Key Points
Lenalidomide integrated into standard induction therapy for newly diagnosed AML does not improve survival except for the SRSF2 genotype.
Treatment of AML with 3 cycles of intensive therapy applied according to a prospective MRD risk-guided approach impacts patient outcome.
Abstract
Lenalidomide, an antineoplastic and immunomodulatory drug, has therapeutic activity in acute myeloid leukemia (AML), but definitive studies about its therapeutic utility have been lacking. In a phase 3 study, we compared 2 induction regimens in newly diagnosed patients age 18 to 65 years with AML: idarubicine-cytarabine (cycle 1) and daunorubicin and intermediate-dose cytarabine (cycle 2) without or with lenalidomide (15 mg orally on days 1-21). One final consolidation cycle of chemotherapy or autologous stem cell transplantation (auto-SCT) or allogeneic SCT (allo-SCT) was provided according to a prognostic risk and minimal residual disease (MRD)–adapted approach. Event-free survival (EFS; primary end point) and other clinical end points were assessed. A second random assignment in patients in complete response or in complete response with incomplete hematologic recovery after cycle 3 or auto-SCT involved 6 cycles of maintenance with lenalidomide (10 mg on days 1-21) or observation. In all, 392 patients were randomly assigned to the control group, and 388 patients were randomly assigned to lenalidomide induction. At a median follow-up of 41 months, the study revealed no differences in outcome between the treatments (EFS, 44% ± 2% standard error and overall survival, 54% ± 2% at 4 years for both arms) although in an exploratory post hoc analysis, a lenalidomide benefit was suggested in SRSF2-mutant AML. In relation to the previous Dutch-Belgian Hemato-Oncology Cooperative Group and Swiss Group for Clinical Cancer Research (HOVON-SAKK) studies that used a similar 3-cycle regimen but did not pursue an MRD-guided approach, these survival estimates compare markedly more favorably. MRD status after cycle 2 lost prognostic value in intermediate-risk AML in the risk-adjusted treatment context. Maintenance with lenalidomide showed no apparent effect on relapse probability in 88 patients randomly assigned for this part of the study.
Introduction
Treatment for acute myeloid leukemia (AML) in adults younger than age 65 years with anthracycline-cytarabine–based combination chemotherapy results in average complete remission (CR) rates of 70% to 85% and a long-term survival rate of about 40%. An inferior outcome is seen in patients with unfavorable prognosis (as defined by cytogenetic and molecular criteria) with a 5-year survival probability of only 10% to 25%. In patients with comparatively favorable subtypes of AML for whom treatment outcome is better, the relapse rate may still be only 30% to 40%.1,2 Thus, in general, there is a considerable need for more effective therapies in newly diagnosed patients with AML. One approach aimed at improving outcome focuses on combining drugs with distinct mechanisms of action with other effective drugs as early in the treatment as possible to tackle leukemia cells at multiple targets.
Lenalidomide belongs to the class of immunomodulatory drugs. It is an orally available compound with antineoplastic, immunomodulatory, and antiangiogenic properties.3 Several studies, all of limited size, have shown therapeutic activity of single-agent lenalidomide in relapsed or refractory AML and previously untreated AML.4-6 Furthermore, antileukemic efficacy has been suggested in studies that examined lenalidomide in combination with azacitidine7-11 or chemotherapy.12,13 Previously reported data regarding the clinical value of adding lenalidomide to chemotherapy at various levels of dose intensity have been conflicting.12-15 But more definitive studies exploring the therapeutic value of lenalidomide are lacking, especially in the context of combination chemotherapy.
In the phase 3 study with mature follow-up reported here, lenalidomide at a dose level of 15 mg/day in combination with idarubicin-cytarabine (cycle 1) and intermediate-dose daunorubicin-cytarabine (cycle 2) was evaluated in 780 newly diagnosed patients (age 65 years or younger) with AML.
Patients and methods
Eligibility
Adults age 18 to 65 years with a diagnosis of AML or with refractory anemia with excess blasts (RAEB) and a Revised International Prognostic Scoring System score >4.5 16 or with acute leukemia of ambiguous lineage according to World Health Organization 2008 criteria, an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, and a written informed consent were eligible. Eligibility and exclusion criteria are specified in detail in the supplemental Data.
Study design and treatments
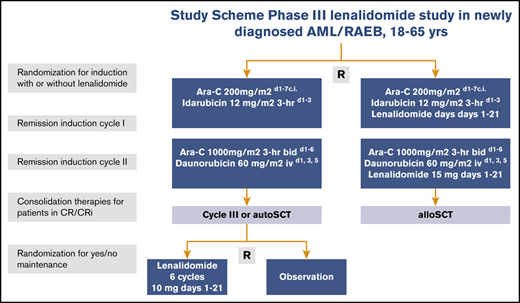
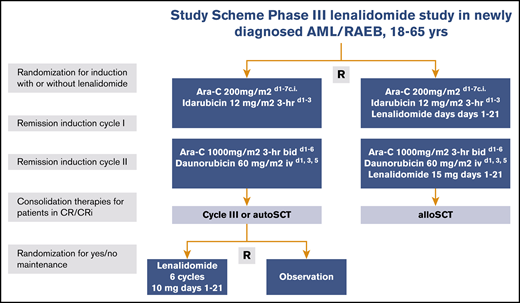
Eligible patients were randomly assigned in a 1:1 ratio to receive remission induction therapy with or without lenalidomide (Figure 1). Random assignment was performed by using a minimization procedure to ensure balance in the number of patients enrolled to each treatment arm overall, within each registration center and diagnostic subgroup (AML, RAEB, or leukemia with ambiguous lineage). Cycle 1 included idarubicin at 12 mg/m2 (3-hour infusion on days 1, 2, and 3) and cytarabine at a dose of 200 mg/m2 (per continuous infusion on days 1-7) with or without lenalidomide. Cycle 2 contained daunorubicin 60 mg/m2 per 1-hour infusion on days 1, 3, and 5 plus cytarabine 1000 mg/m2 given intravenously for 3 hours twice per day on days 1 to 6 with or without the addition of lenalidomide. The study began with a dose-selection run-in phase with an initial dose of lenalidomide of 20 mg/day on days 1 to 21 in cycles 1 and 2 (details are provided in the supplemental Data) and then continued with lenalidomide at 15 mg/day as an open-label phase 3 trial (part A of the trial), which is reported here.
