Key Points
ASCT after PD-1-blockade resulted in very favorable outcomes among high-risk patients with multiply relapsed/refractory HL.
Response to PD-1 blockade, and not prior chemosensitivity, best predicted post-ASCT PFS in this cohort.
Abstract
Autologous stem cell transplantation (ASCT) can be curative for patients with relapsed/refractory Hodgkin lymphoma (HL). Based on studies suggesting that anti-PD-1 monoclonal antibodies (mAbs) can sensitize patients to subsequent chemotherapy, we hypothesized that anti-PD-1 therapy before ASCT would result in acceptable outcomes among high-risk patients who progressed on or responded insufficiently to ≥1 salvage regimen, including chemorefractory patients who are traditionally considered poor ASCT candidates. We retrospectively identified 78 HL patients who underwent ASCT after receiving an anti-PD-1 mAb (alone or in combination) as third-line or later therapy across 22 centers. Chemorefractory disease was common, including 42 patients (54%) refractory to ≥2 consecutive systemic therapies immediately before anti-PD-1 treatment. Fifty-eight (74%) patients underwent ASCT after anti-PD-1 treatment, while 20 patients (26%) received additional therapy after PD-1 blockade and before ASCT. Patients received a median of 4 systemic therapies (range, 3-7) before ASCT, and 31 patients (41%) had a positive pre-ASCT positron emission tomography (PET) result. After a median post-ASCT follow-up of 19.6 months, the 18-month progression-free survival (PFS) and overall survival were 81% (95% CI, 69-89) and 96% (95% confidence interval [CI], 87-99), respectively. Favorable outcomes were observed for patients who were refractory to 2 consecutive therapies immediately before PD-1 blockade (18-month PFS, 78%), had a positive pre-ASCT PET (18-month PFS, 75%), or received ≥4 systemic therapies before ASCT (18-month PFS, 73%), while PD-1 nonresponders had inferior outcomes (18-month PFS, 51%). In this high-risk cohort, ASCT after anti-PD-1 therapy was associated with excellent outcomes, even among heavily pretreated, previously chemorefractory patients.
Introduction
High-dose chemotherapy and autologous stem cell transplantation (ASCT) can be curative for many patients with relapsed or refractory (R/R) Hodgkin lymphoma (HL),1,2 but outcomes depend on disease status at ASCT. More favorable outcomes are observed for patients with chemosensitive disease, especially those who achieve a complete metabolic response (CMR) on pre-ASCT positron emission tomography (PET).3-12 In contrast, patients who have chemorefractory disease, particularly those who fail to respond to ≥2 lines of salvage therapy, are typically considered poor candidates for ASCT.13-18
The anti-PD-1 monoclonal antibodies (mAbs) nivolumab and pembrolizumab achieve high objective response rates (ORRs) in patients with HL who relapse after or are ineligible for ASCT, including patients with chemorefractory HL. However, extended follow-up from phase 2 trials suggests that a large majority of patients who respond to PD-1 blockade will eventually relapse.19-22 While ASCT has traditionally been reserved for chemosensitive patients, preliminary evidence suggests that treatment with PD-1 blockade may result in higher-than-expected response rates to subsequent cytotoxic therapy.23-29 The mechanism of this effect is not clear but could be related to the immunomodulatory effects of chemotherapy, including reduction in suppressive immune cell populations (ie, regulatory T cells or myeloid-derived suppressor cells), recruitment of tumor-infiltrating immune cells, or augmentation of antigen presentation on tumor cells.30-32 In 2 retrospective studies, high response rates and durable remissions were observed with chemotherapy following PD-1 blockade among heavily pretreated HL patients.23,24 Further supporting the concept of resensitization to chemotherapy, one series reported frequent objective responses (ORR, 80%) for 15 patients who were retreated with a chemotherapy agent that they had received and progressed on prior to PD-1 blockade.23
Based on this rationale, we hypothesized that treatment with anti-PD-1 mAbs may sensitize previously chemorefractory HL patients to subsequent high-dose chemotherapy and ASCT. We identified high-risk HL patients who were treated with anti-PD-1 therapy after receiving at least 2 systemic chemotherapy regimens. In addition, we performed a planned subgroup analysis among the highest risk patients who received ≥3 lines of therapy before PD-1 treatment, many of whom would typically be considered poor candidates for ASCT. Here, we report the outcomes of 78 patients with multiply R/R HL who received anti-PD-1 therapy before ASCT.
Methods
Patients
Twenty-two transplant centers in the United States participated in this retrospective study. Individual centers identified patients with a diagnosis of classic HL who (1) had insufficient responses to proceed to ASCT after ≥2 systemic therapies, (2) were treated with a PD-1 or PD-L1 mAb as third-line or later therapy, and (3) subsequently underwent ASCT before 1 October 2019. Patients could receive an anti-PD-1 mAb as monotherapy or as part of a combination regimen, and patients who received intervening salvage therapy between anti-PD-1 treatment and ASCT were eligible. No patients underwent ASCT prior to anti-PD-1 therapy. In this study, patients were considered to have primary refractory HL if they failed to achieve a complete response (CR) to frontline treatment or relapsed within 90 days of completing therapy. Patients were deemed to be refractory to subsequent therapies if they failed to achieve an objective response (ie, best response was stable disease [SD] or progressive disease [PD]). Patients were considered brentuximab vedotin (BV) refractory if they failed to achieve an objective response to their first BV-containing line of therapy. The study protocol was approved by institutional review boards at all sites, and a waiver of informed consent was granted for this analysis. Response assessment was performed by local investigators according to Lugano 2014 criteria.33 Timing and eligibility for ASCT were determined by the treating physician based on institutional guidelines. Likewise, stem cell mobilization, leukapheresis, and conditioning chemotherapy were based on institutional standards. Radiographic assessment, maintenance therapy, and consolidative radiation after ASCT were at the discretion of treating physicians.
