that 54% to 72% of KSHV-MCD participants have concurrent KS.9,12 However, it is unclear how many patients with KS have concurrent MCD, and this requires further study. While KS is a prominent cutaneous tumor that is generally not difficult to recognize, the diagnosis of KSHV-MCD (with or without PEL) requires a high level of suspicion, invasive diagnostic procedures, and specialist pathology review. Moreover, the inflammatory signs and symptoms of KSHV-MCD can mimic a number of other diseases that occur in people living with HIV, such as lymphoma, uncontrolled HIV, or infections, such as tuberculosis. Based on our experience, it is particularly important to consider KSHV-MCD in patients with HIV infection with or without KS who experience fevers, fatigue, effusions, edema, and laboratory abnormalities such as cytopenias, hypoalbuminemia, CRP elevations, or circulating KSHV levels. Additionally, concurrent PEL should be considered if these symptoms (particularly effusions) persist despite treatment of KSHV-MCD with rituximab-based therapies.
These study findings may be applicable beyond the United States. Eleven percent of the participants were from sub-Saharan Africa. While KSHV-infection and KS are common in many parts of sub-Saharan Africa,2 there are very few reported cases of KSHV-MCD in this region, and it is likely that many cases are misdiagnosed as tuberculosis or other infections. Where KSHV-MCD has been diagnosed in sub-Saharan Africa, patients presented with severe symptoms, and high mortality rates were reported.39,40 This reinforces the need for physicians to look for KSHV-MCD and other KSHV-associated diseases in systemically unwell patients with concurrent HIV infection.
This is the largest single-center cohort of participants treated in the United States for KSHV-MCD with or without other KSHV-associated diseases and the first to compare patients with KSHV-MCD alone and concurrent KSHV-associated diseases. A possible limitation of this study is referral bias, as some of these participants were treated in other institutions for KSHV-MCD or KS prior to referral, which may have impacted our observations of the frequency of comorbid KSHV-associated diseases, cytokine profiles, or survival outcomes. Furthermore, the treatments offered for KSHV-MCD in this study were not randomized; therefore, differences in treatment-specific PFS outcomes should be considered hypothesis generating. Despite these limitations, this study conducted over a 16-year period provides valuable insights on the pathogenesis of KSHV-MCD, PEL, and KS.
In summary, KSHV-MCD remains an important and underdiagnosed condition among people living with HIV on ART. It often coexists with other KSHV-associated diseases, which can pose challenges in diagnosis and therapy. A high index of suspicion and proper diagnosis of KSHV-MCD and associated diseases is important, as these patients can have a good prognosis if appropriately treated.
A part of this study was presented at the 26th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 4-7 March 2019.
For original data, please e-mail ramya.ramaswami@nih.gov and robert.yarchoan@nih.gov.
Acknowledgments
The authors thank the individuals who volunteered for this study and their families. They thank the nurses, physicians, and patient care and clinical research support staff at the NCI and the National Institutes of Health Clinical Center, as well as the physicians who referred participants. They also thank Randy Stevens and other members of the Leidos AIDS Monitoring Laboratory.
This work was supported by the Intramural Research Program of the NCI, National Institutes of Health, and in part by the Intramural Program of the NCI, National Institutes of Health, Department of Health and Human Services. This project has also been funded in part with federal funds from the NCI, National Institutes of Health (75N91019D00024 and HHSN261200800001E).
Authorship
Contribution: R.F.L., T.S.U., M.N.P., and R.Y. designed the study; A.W., I.E., R.M., V.A.M., D.W., K.W., A.R., J.G., and R.R. collected and analyzed the data; E.S.J., H.-W.W., and S.P. confirmed the diagnosis of MCD by pathology; I.E., R.M., A.W., K.W., P.H.G., T.S.U., R.R., K.L., R.F.L, M.N.P, and R.Y. cared for the participants on the study; S.M.S. analyzed data and provided statistical support; and R.R. and R.Y. wrote the manuscript.
Conflict-of-interest disclosure: R.R., T.S.U., K.L., and R.Y. report receiving research support from Celgene through a cooperative research and development agreement (CRADA) at the NCI. R.R., T.S.U., K.L., and R.Y. report receiving a drug for a clinical trial from Merck through a CRADA with the NCI. R.R., K.L., and R.Y. report receiving a drug for a clinical trial from EMD-Serano through a CRADA at the NCI. R.Y. reports receiving a drug for preclinical studies from Janssen and CTI BioPharma. T.S.U. reports receiving other commercial research support from Roche through a clinical trial agreement with Fred Hutchinson Cancer Research Center. T.S.U., R.Y., and D.W. are co-inventors on US Patent 10 001,483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds, and uses of biomarkers.” R.Y. is also a coinventor on patents on a peptide vaccine for HIV and the treatment of Kaposi sarcoma with IL-12, and an immediate family member of R.Y. is a co-inventor on patents related to internalization of target receptors, KSHV vIL-6, and the use of calreticulin and calreticulin fragments to inhibit angiogenesis. All rights, title, and interest to these patents have been or should by law be assigned to the US Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). The remaining authors declare no competing financial interests.
Correspondence: Ramya Ramaswami, HIV/AIDS Malignancy Branch, Center for Cancer Research, 10 Center Dr, 6N106, Bethesda, MD 20892; e-mail: ramya.ramaswami@nih.gov.
References
Author notes
The full-text version of this article contains a data supplement.