Study schema. Ara-C, cytarabine; bid, twice per day; c.i., continuous infusion; d, day; hr, hour; iv, intravenous; R, random assignment.
Study schema. Ara-C, cytarabine; bid, twice per day; c.i., continuous infusion; d, day; hr, hour; iv, intravenous; R, random assignment.
Patients in CR or CR with incomplete hematologic recovery (CRi) after cycle 2 received consolidation with 1 final additional cycle of intensive chemotherapy with mitoxantrone-etoposide (cycle 3) or autologous stem cell transplantation (auto-SCT) after conditioning with busulfan-cyclophosphamide or total body irradiation-cyclophosphamide,2 or allogeneic SCT (allo-SCT), depending on their baseline prognostic risk status and minimal residual disease (MRD) status after the first 2 induction cycles as described (details are provided in the supplemental Data).
After consolidation treatment with cycle 3 or auto-SCT and meeting predefined eligibility criteria for maintenance treatment (supplemental Data), patients were eligible for a second random assignment (part B of the trial) to receive either 6 cycles of lenalidomide maintenance (at a fixed dose of 10 mg/day on days 1-21 followed by 14 days of rest) or observation (Figure 1), regardless of whether they had been assigned to the control or lenalidomide induction treatment at the first random assignment. Patients who received an allo-SCT as consolidation therapy were not eligible for the second random assignment. Randomization minimization factors in part B of the trial were registration center, diagnostic subgroup, treatment arm of the induction randomization, and type of consolidation treatment.
The study was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All patients gave their written informed consent.
Risk classification and clinical characteristics
On the basis of the European LeukemiaNet (ELN) risk classification,1 patients were classified into prognostic categories. Patients with previous myelodysplastic syndrome (MDS) or other antecedent hematologic disease (including myeloproliferative diseases) diagnosed 3 months or more before the AML/RAEB diagnosis were classified as secondary AML (sAML). Patients with previous chemotherapy or radiotherapy (and no sAML) were classified as therapy-related AML (tAML). Clinical and hematologic parameters, including ECOG performance status, extramedullary disease, and white blood cell count (WBC) were registered at diagnosis.
Criteria for response and end points
Criteria for CR, CRi, and relapse were as described.2 The primary end point for part A of the trial, which evaluated the added value of lenalidomide during induction, was event-free survival (EFS), which refers to the interval from induction randomization to the date of failure to achieve CR/CRi within 2 induction cycles, death without relapse after achieving CR/CRi, or relapse after achieving CR/CRi, whichever occurred first. Secondary efficacy end points for part A of the trial included overall survival (OS), relapse-free survival (RFS), rate of hematologic response during and after induction, and MRD negativity rate after induction cycle 2.
The primary end point for part B of the trial, which evaluated the value of maintenance treatment with lenalidomide, was cumulative incidence of relapse (CIR) defined as the time from the date of second random assignment until the date of relapse. Secondary end points for part B are cumulative incidence of death, RFS, and OS, all measured from the second random assignment. Safety was evaluated by observed adverse events (AEs), including pulmonary embolism, thromboembolic events, second primary malignancy, (early) mortality, and the need for transfusions, hospitalizations, and time to hematologic recovery after each chemotherapy cycle.
Molecular analysis and MRD assessment
Targeted sequencing and other methods used for the molecular assessment of common AML-associated gene mutations and high EVI1 messenger RNA expression on bone marrow or blood specimens at diagnosis were performed as previously reported2 with minor changes (specified in supplemental Data), and the molecular biomarkers are listed in Table 1. MRD was assessed after cycle 2 in patients in morphologic CR/CRi with multiparameter flow cytometry (MFC) and reverse transcriptase polymerase chain reaction (RT-PCR) for mutated NPM1 as described.17-19
Patient characteristics at baseline according to random assignment for induction with or without lenalidomide
| . | No. of patients evaluated . | Control induction treatment . | Lenalidomide induction treatment . |
|---|---|---|---|
| Total | 392 (100) | 388 (100) | |
| Male sex | 210 (54) | 233 (60) | |
| Age, y | |||
| Median (range) | 53 (18-65) | 54 (18-65) | |
| ≤45 | 110 (28) | 99 (26) | |
| 46-60 | 194 (49) | 195 (50) | |
| >60 | 88 (22) | 94 (24) | |
| WHO/ECOG performance status | |||
| 0 | 239 (61) | 264 (66) | |
| 1 | 138 (35) | 113 (29) | |
| 2 | 15 (4) | 11 (3) | |