Statistical analysis
Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier (KM) method, and differences in survival between groups were assessed using the log-rank test. PFS was defined as the time from day 0 of ASCT to death from any cause, relapse, or progression, with patients censored at the last time seen alive and progression-free. OS was defined as the time from day 0 of ASCT to death from any cause, with patients censored at the last time seen alive. Median follow-up time was estimated using the reverse KM method. Descriptive statistics were used to summarize variables of interest. Univariable Cox proportional hazards regression were used to evaluate associations between prognostic factors and PFS and OS, and Wald P values were reported for covariates. Multivariable Cox proportional hazard models were used to determine the effects of pre-ASCT variables on PFS. The final Cox model was selected using a penalized maximum likelihood model (LASSO), and a k-fold cross-validation was performed to select a subset of the predictive variables. Finally, a stepwise forward/backward model selection by Akaike information criterion was used to determine the predictive variables for inclusion in the model. Two-sided P values of < .05 were considered significant. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) are reported. All analyses were performed using R v4.0 and the package glmnet v4.0-2 for LASSO models.
Results
Patients
Seventy-eight eligible patients were identified at 22 US transplant centers for this analysis. The median age at ASCT was 35 years (range, 19-73 years), and 67% of patients were male. First-line therapy consisted of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) (n = 71), AVD (n = 2), Stanford V (n = 2), BV + AVD (n = 2), and BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone) (n = 1). Forty-eight patients (62%) had primary refractory disease, and 14 additional patients (18%) had an initial remission duration of <12 months. At the time of first progression, 57% of patients had extranodal disease, 28% had B symptoms, and 64% had advanced-stage disease (Table 1).
Baseline characteristics
| . | All patients . | ASCT immediately after PD-1 treatment . | Received intervening therapy between PD-1 and ASCT . |
|---|---|---|---|
| N (%) | 78 (100) | 58 (74) | 20 (26) |
| Male, n (%) | 52 (67) | 40 (69) | 12 (60) |
| Age at ASCT, median (range), y | 35 (19-73) | 33 (21-65) | 40 (19-73) |
| HL subtype, n (%) | |||
| Nodular sclerosing | 61 (78) | 47 (81) | 14 (70) |
| Classic HL NOS | 10 (13) | 5 (9) | 5 (25) |
| Mixed cellularity | 6 (8) | 6 (10) | 0 (0) |
| Lymphocyte-rich | 1 (1) | 0 (0) | 1 (5) |
| Stage at diagnosis, n (%) | |||
| II | 26 (33) | 22 (38) | 4 (20) |
| III | 24 (31) | 17 (29) | 7 (35) |
| IV | 28 (36) | 19 (33) | 9 (45) |
| Primary refractory, n (%) | 48 (62) | 39 (67) | 9 (45) |
| DOR to first-line treatment <12 mo, n (%) | 62 (79) | 49 (84) | 13 (65) |
| Extranodal disease at relapse, n (%) | 33/76 (57) | 20/57 (35) | 11/19 (58) |
| B symptoms at relapse, n (%) | 21/74 (28) | 13/56 (23) | 8/18 (44) |
| Received BV prior to ASCT, n (%) | 69 (88) | 50 (86) | 19 (95) |
| Refractory to BV, n (%) | 45 (58) | 31 (53) | 14 (70) |
| Refractory to line of therapy before anti-PD-1 mAb, n (%) | 55 (71) | 42 (72) | 13 (65) |
| Anti-PD-1 mAb, n (%) | |||
| Nivolumab | 51 (65) | 38 (66) | 13 (65) |
| Pembrolizumab | 26 (33) | 19 (33) | 7 (35) |
| Avelumab | 1 (1) | 1 (2) | 0 (0) |
| PD-1 therapy, n (%) | |||
| Monotherapy | 59 (76) | 43 (72) | 17 (85) |
| Combination therapy | 18 (23) | 16 (28) | 2 (10) |
| Both monotherapy and combination therapy | 1 (1) | 0 (0) | 1 (5) |
| Number of cycles of anti-PD-1 treatment, median (range) | 6.5 (2-32) | 6 (3-28) | 8 (2-32) |
| Best response to anti-PD-1-based treatment, n (%) | |||
| CR | 33 (42) | 31 (53) | 2 (10) |
| PR | 30 (38) | 19 (33) | 11 (55) |
| SD | 9 (12) | 6 (10) | 3 (15) |
| PD | 6 (8) | 2 (3) | 4 (20) |
| Days from last dose of anti-PD-1 mAb to ASCT, median (range) | 52 (12-934) | 42 (12-194) | 139 (42-934) |