| Diagnostic subgroup | |||
| AML | 356 (91) | 351 (90) | |
| High-risk RAEB | 33 (8) | 35 (9) | |
| Leukemia with ambiguous lineage | 3 (1) | 2 (1) | |
| AML type | |||
| De novo | 358 (91) | 351 (90) | |
| sAML | 19 (5) | 24 (6) | |
| tAML | 15 (4) | 13 (3) | |
| WBC, × 109/L | |||
| Median (range) | 8.0 (0-265) | 6.7 (0.4-297) | |
| ≤20 | 259 (66) | 257 (66) | |
| 20-100 | 109 (28) | 103 (27) | |
| >100 | 23 (6) | 27 (7) | |
| Unknown | 1 (0) | 1 (0) | |
| Median blasts in bone marrow, % | 52 | 52 | |
| Cytogenetics* | |||
| t(8;21) | 11 (3) | 22 (6) | |
| inv(16) or t(16;16) | 23 (6) | 26 (7) | |
| CN-X-Y | 211 (54) | 199 (51) | |
| CA rest | 97 (25) | 95 (24) | |
| Monosomal karyotype | 39 (10) | 34 (9) | |
| Unknown | 11 (3) | 12 (3) | |
| Gene mutations† | |||
| NPM1-mut | 744 | 129 (33) | 115 (30) |
| FLT3-ITD-mut | 742 | 85 (22) | 69 (18) |
| FLT3-TKD835-mut | 742 | 34 (9) | 33 (9) |
| NPM1-mut FLT3-ITD-negative | 741 | 69 (18) | 77 (20) |
| NPM1-mut FLT3-ITD-mut | 59 (15) | 38 (10) | |
| NPM1-wt FLT3-ITD-negative | 217 (55) | 224 (58) | |
| NPM1-wt FLT3-ITD-mut | 26 (7) | 31 (8) | |
| DNMT3A-mut | 739 | 108 (28) | 105 (27) |
| IDH1-mut | 739 | 35 (9) | 34 (9) |
| IDH2-mut | 739 | 45 (11) | 45 (12) |
| TET2-mut | 739 | 54 (14) | 41 (11) |
| Biallelic CEBPA-mut | 739 | 16 (4) | 12 (3) |
| RUNX1-mut | 742 | 41 (10) | 49 (13) |
| ASXL1-mut | 742 | 39 (10) | 31 (8) |
| TP53-mut | 742 | 28 (7) | 29 (7) |
| SF3B1-mut or SRSF2-mut‡ | 739 | 46 (12) | 43 (11) |
| SRSF2-mut‡ | 739 | 30 (8) | 28 (7) |
| PTPN11-mut | 739 | 44 (11) | 33 (9) |
| KRAS-mut | 739 | 23 (6) | 18 (5) |
| NRAS-mut | 739 | 60 (15) | 69 (18) |
| JAK2-mut | 739 | 3 (1) | 4 (1) |
| BCOR-mut or BCOR1-mut | 739 | 29 (7) | 25 (6) |
| EVI1 overexpression | 594 | 21 (5) | 28 (7) |
| Prognostic risk according to 2017 ELN criteria§ | |||
| Favorable | 134 (34) | 150 (39) | |
| Intermediate | 127 (32) | 94 (24) | |
| Adverse | 119 (30) | 139 (36) | |
| Unknown | 12 (3) | 5 (1) |
| . | No. of patients evaluated . | Control induction treatment . | Lenalidomide induction treatment . |
|---|---|---|---|
| Total | 392 (100) | 388 (100) | |
| Male sex | 210 (54) | 233 (60) | |
| Age, y | |||
| Median (range) | 53 (18-65) | 54 (18-65) | |
| ≤45 | 110 (28) | 99 (26) | |
| 46-60 | 194 (49) | 195 (50) | |
| >60 | 88 (22) | 94 (24) | |
| WHO/ECOG performance status | |||
| 0 | 239 (61) | 264 (66) | |
| 1 | 138 (35) | 113 (29) | |
| 2 | 15 (4) | 11 (3) | |
| Diagnostic subgroup | |||
| AML | 356 (91) | 351 (90) | |
| High-risk RAEB | 33 (8) | 35 (9) | |
| Leukemia with ambiguous lineage | 3 (1) | 2 (1) | |
| AML type | |||
| De novo | 358 (91) | 351 (90) | |
| sAML | 19 (5) | 24 (6) | |
| tAML | 15 (4) | 13 (3) | |
| WBC, × 109/L | |||
| Median (range) | 8.0 (0-265) | 6.7 (0.4-297) | |
| ≤20 | 259 (66) | 257 (66) | |
| 20-100 | 109 (28) | 103 (27) | |
| >100 | 23 (6) | 27 (7) | |
| Unknown | 1 (0) | 1 (0) | |
| Median blasts in bone marrow, % | 52 | 52 | |
| Cytogenetics* | |||
| t(8;21) | 11 (3) | 22 (6) | |
| inv(16) or t(16;16) | 23 (6) | 26 (7) | |
| CN-X-Y | 211 (54) | 199 (51) | |
| CA rest | 97 (25) | 95 (24) | |
| Monosomal karyotype | 39 (10) | 34 (9) | |
| Unknown | 11 (3) | 12 (3) | |
| Gene mutations† | |||
| NPM1-mut | 744 | 129 (33) | 115 (30) |
| FLT3-ITD-mut | 742 | 85 (22) | 69 (18) |
| FLT3-TKD835-mut | 742 | 34 (9) | 33 (9) |
| NPM1-mut FLT3-ITD-negative | 741 | 69 (18) | 77 (20) |
| NPM1-mut FLT3-ITD-mut | 59 (15) | 38 (10) | |
| NPM1-wt FLT3-ITD-negative | 217 (55) | 224 (58) | |
| NPM1-wt FLT3-ITD-mut | 26 (7) | 31 (8) | |
| DNMT3A-mut | 739 | 108 (28) | 105 (27) |
| IDH1-mut | 739 | 35 (9) | 34 (9) |
| IDH2-mut | 739 | 45 (11) | 45 (12) |
| TET2-mut | 739 | 54 (14) | 41 (11) |
| Biallelic CEBPA-mut | 739 | 16 (4) | 12 (3) |
| RUNX1-mut | 742 | 41 (10) | 49 (13) |
| ASXL1-mut | 742 | 39 (10) | 31 (8) |
| TP53-mut | 742 | 28 (7) | 29 (7) |
| SF3B1-mut or SRSF2-mut‡ | 739 | 46 (12) | 43 (11) |
| SRSF2-mut‡ | 739 | 30 (8) | 28 (7) |
| PTPN11-mut | 739 | 44 (11) | 33 (9) |
| KRAS-mut | 739 | 23 (6) | 18 (5) |
| NRAS-mut | 739 | 60 (15) | 69 (18) |
| JAK2-mut | 739 | 3 (1) | 4 (1) |
| BCOR-mut or BCOR1-mut | 739 | 29 (7) | 25 (6) |
| EVI1 overexpression | 594 | 21 (5) | 28 (7) |
| Prognostic risk according to 2017 ELN criteria§ | |||
| Favorable | 134 (34) | 150 (39) | |
| Intermediate | 127 (32) | 94 (24) | |
| Adverse | 119 (30) | 139 (36) | |
| Unknown | 12 (3) | 5 (1) |
All data are n (%) unless otherwise indicated. RAEB is defined as having a Revised International Prognostic Scoring System score >4.5.16
CA, abnormal cytogenetics; CN, normal cytogenetics; ECOG, Eastern Cooperative Oncology Group; mut, mutation; sAML, secondary AML (after myelodysplastic syndrome and antecedent hematologic disease); tAML, therapy-related AML (in case of previous chemotherapy or radiotherapy) (for details see “Patients and methods”); WHO, World Health Organization; wt, wild-type.
AML with core binding factor abnormalities: t(8;21)(q22;q22), inv(16)(p13.1;q22), or t(16;16)(p13.1;q22); monosomal karyotype defined as described in Breems et al.20
Gene mutations include ASXL1, additional sex combs–like 1; CEPBA, CCAAT/enhancer-binding protein α; DNMT3A, DNA methyltransferase 3 α; EVI1, ecotropic virus integration 1; FLT3, fms-like tyrosine kinase-3; FLT3-ITD-negative, FLT3 without internal tandem duplications (ITDs); FLT3-TKD835, FLT3-tyrosine kinase domain 835, FLT3 gene with point mutation at position D835; IDH1/IDH2, isocitrate dehydrogenase 1 and 2; NPM1, nuclephosmin-1; PTPN11, protein tyrosine phosphatase nonreceptor type 11; RUNX1, runt-related transcription factor 1; SF3B1, splicing factor 3B subunit; SRSF2, serine/arginine-rich splicing factor 2; TET2, Tet methylcytosine dioxygenase 2.
We considered the frequencies of SRSF2 alone and SRSF2 and SF3B1 in combination.
The ELN prognostic risk categories as described in Döhner et al.1
For the purpose of the analysis, MRD negativity is defined as the absence of mutant NPM1 by quantitative RT-PCR (<10−4) and in NPM1-wild-type patients as negative by MFC (below 0.1%). If RT-PCR assessment for mutated NPM1 AML was not available, the MFC results became the leading results.
Statistical analysis
The trial was powered for EFS, the primary end point for part A of the trial. Assuming the target sample size of 800 patients was to be accrued in 3 years with an additional follow-up of 1 year after the end of enrollment, we estimated that 441 EFS events would be observed by the time of the analysis, and the trial would have 82% power, at a 2-sided significance level of 5% by a log-rank test, to detect a hazard ratio (HR) for EFS of 0.76 (corresponding to an increase in the 3-year EFS rate from 38% in the control arm to 48% in the lenalidomide arm). But when after 2.5 years after the last patient enrollment the target number of events had not yet been reached (427 events reported by February 2020), the Data and Safety Monitoring Board (DSMB) recommended not waiting for the additional events and performing the final trial analysis as soon as all relevant clinical data were clean and as up-to-date as possible.
In all, 253 of 800 enrolled patients were expected to proceed to part B of the trial. Ultimately, however, only 88 patients were randomly assigned for maintenance with lenalidomide vs observation, which means that the analyses of this part of the trial are greatly underpowered and hence the significance of the latter results is limited.
All analyses were performed according to the intention-to-treat principle, irrespective of protocol compliance, but 20 of 800 patients who seemed ineligible after registration were excluded (7 in the control arm and 13 in lenalidomide arm; reasons for ineligibility are provided in the supplemental Data).
Cox regression analysis was used to analyze the effect of treatment on EFS, OS, and RFS with and without adjustment for other covariates, and the response rate variables were analyzed with the use of logistic regression. Because only 2 patients died between the date of second random assignment and the time of the analysis, cumulative incidence of death from the second random assignment was not evaluated, and the CIR from the second random assignment was analyzed using Cox regression. The between-arm difference of the time to recovery after each chemotherapy cycle was tested by means of Fine and Gray regression.
All reported P values are 2-sided, and values of P < .05 were considered statistically significant. No corrections were made for multiple testing. Statistical analyses were performed with STATA Statistical Software, Release 15.1 (STATA, College Station, TX). Details of the statistical analyses are provided in the supplemental Data. Data for this analysis were locked as of 19 May 2020. At the time of the analysis, no patients were receiving the trial treatment and no patients were awaiting random assignment to maintenance therapy. The trial treatment was discontinued in the last patient in June 2019.
Results
Patients
Between 2 February 2015, and 9 August 2017, 800 patients, including 68 with MDS-RAEB, were randomly assigned to induction treatment with or without lenalidomide; 20 patients turned out to be ineligible (see the supplemental Data for details), leaving 780 eligible and evaluable patients for the final analysis. In all, 392 patients were assigned to the standard induction regimen and 388 were assigned to the lenalidomide induction regimen (supplemental Figure 1A-B). Median follow-up time for patients still alive at the date of last contact (n = 436) was 41 months. Table 1 presents the demographic and molecular characteristics of the patients. Median age was 54 years with 23% of patients between age 61 and 66 years. Both treatment groups were comparable regarding clinical, hematologic, cytogenetic, and molecular features.