| Received intervening salvage therapy between PD-1 and ASCT, n (%) | 20 (26) | 0 (0) | 20 (100) |
| Pre-ASCT PET response status, n (%) | |||
| CR | 47 (59) | 33 (57) | 14 (65) |
| PR | 24 (31) | 19 (33) | 5 (25) |
| SD | 4 (5) | 3 (5) | 1 (5) |
| PD | 3 (4) | 3 (5) | 0 (0) |
| Systemic lines of therapy before ASCT, median (range) | 4 (3-7) | 4 (3-7) | 5 (4-7) |
| Number of systemic lines of therapy before ASCT, n (%) | |||
| 3 | 24 (31) | 24 (41) | 0 (0) |
| 4 | 28 (36) | 25 (43) | 3 (15) |
| ≥5 | 26 (33) | 9 (16) | 17 (85) |
| Number of modified AETHERA risk factors, n (%) | |||
| 1 | 4 (5) | 3 (5) | 1 (5) |
| 2 | 23 (29) | 17 (29) | 6 (30) |
| 3 | 30 (38) | 24 (41) | 6 (30) |
| ≥4 | 21 (27) | 14 (24) | 7 (35) |
| Conditioning regimen, n (%) | |||
| BEAM (BCNU, etoposide, cytarabine, melphalan) | 53 (68) | 42 (72) | 11 (55) |
| GemBuMel (gemcitabine, busulfan, melphalan) | 12 (15) | 6 (10) | 6 (30) |
| CBV (cyclophosphamide, BCNU, etoposide) | 7 (9) | 5 (9) | 2 (10) |
| CBV+Gem/Nav (cyclophosphamide, BCNU, etoposide, gemcitabine, navelbine) | 4 (5) | 3 (5) | 1 (5) |
| BEAC (BCNU, etoposide, cytarabine, cyclophosphamide) | 1 (1) | 1 (2) | 0 (0) |
| CBV + Mel (cyclophosphamide, BCNU, etoposide, melphalan) | 1 (1) | 1 (2) | 0 (0) |
| . | All patients . | ASCT immediately after PD-1 treatment . | Received intervening therapy between PD-1 and ASCT . |
|---|---|---|---|
| N (%) | 78 (100) | 58 (74) | 20 (26) |
| Male, n (%) | 52 (67) | 40 (69) | 12 (60) |
| Age at ASCT, median (range), y | 35 (19-73) | 33 (21-65) | 40 (19-73) |
| HL subtype, n (%) | |||
| Nodular sclerosing | 61 (78) | 47 (81) | 14 (70) |
| Classic HL NOS | 10 (13) | 5 (9) | 5 (25) |
| Mixed cellularity | 6 (8) | 6 (10) | 0 (0) |
| Lymphocyte-rich | 1 (1) | 0 (0) | 1 (5) |
| Stage at diagnosis, n (%) | |||
| II | 26 (33) | 22 (38) | 4 (20) |
| III | 24 (31) | 17 (29) | 7 (35) |
| IV | 28 (36) | 19 (33) | 9 (45) |
| Primary refractory, n (%) | 48 (62) | 39 (67) | 9 (45) |
| DOR to first-line treatment <12 mo, n (%) | 62 (79) | 49 (84) | 13 (65) |
| Extranodal disease at relapse, n (%) | 33/76 (57) | 20/57 (35) | 11/19 (58) |
| B symptoms at relapse, n (%) | 21/74 (28) | 13/56 (23) | 8/18 (44) |
| Received BV prior to ASCT, n (%) | 69 (88) | 50 (86) | 19 (95) |
| Refractory to BV, n (%) | 45 (58) | 31 (53) | 14 (70) |
| Refractory to line of therapy before anti-PD-1 mAb, n (%) | 55 (71) | 42 (72) | 13 (65) |
| Anti-PD-1 mAb, n (%) | |||
| Nivolumab | 51 (65) | 38 (66) | 13 (65) |
| Pembrolizumab | 26 (33) | 19 (33) | 7 (35) |
| Avelumab | 1 (1) | 1 (2) | 0 (0) |
| PD-1 therapy, n (%) | |||
| Monotherapy | 59 (76) | 43 (72) | 17 (85) |
| Combination therapy | 18 (23) | 16 (28) | 2 (10) |
| Both monotherapy and combination therapy | 1 (1) | 0 (0) | 1 (5) |
| Number of cycles of anti-PD-1 treatment, median (range) | 6.5 (2-32) | 6 (3-28) | 8 (2-32) |
| Best response to anti-PD-1-based treatment, n (%) | |||
| CR | 33 (42) | 31 (53) | 2 (10) |
| PR | 30 (38) | 19 (33) | 11 (55) |
| SD | 9 (12) | 6 (10) | 3 (15) |
| PD | 6 (8) | 2 (3) | 4 (20) |
| Days from last dose of anti-PD-1 mAb to ASCT, median (range) | 52 (12-934) | 42 (12-194) | 139 (42-934) |
| Received intervening salvage therapy between PD-1 and ASCT, n (%) | 20 (26) | 0 (0) | 20 (100) |
| Pre-ASCT PET response status, n (%) | |||
| CR | 47 (59) | 33 (57) | 14 (65) |
| PR | 24 (31) | 19 (33) | 5 (25) |
| SD | 4 (5) | 3 (5) | 1 (5) |
| PD | 3 (4) | 3 (5) | 0 (0) |
| Systemic lines of therapy before ASCT, median (range) | 4 (3-7) | 4 (3-7) | 5 (4-7) |
| Number of systemic lines of therapy before ASCT, n (%) | |||
| 3 | 24 (31) | 24 (41) | 0 (0) |
| 4 | 28 (36) | 25 (43) | 3 (15) |
| ≥5 | 26 (33) | 9 (16) | 17 (85) |
| Number of modified AETHERA risk factors, n (%) | |||
| 1 | 4 (5) | 3 (5) | 1 (5) |
| 2 | 23 (29) | 17 (29) | 6 (30) |
| 3 | 30 (38) | 24 (41) | 6 (30) |
| ≥4 | 21 (27) | 14 (24) | 7 (35) |
| Conditioning regimen, n (%) | |||
| BEAM (BCNU, etoposide, cytarabine, melphalan) | 53 (68) | 42 (72) | 11 (55) |
| GemBuMel (gemcitabine, busulfan, melphalan) | 12 (15) | 6 (10) | 6 (30) |
| CBV (cyclophosphamide, BCNU, etoposide) | 7 (9) | 5 (9) | 2 (10) |