Treatment, response, and outcome
Of 780 patients, 777 (99.6%) received induction cycle 1 starting at a median of 1 day after study registration. At a median of 37 days after registration, 666 (85%) of 780 patients began treatment with induction cycle 2 (Table 2; supplemental Figure 1A-B). The actual delivery of total doses of anthracycline, cytarabine, and lenalidomide relative to the intended protocol dose levels are given in the supplemental Data. The percentage of patients attaining CR or CRi after induction on protocol was 87% for the control group and 82% for the lenalidomide treatment group, with no statistically significant difference (odds ratio [OR], 0.71; 95% confidence interval [CI], 0.48-1.05; P = .08) (Table 2). Subsequently, 59 (8%) of 780 patients received chemotherapy cycle 3, 170 (22%) underwent auto-SCT, and 304 (39%) proceeded to allo-SCT. The percentages of complete responders (CR/CRi) who received cycle 3, auto-SCT, or allo-SCT were 9%, 26%, and 46%, respectively.
Treatment and outcomes comparison between control and lenalidomide remission induction
| . | Control induction therapy . | Lenalidomide induction therapy . | Logistic/Cox regression* . | ||
|---|---|---|---|---|---|
| OR/HR . | 95% CI . | P . | |||
| Total no. of patients | 392 (100) | 388 (100) | |||
| Treatment | |||||
| Remission induction | |||||
| Cycle 1 | 391 (100) | 386 (99) | |||
| Cycle 2 | 340 (87) | 326 (84) | |||
| Consolidation therapy after CR/CRi | 279 (71) | 254 (65) | |||
| Cycle 3 | 25 (6) | 34 (9) | |||
| Auto-SCT | 94 (24) | 76 (20) | |||
| Allo-SCT | 160 (41) | 144 (37) | |||
| Outcomes | |||||
| CR/CRi after induction | 340 (87) | 319 (82) | 0.71 | 0.48-1.05 | .08 |
| Early CR/CRi (attained before remission induction cycle 2) | 276 (70) | 254 (65) | |||
| Early death | |||||
| Death within 30 days | 11 (3) | 18 (5) | |||
| Death within 60 days | 20 (5) | 26 (7) | |||
| At 4 years (% ± SE): | |||||
| EFS† | 44 ± 3 | 44 ± 3 | 0.99 | 0.82-1.20 | .96 |
| OS† | 54 ± 3 | 54 ± 3 | 0.98 | 0.79-1.21 | .83 |
| RFS† | |||||
| RFS | 49 ± 3 | 51 ± 3 | 0.95 | 0.77-1.18 | .66 |
| Relapse | 39 ± 3 | 36 ± 3 | |||
| Death | 12 ± 2 | 13 ± 2 | |||
| MRD negativity after cycle 2‡ | 167 (78) | 161 (77) | 0.92 | 0.59-1.46 | .73 |
| . | Control induction therapy . | Lenalidomide induction therapy . | Logistic/Cox regression* . | ||
|---|---|---|---|---|---|
| OR/HR . | 95% CI . | P . | |||
| Total no. of patients | 392 (100) | 388 (100) | |||
| Treatment | |||||
| Remission induction | |||||
| Cycle 1 | 391 (100) | 386 (99) | |||
| Cycle 2 | 340 (87) | 326 (84) | |||
| Consolidation therapy after CR/CRi | 279 (71) | 254 (65) | |||
| Cycle 3 | 25 (6) | 34 (9) | |||
| Auto-SCT | 94 (24) | 76 (20) | |||
| Allo-SCT | 160 (41) | 144 (37) | |||
| Outcomes | |||||
| CR/CRi after induction | 340 (87) | 319 (82) | 0.71 | 0.48-1.05 | .08 |
| Early CR/CRi (attained before remission induction cycle 2) | 276 (70) | 254 (65) | |||
| Early death | |||||
| Death within 30 days | 11 (3) | 18 (5) | |||
| Death within 60 days | 20 (5) | 26 (7) | |||
| At 4 years (% ± SE): | |||||
| EFS† | 44 ± 3 | 44 ± 3 | 0.99 | 0.82-1.20 | .96 |
| OS† | 54 ± 3 | 54 ± 3 | 0.98 | 0.79-1.21 | .83 |
| RFS† | |||||
| RFS | 49 ± 3 | 51 ± 3 | 0.95 | 0.77-1.18 | .66 |
| Relapse | 39 ± 3 | 36 ± 3 | |||
| Death | 12 ± 2 | 13 ± 2 | |||
| MRD negativity after cycle 2‡ | 167 (78) | 161 (77) | 0.92 | 0.59-1.46 | .73 |
All data are n (%) unless otherwise indicated. OR is estimated for CR/CRi after induction and for MRD negativity rate after cycle 2. HR applies to EFS, OS, and RFS.
Logistic/Cox regression included induction treatment arm and diagnostic subgroup (AML or RAEB) as covariates.
Percentages are actuarial 4-year probabilities.
MRD negativity rate is evaluated relative to 424 patients with evaluable MRD after induction cycle 2 (214 in the control arm and 210 in the lenalidomide arm).
Table 2 summarizes the values for the distinct clinical end points split by treatment group. For the analysis of EFS, 430 events were observed. The median EFS was 24 months for the control group and 21 months for the lenalidomide treatment group, which corresponds with respective EFS rates at 4 years of 44% ± 3% (standard error [SE]) and 44% ± 3%, which implies no advantage for lenalidomide (HR, 0.99; 95% CI, 0.82-1.20; P = .96) (Table 2; Figure 2). The latter conclusion remains unchanged after adjustment for known prognostic factors at diagnosis (WBC [log-transformed], age, 2017 ELN risk group, AML type) (Table 3). When we accounted for allo-SCT and for consolidation treatment in general, no difference in EFS estimates between the control and lenalidomide treatment groups became apparent either. Further, no differential effect of treatment on competing risks of EFS was observed.
EFS and OS following lenalidomide remission induction therapy vs control induction treatment. EFS (A) and OS (B) for patients receiving remission induction therapy (control group vs lenalidomide [Lena] therapy group). Patients were randomly assigned for their first and second induction cycles of combination chemotherapy without additional lenalidomide (control treatment) or with lenalidomide at 15 mg on days 1 to 21 of both cycles. F, failure (or event).
EFS and OS following lenalidomide remission induction therapy vs control induction treatment. EFS (A) and OS (B) for patients receiving remission induction therapy (control group vs lenalidomide [Lena] therapy group). Patients were randomly assigned for their first and second induction cycles of combination chemotherapy without additional lenalidomide (control treatment) or with lenalidomide at 15 mg on days 1 to 21 of both cycles. F, failure (or event).
Multivariable Cox regression analysis for EFS
| Variable . | HR ± SE . | 95% CI . | P . |
|---|---|---|---|
| Lenalidomide treatment | 0.96 ± 0.09 | 0.79-1.17 | .70 |
| Age | 1.00 ± 0.00 | 1.00-1.01 | .30 |
| WBC | 1.07 ± 0.04 | 1.01-1.15 | .03 |
| 2017 ELN risk group | <.001 | ||
| Favorable | 0.55 ± 0.07 | 0.42-0.71 | <.001 |
| Adverse | 1.48 ± 0.17 | 1.18-1.87 | <.001 |
| Unknown | 0.57 ± 0.21 | 0.27-1.16 | .10 |
| AML type | .04 | ||
| sAML | 1.61 ± 0.30 | 1.11-2.33 | .01 |
| tAML | 1.09 ± 0.28 | 0.66-1.82 | .73 |
| Variable . | HR ± SE . | 95% CI . | P . |
|---|---|---|---|
| Lenalidomide treatment | 0.96 ± 0.09 | 0.79-1.17 | .70 |
| Age | 1.00 ± 0.00 | 1.00-1.01 | .30 |
| WBC | 1.07 ± 0.04 | 1.01-1.15 | .03 |
| 2017 ELN risk group | <.001 | ||
| Favorable | 0.55 ± 0.07 | 0.42-0.71 | <.001 |
| Adverse | 1.48 ± 0.17 | 1.18-1.87 | <.001 |
| Unknown | 0.57 ± 0.21 | 0.27-1.16 | .10 |
| AML type | .04 | ||
| sAML | 1.61 ± 0.30 | 1.11-2.33 | .01 |
| tAML | 1.09 ± 0.28 | 0.66-1.82 | .73 |
The following covariates were considered for this analysis: patient age, WBC × 109/L at diagnosis (log transformation), 2017 ELN risk category1 (values expressed relative to intermediate-risk group), sAML and tAML (relative to de novo AML).
Median OS was 56 months for the control treatment, and it was not yet reached in the lenalidomide group. For the combined treatment groups, the 4-year OS was estimated at 54% ± 2% (SE) with no difference between the 2 arms (HR, 0.98; 95% CI, 0.79-1.21; P = .83) (Figure 2). Further adjustment for allo-SCT and for consolidation treatment did not alter the results.
In those with CR or CRi, the 4-year RFS was 50% ± 2% with no difference between the 2 arms (HR, 0.95; 95% CI, 0.77-1.18; P = .66). Nor was a between-arm difference observed for the competing risks of RFS (ie, relapse and nonrelapse mortality) (Table 2).
Prognostic factors and subgroup analysis
In an exploratory analysis, the possible differential effect of lenalidomide treatment on EFS and OS was evaluated in a variety of subgroups distinguished by prognostic factors for treatment outcome (patient age at registration, AML type (de novo AML, sAML, tAML), disease type (AML, RAEB), WBC at diagnosis, and 2017 ELN risk group; EFS results are provided in supplemental Figure 2) and also according to molecular genotypes. No convincing indications were found that selected prognostic subgroups benefit from addition of lenalidomide (compared with the control treatment), except for a statistically significant survival advantage for treatment with lenalidomide in 58 patients with SRSF2-mutated AML (57% vs 33% EFS at 4 years; HR, 0.47; 95% CI, 0.23-0.96; P = .04; supplemental Figure 2). This difference is explained by a lower relapse rate in the lenalidomide treatment group (67% vs 42% RFS at 4 years; HR, 0.39; 95% CI, 0.17-0.93; P = .03). The advantage of lenalidomide treatment in the SRSF2-mutated subset was also apparent regarding OS (68% vs 43% at 4 years; HR, 0.42; 95% CI, 0.19-0.94; P = .03). Remarkably, in the current risk-adjusted treatment study without the use of an FLT3 inhibitor, FLT3-internal tandem duplication (FLT-ITD) does not express any prognostic value or for either high or low allelic ratios (EFS: 43% FLT3-ITD+ vs 45% FLT3-ITD– at 4 years; HR, 1.12; 95% CI, 0.88-1.42; P = .35; OS: 59% FLT3-ITD+ vs 53% FLT3-ITD– at 4 years; HR, 0.90; 95% CI, 0.68-1.19; P = .47; supplemental Figure 3).
Assessment of MRD in CR/CRi after cycle 2
MRD status after induction cycle 2 was evaluated centrally according to protocol with NPM1 qRT-PCR and MFC in 424 (64%) of 666 patients who had received cycle 2 and continued in CR/CRi. Among these patients, the overall MRD negativity rate after induction cycle 2 was 77% with no difference between the comparative treatment arms (OR, 0.92; 95% CI, 0.59-1.46; P = .73) (Table 2).