| CBV+Gem/Nav (cyclophosphamide, BCNU, etoposide, gemcitabine, navelbine) | 4 (5) | 3 (5) | 1 (5) |
| BEAC (BCNU, etoposide, cytarabine, cyclophosphamide) | 1 (1) | 1 (2) | 0 (0) |
| CBV + Mel (cyclophosphamide, BCNU, etoposide, melphalan) | 1 (1) | 1 (2) | 0 (0) |
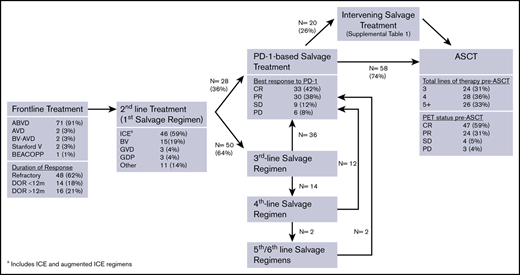
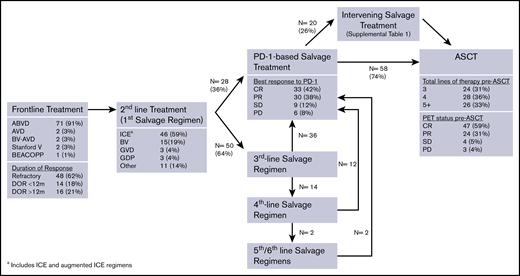
Patients received a median of 3 lines of systemic therapy before receiving anti-PD-1 treatment (range, 2-6). Figure 1 depicts the sequence of pre-ASCT therapies. Fifty-five patients (71%) were refractory to the line of therapy before anti-PD-1 treatment, 42 patients (54%) were refractory to 2 consecutive lines of therapy immediately before anti-PD-1 treatment, and 18 patients (23%) were refractory to 3 consecutive lines of therapy before anti-PD-1 treatment. Sixty-nine patients (88%) received BV prior to ASCT, and 45 of these patients (65%) were BV refractory, including 39 of 50 patients (78%) treated with BV monotherapy. Among 50 patients who received ≥3 lines of therapy before anti-PD-1 treatment, 30 patients (60%) were refractory to ≥2 salvage regimens. In total, 29 patients (37%) were refractory to all lines of therapy prior to anti-PD-1 treatment.
Consort diagram of pre-ASCT treatments. GDP, gemcitabine, dexamethasone, cisplatin; GVD, gemcitabine, vinorelbine, doxil; ICE, ifosfamide, carboplatin, etoposide.
Consort diagram of pre-ASCT treatments. GDP, gemcitabine, dexamethasone, cisplatin; GVD, gemcitabine, vinorelbine, doxil; ICE, ifosfamide, carboplatin, etoposide.
Patients received a median of 6.5 doses (range, 2-32 doses) of an anti-PD-1 mAb as monotherapy (n = 59), combination therapy (n = 18), or monotherapy followed by combination therapy (n = 1). Among 60 patients who received monotherapy, 37 received nivolumab (62%), 22 received pembrolizumab (37%), and 1 received avelumab (1%). Most patients treated with a PD-1-based combination received nivolumab (15/19, 79%), with nivolumab plus BV being the most common combination regimen (n = 8) (supplemental Table 2). The median time from last dose of an anti-PD-1 mAb to ASCT was 52 days (range, 12-934 days; interquartile range, 34-107 days). For 68 patients (87%), the time from last dose of PD-1 and ASCT was <20 weeks (or ∼5 half-lives of nivolumab or pembrolizumab). Investigator-reported best response to anti-PD-1 therapy was CR in 33 patients (42%), partial response (PR) in 30 patients (38%), SD in 9 patients (11%), and PD in 6 patients (8%). Patients who received an anti-PD-1 mAb-based combination had a higher ORR (100% vs 75%) and CR rate (58% vs 33%) compared with patients receiving anti-PD-1 monotherapy. Twenty patients (26%) received intervening salvage therapy between anti-PD-1 treatment and ASCT (supplemental Table 1). Among these 20 patients, the best response to anti-PD-1 treatment was PD in 4 patients, SD in 3 patients, PR in 11 patients, and CR in 2 patients.
ASCT and maintenance treatments
The median number of systemic therapies before ASCT was 4 (range, 3-7). Pre-ASCT PET status was CR in 47 patients (59%), PR in 24 patients (31%), SD in 4 patients (5%), and PD in 3 patients (4%). At the time of ASCT, 51 patients (65%) had ≥3 of the 5 pre-ASCT modified AETHERA high-risk factors (ie, primary refractory disease or initial remission duration <12 months, extranodal disease at relapse, B symptoms at relapse, positive pre-ASCT PET scan, and receipt of ≥2 salvage regimens) (Table 1).
Conditioning regimens varied across institutions with BEAM (BCNU, etoposide, cytarabine, and melphalan) (68%), GemBuMel (gemcitabine, busulfan, and melphalan) (15%), and CBV (cyclophosphamide, BCNU, and etoposide) (9%) being used most frequently (Table 1). In total, 24 patients (31%) received systemic maintenance therapy following ASCT; 14 received BV monotherapy, 2 received BV + nivolumab, and 8 received PD-1 monotherapy. Among the 33 patients who were either BV naive or BV nonrefractory, 12 patients (36%) received BV maintenance. In addition, 6 patients (8%) received involved-site radiation consolidation following ASCT.