MRD positivity after induction cycle 2 correlated with a significant negative impact on RFS (43% vs 57% at 4 years; HR, 1.75; 95% CI, 1.27-2.40; P = .001) and OS (51% vs 66% at 4 years; HR, 1.98; 95% CI, 1.39-2.81; P < .001); however, there was no apparent difference in outcome between the control and lenalidomide treatment groups or in MRD-positive patients or in MRD-negative patients (Figure 3). MRD information is generally considered most useful for guiding treatment choice in intermediate-risk AML. It seems that when choosing MRD-guided treatment, the prognostic significance of MRD in intermediate-risk AML is entirely lost (Figure 4). Furthermore, especially among the consolidated intermediate-risk patients with negative MRD, 31% proceeded to auto-SCT and 55% proceeded to allo-SCT, whereas in those with positive MRD, only 8% proceeded to auto-SCT and 88% proceeded to allo-SCT. The data suggest that, in MRD-negative intermediate-risk patients, auto-SCT furnishes an effective alternative to allo-SCT in terms of OS (OS in the MRD-negative subgroup: 76% auto-SCT vs 68% allo-SCT at 4 years; HR, 0.71; 95% CI, 0.27-1.86; P = .49; RFS in the MRD-negative group: 52% auto-SCT vs 62% allo-SCT at 4 years; HR, 1.45; 95% CI, 0.69-3.07; P = .33; for survival curves, see supplemental Figure 4). Of note, analyses showed similar relationships for patients with favorable-risk disease (supplemental Figure 5). Obviously, these conclusions should be interpreted with some caution because of low numbers of patients and the nonrandomized comparison of the consolidation options.
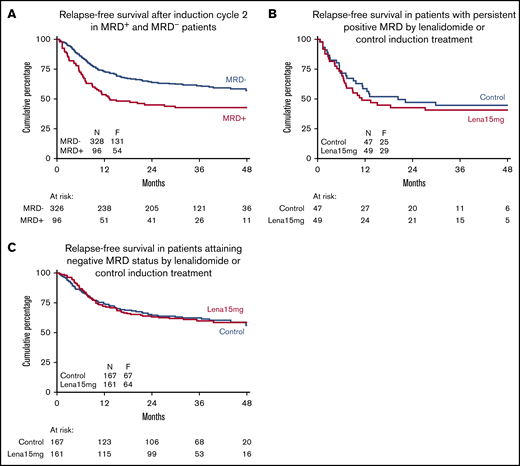
Relapse-free survival of complete responders after remission induction therapy according to MRD-negative/positive status. RFS according to attainment of MRD negativity for all patients (A), in the subgroup of patients with persistent positive MRD (B), and in those attaining a status of MRD negativity according to remission induction therapy (control group vs lenalidomide therapy group) (C). The unfavorable effect of MRD positivity is evident, but there is no apparent effect of lenalidomide treatment on outcome in patients attaining a status of MRD negativity or in patients with persistent MRD.
Relapse-free survival of complete responders after remission induction therapy according to MRD-negative/positive status. RFS according to attainment of MRD negativity for all patients (A), in the subgroup of patients with persistent positive MRD (B), and in those attaining a status of MRD negativity according to remission induction therapy (control group vs lenalidomide therapy group) (C). The unfavorable effect of MRD positivity is evident, but there is no apparent effect of lenalidomide treatment on outcome in patients attaining a status of MRD negativity or in patients with persistent MRD.
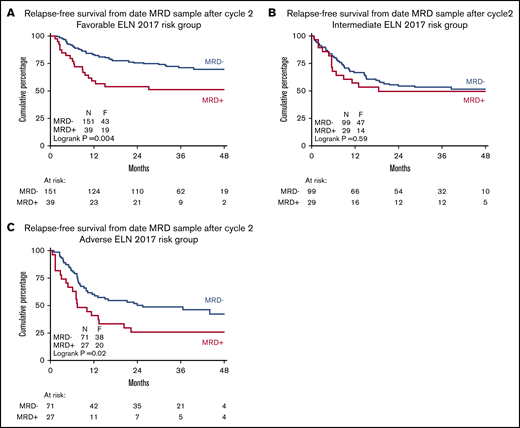
Prognostic value of MRD status after remission induction in distinct AML risk groups in context of MRD-guided consolidation treatment choice. RFS by MRD status after induction cycle 2 in favorable risk (A), intermediate risk (B), and adverse risk (C) 2017 ELN risk classification groups. The prognostic significance of MRD positivity is lost in intermediate-risk AML in the context of MRD-guided treatment choice.
Prognostic value of MRD status after remission induction in distinct AML risk groups in context of MRD-guided consolidation treatment choice. RFS by MRD status after induction cycle 2 in favorable risk (A), intermediate risk (B), and adverse risk (C) 2017 ELN risk classification groups. The prognostic significance of MRD positivity is lost in intermediate-risk AML in the context of MRD-guided treatment choice.
Safety and tolerability
The 2 treatment arms were compared with respect to AEs, time of neutrophil and platelet recovery, platelet transfusion requirements, and number of nights spent in the hospital (supplemental Table 1). The incidence and severities of AEs were comparable between the arms during induction and maintenance phase with no apparent differences in the frequencies of AEs of special interest (eg, pulmonary embolism and thrombosis with a frequency of 5% after cycle 1 and 6% after induction cycle 2). The frequencies of patients presenting with second primary malignancies registered during study follow-up was 4% and did not differ between the treatment arms.
Time to neutrophil and platelet recovery and platelet transfusion requirements after induction cycle 1 did not differ, but after cycle 2 and cycle 3, both neutrophil and platelet recovery became progressively delayed in patients assigned to lenalidomide treatment. By comparison, the median number of days to neutrophil and platelet recovery was prolonged by an additional 2 to 4 days after cycle 2, and the median recovery intervals for neutrophils and platelets after chemotherapy in cycle 3 were delayed by an extra 11 and 24 days, respectively (supplemental Table 1). Patients in the lenalidomide treatment group remained dependent on platelet transfusions during prolonged intervals after cycle 2 and cycle 3. Cycle 3 patients who received lenalidomide during induction spent more nights in the hospital (supplemental Table 1). Early mortality rates (at 30 and 60 days) were 4% and 6%, respectively, with no differences between the treatment groups.