There were no ASCT-related deaths or frequent unexpected post-ASCT complications. Three patients (4%) developed BCNU pneumonitis, which resolved with steroids in all cases. In addition, 1 patient (1%) developed each of the following: diarrhea and rash, which was felt to be consistent with engraftment syndrome and was responsive to steroids; adrenal insufficiency; and steroid-responsive diarrhea.
Survival outcomes and predictive factors
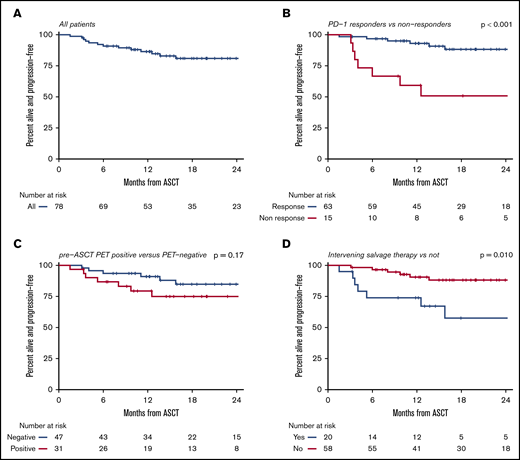
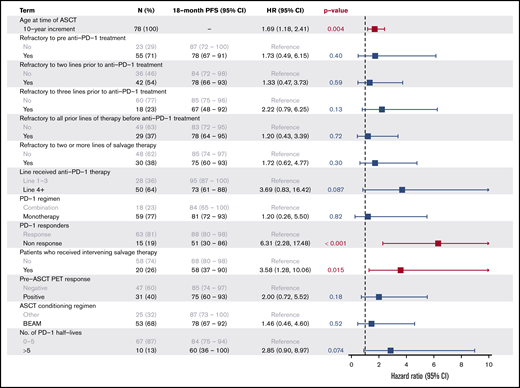
After a median post-ASCT follow-up of 19.6 months (range, 3.3-52.0 months), the 18-month PFS for the entire cohort was 81% (95% CI, 69% to 89%) (Figure 2). Lack of response to anti-PD-1 therapy (18-month PFS, 51% vs 88%; P < .001), receipt of intervening salvage therapy (18-month PFS, 58% vs 88%; P = .015), and advanced age (increasing 10-year increments HR, 1.69; P = .004) were all significant predictors of inferior PFS on univariate analysis. Notably, outcomes for patients who were refractory to 1 or 2 consecutive lines of therapy immediately before anti-PD-1 treatment were similar to the entire cohort (18-month PFS, 78% for both groups) (Figure 3). Even the most chemorefractory patients (those refractory to ≥2 salvage regimens and those refractory to 3 consecutive systemic therapies before anti-PD-1 treatment) had favorable outcomes, with 18-month PFS of 75% and 67%, respectively (Figure 3). The 18-month PFS in heavily pretreated patients who received anti-PD-1 therapy in the fourth line or later was 73% compared with 95% for those receiving anti-PD-1 therapy as third-line treatment (P = .087). Among all patients, there was no significant difference in PFS for patients with a positive pre-ASCT PET compared with those who achieved a CMR on pre-ASCT PET (18-month PFS, 75% vs 85%, P = .18). In this cohort, the predictive value of pre-ASCT PET depended upon the final line of therapy before ASCT. Among the 58 patients who had PD-1 blockade as their most recent therapy before ASCT, there was no difference in outcomes between PET-positive (n = 25) and PET-negative patients (n = 33) (18-month PFS 91% vs 86%, P = .87). In contrast, pre-ASCT PET appeared to retain its prognostic value for the 20 patients who had intervening salvage therapy after anti-PD-1 therapy and before ASCT (18-month PFS, 79% for PET negative [n = 14] vs 17% for PET-positive [n = 6]; P < .001). Patients with an interval of >20 weeks (∼5 PD-1 mAb half-lives) from PD-1 to ASCT had an 18-month PFS of 60% as compared with 84% in patients with <20 weeks between ASCT and anti-PD-1 therapy (P = .06). No differences in PFS were observed based on receipt of BV (P = .27) or PD-1 mAb (P = .37) maintenance therapy after ASCT, treatment with post-ASCT radiation (P = .88), treatment with anti-PD-1 monotherapy vs anti-PD-1-based combination treatments (P = .82), or receipt of BEAM vs alternative conditioning regimens (P = .51), although our study was underpowered to detect these differences.
PFS. All patients (A), PD-1 responders vs nonresponders (B), pre-ASCT PET positive vs negative (C), and treatment with and without intervening salvage therapy (D).
PFS. All patients (A), PD-1 responders vs nonresponders (B), pre-ASCT PET positive vs negative (C), and treatment with and without intervening salvage therapy (D).
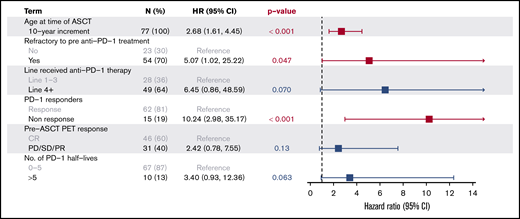
A multivariate analysis confirmed that age (HR, 2.7 for increasing 10-year increments; P < .001), lack of response to anti-PD-1 therapy (HR, 10.2; P < .001), and refractory disease to the line prior to anti-PD-1 therapy (HR, 5.1; P = .047) were significant predictors of inferior PFS, while other factors, including pre-ASCT PET status (HR, 2.4; P = .13), receipt of anti-PD-1 treatment as fourth-line or later therapy (HR, 6.5; P = .07), and an interval >20 weeks from PD-1 to ASCT (HR, 3.4; P = .063) failed to reach statistical significance (Figure 4).
The median 18-month OS for this cohort was 96% (95% CI, 87% to 99%) (Figure 5). All deaths were due to progressive HL. In univariate analysis, inferior OS was observed for PD-1 nonresponders (18-month OS, 86% vs 98%; P = .002), patients who received intervening salvage therapy (18-month OS, 83% vs 100%; P = .028), and patients refractory to 3 consecutive lines of therapy immediately before anti-PD-1 therapy (89% vs 98%, P = .023). Only failure to respond to anti-PD-1 therapy (HR, 12.8; P = .003) retained significance on a multivariable analysis.
Discussion
ASCT following anti-PD-1 therapy was associated with favorable PFS in this high-risk cohort of patients with heavily treated, multiply R/R HL. Based on a lack of demonstrated chemosensitivity, many patients in our cohort would traditionally be considered poor candidates for ASCT, particularly those who received ≥4 lines of systemic therapy before ASCT and those refractory to multiple consecutive chemotherapy regimens. Pursuing ASCT in such patients with multiply R/R HL contradicts the dogma that ASCT should only be used in patients with chemosensitive lymphoma. Nevertheless, patients who were sensitive to anti-PD-1 therapy had a high rate of durable remission following ASCT with the majority not receiving any post-ASCT maintenance or consolidation therapy.
It is difficult to find a modern cohort with a similar risk profile for useful comparison. Unlike the study population in the AETHERA trial, which evaluated placebo vs BV maintenance therapy following ASCT in high-risk R/R HL, a large majority of patients in our study (88%) had received BV prior to ASCT and most of these patients (65%) were BV-refractory. Despite high rates of BV treatment before ASCT and the lack of maintenance therapy for most patients in our study, the 18-month PFS of 81% is higher than that observed in both arms of the AETHERA trial, and is considerably better than that observed for similarly high-risk patients in the AETHERA trial who did not receive BV maintenance (18-month PFS 30% to 40% for patients with 2+ or 3+ risk factors).34 Retrospective series also report poor outcomes among heavily pretreated patients who were refractory to salvage chemotherapy. While high-dose chemotherapy and ASCT can overcome chemoresistance in a subset of patients with R/R HL, the rate of long-term remissions among chemoresistant patient in these studies (11% to 51%) is much lower than what we observed in our cohort.17,18,35
In our study, we noted particularly favorable outcomes for patients who did not receive intervening treatments between anti-PD-1 therapy and ASCT (18-month PFS, 88%). A similar pattern was observed in a phase 2 trial of second-line BV + nivolumab combination therapy. In that trial, the 67 patients who underwent ASCT after nivolumab-based salvage therapy achieved a 2-year PFS of 91%.36 So far, studies in the pre-ASCT salvage setting have focused on PD-1 blockade as part of second-line therapy. However, our study, which included patients who received anti-PD-1 treatment as third-line or later therapy, supports the strategy of using PD-1 blockade as a bridge to a potentially curative ASCT in more heavily pretreated, chemorefractory patients. Collectively, these findings support the hypothesis that PD-1 blockade may augment chemosensitivity in patients with R/R HL. In other retrospective studies, higher-than-expected response rates to chemotherapy regimens were observed following PD-1 blockade in HL,23,24 non-HL,25 and solid tumors.26-29 Our data suggest a similar phenomenon may occur when PD-1 blockade precedes high-dose chemotherapy and ASCT with better-than-expected PFS among previously chemorefractory patients. Based on these findings, additional trials incorporating PD-1 mAbs into salvage therapy are warranted. The proximity of anti-PD1 salvage therapy to ASCT may be an important factor to consider in trial design or clinical decision-making, as we observed a trend toward worse outcomes in patients with an interval of greater than 5 PD-1 mAb half-lives from last dose of PD-1 to ASCT. However, this observation requires further study.
Our data also challenge the importance of pre-ASCT PET negativity among patients receiving PD-1 blockade. Prior studies have consistently demonstrated that a positive pre-ASCT PET scan following chemotherapy-based salvage treatment predicts significantly inferior outcomes with an expected 18-month PFS ranging from 30% to 45%.4-6,8,9,11,12 In our study, outcomes among patients who underwent ASCT directly after anti-PD-1 therapy were equivalent for PET-positive and PET-negative patients with an 18-month PFS of >85% in both groups. This better-than-expected outcome could again argue for a chemosensitization effect for PD-1 blockade but may also be due to false-positive PET scan results after anti-PD-1 therapy. Investigators have reported false-positive PET scan results and pseudoprogression for HL patients receiving PD-1 mAbs in other clinical settings.20,37,38 The Checkmate-205 trial, which tested nivolumab in combination with AVD for patients with untreated advanced-stage HL, found that a subset of patients with positive end-of-treatment PET scan results achieved durable remissions.39 Similarly, 4 patients enrolled in the second-line trial of BV + nivolumab had PET-positive lesions (Deauville 4 or 5) on an end-of-treatment PET but had no evidence of lymphoma on biopsy.36 Additional experience is necessary to confirm our findings, but our study suggests that patients who achieve a PR following PD-1 therapy may have high rates of durable remissions with consolidative ASCT.
Patients considered ineligible for ASCT due to chemorefractory disease have gained more effective treatment options with the approvals of BV, nivolumab, and pembrolizumab. While these drugs are associated with high response rates, long-term follow-up suggests that most patients will not be cured and many will require continuous treatment of disease control.40,41 In our study, a large majority of patients achieved durable remissions following ASCT without the need for ongoing treatment. Patients with multiply relapsed HL may also be considered for treatment with allogeneic hematopoietic stem cell transplantation, which can be curative (2-year PFS, ∼45%)42-44 but carries a significant risk of treatment-related mortality and long-term morbidity, with several studies suggesting higher rates of early toxicity following PD-1 blockade.45,46 Our results suggest that consolidation with ASCT rather than allogeneic hematopoietic stem cell transplantation may be a reasonable treatment approach for patients with multiply relapsed HL, even for those who were previously refractory to multiple chemotherapy regimens.
Instead of consolidation with autologous or allogeneic transplantation, patients with R/R HL could be managed with either observation off of PD-1 therapy or ongoing treatment with a PD-1 mAb. There are limited data that some patients who achieve an excellent response to PD-1 blockade may have durable responses even after PD-1 discontinuation. However, this benefit is primarily confined to patients achieving a CR, and the duration of response (DOR) following PD-1 discontinuation for these patients does not appear to be as favorable as that observed with consolidative ASCT in our study.40 Among PD-1 responders treated with ongoing PD-1 blockade, the median DOR in phase 2 trials of nivolumab and pembrolizumab was ∼16 months, with a shorter DOR for partial responders.20,22 In our study, patients who achieved a PR or CR to anti-PD-1 treatment prior to ASCT both had a post-ASCT 18-month PFS of >85% (which reflects an ∼24-month PFS end point from PD-1 initiation). These data strongly suggest that ASCT provides additional benefit. Even so, longer follow-up will be helpful to confirm that these excellent results among high-risk HL patients are durable.
While most patients in our cohort had excellent outcomes, the 15 patients (19%) who failed to respond to anti-PD-1-based salvage treatment had poor long-term PFS and OS after ASCT (Figures 2B and 5B). Poor outcomes for this group of patients have also been reported in the phase 2 trials of nivolumab and pembrolizumab.20,22 These data suggest that PD-1 nonresponders, who typically make up ∼30% of patients in studies of PD-1 mAb monotherapy, are a particularly high-risk group who should not be considered for ASCT and are in need of alternative therapeutic strategies. Patients who required intervening salvage therapy after anti-PD-1 therapy also had inferior outcomes, although the heightened risk for relapse was primarily among patients who failed to achieve a CMR to their post-PD-1 salvage treatment, and these patients appear to be poor candidates for ASCT. Our data suggest that patients who achieve a CMR with post-PD-1 salvage treatment may also have good outcomes after ASCT, although this cohort in our study was small and requires validation.
Our study has several limitations inherent to its retrospective design. Foremost, we acknowledge the possibility of selection bias. While patients in our cohort had numerous high-risk features, including frequent chemorefractory disease, it is possible that clinicians at the participating centers did not offer ASCT following anti-PD-1 therapy to their most chemorefractory patients. Due to the large number of participating centers, the cohort is heterogeneous with regards to timing and mode of PD-1 blockade (ie, monotherapy vs combination), conditioning regimen, transplant practices (including timing of ASCT and approach to patients achieving a PR to salvage regimens), and use of post-ASCT maintenance therapy, including a small subset of patients (13%) who received investigational anti-PD-1 mAb-based maintenance therapy after ASCT. However, the great majority of patients (>70%) received anti-PD-1 as monotherapy and did not receive PD-1 mAb maintenance after ASCT. Moreover, we demonstrated that the small number of patients who received anti-PD-1 based combination therapies or post-ASCT treatments did not have significantly different PFS, and, therefore, it is unlikely that these account for the favorable results observed among the entire study population. Our study does not include central radiologic review, so we are unable to provide additional details about disease burden or sites of involvement among patients with positive pre-ASCT PET scans. In addition, PET scans were not evaluated using provisional response criteria designed for immune-based treatments in lymphoma (ie, LYRIC37 and RECIL47 ), which we would recommend for future studies in this setting. Despite these shortcomings, our center-based recruitment approach allowed for detailed collection of patient characteristics, prior treatment regimens, anti-PD-1 treatment, and response assessment data, which have added important context to our results and would not have been possible using available transplant databases.
Historically, patients with chemorefractory R/R HL or those who require multiple salvage regimens prior to ASCT have had poor outcomes. In this high-risk cohort, treatment with anti-PD-1 therapy prior to ASCT was associated with excellent post-ASCT PFS, even among heavily pretreated patients who had chemorefractory disease before PD-1 treatment. Patients who responded to anti-PD-1 therapy had particularly favorable outcomes, with an 18-month PFS of 88%. Our study supports the use of PD-1 blockade as salvage therapy and provides initial evidence that anti-PD-1 therapy beyond the second-line setting can serve as a bridge to ASCT. Additional studies to validate these results and clarify the importance of pre-ASCT variables (including pre-ASCT PET status and time from PD-1 to ASCT) are needed. In the meantime, based on these data, ASCT should be considered for patients with multiply R/R HL responding to anti-PD-1 therapy, even those with previously chemorefractory disease.
For original data, please contact aherrera@coh.org or reid_merryman@dfci.harvard.edu.
Acknowledgments
R.W.M. gratefully acknowledges support from an ASH Research Training Award for Fellows, the ASH Clinical Research Training Institute, an ASBMT New Investigator Award, and the LRF Lymphoma Clinical Research Mentoring Program. A.F.H. is supported by the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award.
Authorship
Contribution: R.W.M. designed the research, performed research, analyzed the data, and wrote the paper; R.A.R. analyzed the data; T.N., J.C., Y.N., J.M.D., U.R., M.T.B., D.A.B., K.J.M., M.A.S., R.H.A., H.J.B., J.S., A.K.S., J.P.M., D.M., R.R., J.R., R.B.C., M.J.F., Y.-B.C., A.V.S., J.K., S.A., S. Nathan, M.R., R.M.J., M.S., K.A.D., S.P., A.W.B., A.H., V.B., S. Nakhoda, N.K., R.C.L., S.D.S., V.T.H., A.L., and P.A. performed research and reviewed the paper; and A.F.H. designed the research, performed research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: T.N. received research funding from Novartis and Karyopharm. J.C. received research funding from Merck and consultant fees from Novartis, Genentech, Kite, Humanigen; is on the advisory board at Novartis, Genentech, and Kite; and received other funds from Janssen, Genetech, and Kite. Y.N. received research funding from Affimed, Novartis, AstraZeneca, and Secura Bio and consultant fees from Affimed. J.M.D. is on the advisory Bboard for Morphosys and Kite Pharma. D.A.B. received honoraria from Seattle Genetics. K.J.M. received research funding from BMS; consultant fees from Morphosys, Celgene, Pharmacyclics, Karyopharm, ADC Therapeutics, and BMS; and honoraria from Morphosys, Celgene, and Pharmacyclics. M.A.S. received honoraria from Notable Labs. R.H.A. received research funding from Celgene, Forty Seven, Genentech/Roche, Janssen Pharmaceutical, Kura, Merck, Millennium, Pharmacyclics, Regeneron, Seattle Genetics. for AstraZeneca, Bayer Healthcare Pharmaceuticals, Cell Medica, Celgene, Genentech/Roche, Gilead, Kite Pharma, Kyowa, Portola Pharmaceuticals, Sanofi, Seattle Genetics, and Takeda. J.S. received research funding from AstraZeneca, BMS, Incyte, Merck, Pharmacyclics, Seattle Genetics, and TG and consultant fees from Adaptive, AstraZeneca, BMS, Imbrium, Pharmacyclics, Seattle Genetics, Genmab, and Atara. J.P.M. received research funding from Novartis, Fresenius Biotech, Astellas, Bellicum Pharmaceuticals, Gamida Cell, Kite Pharmaceuticals, Pluristem, Juno Therapeutics, and AlloVir; is on the advisory board and received travel accommodation/expense compensation from Kite Pharmaceuticals, Juno Therapeutics, and AlloVir; and recieved honoraria from Kite Pharmaceuticals and AlloVir. D.M. is on the advisory board for Morphosys. R.R. received research funding from Merck, Seattle Genetics, Janssen, Genentech. Consultancy for Seattle Genetics, Sandoz-Novartis, Pharmacyclics, an AbbVie Company, Janssen, and Bristol-Myers Squibb. J.B.C. received research funding from Genentech, BMS, Novartis, LAM, BioInvent, LRF, ASH, AstraZeneca, Seattle Genetics. Consultancy for Janssen, Adicet, AstraZeneca, Genentech, Aptitude Health, Cellectar, Kite/Gilead, and Loxo. M.J.F. received research funding from Novartis and consultant fees from Novartis, Gilead/Kite, Celgene, and Arcellx. Y.-B.C. received consultant fees from Incyte, Takeda, Magenta, and Kiadis and is on the data safety monitoring board at Actinium, Equillium, and AbbVie. J.K. received research funding from Merck and Verastem; is on the advisory board at Verastem, Karyopharm, and Seattle Genetics; and recieved speakers bureau fees from Kite/Gilead. S.A. received research funding from BMS, Seattle Genetics, Takeda, AI Therapeutics, Regeneron, Affimed, Trillium, and ACD Therapeutics. A.W.B. received research funding from Celgene, LoxoOncology, MorphoSysAb, Roche, Seattle Genetics, and Tessa Therapeutics. V.B. received research funding from Gamide Cell, Incyte, FATE, and Novartis and is on the advisory board of Karyopharm, Kite, Seattle Genetics, and UNUM. N.K. received research funding from BMS, Seattle Genetics, and Celgene and honoraria from Janssen and Pharmacyclics. R.C.L. received research funding from Takeda, Incyte, TG Therapeutics, Rhizen, Bayer, Juno, Cyteir, and Genentech and consultant fees from Morphosys. S.D.S. received research funding from Acerta Pharma BV, AstraZeneca, Bayer, Ayala, BMS, De Novo Biopharma, Genentech, Igynta, Incyte, Merck, Pharmacyclics, Portola, and Seattle Genetics and consultant fees from AstraZeneca, Takeda, and Beigene. A.L. received consultant fees from BMS, speakers bureau fess from Research to Practice, and patents and royalties for UpToDate. P.A. received research funding from Merck. A.F.H. received research funding from BMS, Merck, Genentech/F. Hoffmann-La Roche, Gilead Sciences, Seattle Genetics, Immune Design, AstraZeneca, and Pharmacyclics; consultant fees from BMS, Merck, Genentech/F. Hoffmann-La Roche, Gilead Sciences, Seattle Genetics, and Karyopharm; and travel, accommodation, and expense compensation from BMS. The remaining authors declare no competing financial interests.
Correspondence: Alex Herrera, Department of Hematology/Hematopoietic Cell Transplantation, City of Hope Medical Center, Duarte, CA 91010; e-mail:aherrera@coh.org.
References
Author notes
The full-text version of this article contains a data supplement.