Randomization for maintenance with lenalidomide
In part B of the trial, 45 patients were assigned to lenalidomide maintenance and 43 patients were assigned to observation. These patients had a median age of 47 years and included 65% males and 3 patients with RAEB, but there were no notable differences in clinical or hematologic characteristics between the groups (data not shown). Detailed actual dose delivery of study drug is described in the supplemental Data. The percentage of patients who experienced relapse (primary end point for this part of the trial) was 24% in the lenalidomide maintenance group and 28% in the observation group. The data revealed no differences between maintenance and observation regarding CIR with an HR of 0.81 (95% CI, 0.36-1.85; P = .62) (Figure 5) or differences in death (HR for OS, 0.51; 95% CI, 0.15-1.72; P = .28), implying that there is no suggestion of a benefit for lenalidomide treatment after consolidation (Table 4).
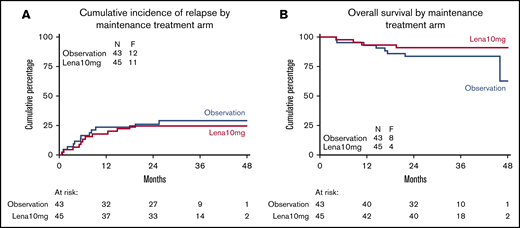
Relapse probability and OS of complete responders following maintenance treatment with lenalidomide or observation. CIR (A) and OS (B) in patients in CR/CRi randomly assigned between lenalidomide maintenance treatment and observation.
Relapse probability and OS of complete responders following maintenance treatment with lenalidomide or observation. CIR (A) and OS (B) in patients in CR/CRi randomly assigned between lenalidomide maintenance treatment and observation.
Patient outcome according to random assignment for lenalidomide maintenance or observation
| . | Observation . | Lenalidomide maintenance . | Cox regression* . | ||
|---|---|---|---|---|---|
| HR . | 95% CI . | P . | |||
| Total | 43 (100) | 45 (100) | |||
| Outcomes | |||||
| Relapse after second randomization | 12 (28) | 11 (24) | |||
| Death after second randomization | 8 (19) | 4 (9) | |||
| Relapse-free mortality after second randomization | 2 (5) | — | |||
| At 3 years (% ± SE): | |||||
| CIR† | 34 ± 10 | 28 ± 8 | 0.81 | 0.36-1.85 | .62 |
| RFS† | 67 ± 7 | 75 ± 6 | 0.70 | 0.32-1.55 | .38 |
| OS† | 84 ± 6 | 91 ± 4 | 0.51 | 0.15-1.72 | .28 |
| MRD positive | |||||
| Before second randomization | 2 (5) | 4 (9) | |||
| After second randomization | 2 (5) | 1 (2) | |||
| . | Observation . | Lenalidomide maintenance . | Cox regression* . | ||
|---|---|---|---|---|---|
| HR . | 95% CI . | P . | |||
| Total | 43 (100) | 45 (100) | |||
| Outcomes | |||||
| Relapse after second randomization | 12 (28) | 11 (24) | |||
| Death after second randomization | 8 (19) | 4 (9) | |||
| Relapse-free mortality after second randomization | 2 (5) | — | |||
| At 3 years (% ± SE): | |||||
| CIR† | 34 ± 10 | 28 ± 8 | 0.81 | 0.36-1.85 | .62 |
| RFS† | 67 ± 7 | 75 ± 6 | 0.70 | 0.32-1.55 | .38 |
| OS† | 84 ± 6 | 91 ± 4 | 0.51 | 0.15-1.72 | .28 |
| MRD positive | |||||
| Before second randomization | 2 (5) | 4 (9) | |||
| After second randomization | 2 (5) | 1 (2) | |||
Data are n (%) unless otherwise stated.
Cox regression included maintenance treatment arm, induction treatment arm, and type of consolidation treatment as covariates.
Percentages are actuarial 3-year probabilities.
MRD status was also assessed in a small number of patients at 6 months after the second random assignment and compared with the MRD status immediately before the second random assignment. The status of MRD negativity and positivity did not differ according to the randomization (Table 4).
Discussion
Preclinical and early clinical studies with single-agent lenalidomide and lenalidomide plus hypomethylating agent or chemotherapy drug combinations provided contradictory data about the potential therapeutic value of lenalidomide in the treatment of AML.7-15 This article reports the first large phase 3 study with mature follow-up in a head-to-head comparison on the use of lenalidomide as an adjunct integrated into intensive remission chemotherapy in newly diagnosed patients with AML younger than age 66 years. The results of the study fail to furnish indications for a positive therapeutic effect regarding various clinical parameters, including response rates, achievement of MRD negativity, EFS, and other survival end points. Although current established first-line treatments in AML need to be improved in terms of efficacy, it appears that the addition of lenalidomide does not satify this need. One question is whether we have used an optimal dosing schedule. In some other studies, lenalidomide was given at higher daily dose levels (even up to 50 mg) and/or over prolonged intervals for more days, but in several instances the tolerability caused problems.11,14 In our study, the run-in randomized study in 127 patients demonstrated excess toxicity (infections, hematologic effects) for a dose of 20 mg so that based on the recommendations of the independent DSMB, a final dose of 15 mg was selected for the phase 3 study. The safety of lenalidomide at the selected dose was manageable (eg, in terms of AEs and early mortality), but the addition of lenalidomide generated cumulative hematologic toxicities that became apparent from cycle 2 onward as evidenced by the delayed neutrophil and platelet recovery times, the increase in transfusion requirements, and the increase in hospitalization days. A more plausible explanation for the failure of lenalidomide therapeutic efficacy in this study is that lenalidomide is at best a moderately active drug in AML that adds little value to an intensive treatment regimen, at least in the general AML population. This is consistent with previous experiences of failure on multiple occasions of adding cytotoxic drugs to 7+3–like treatment regimens. In light of the considerable genetic heterogeneity of AML, it is an unrealistic logistic and methodologic challenge to test the potential value of a specific drug in individual sybtypes across the diverse disease genotypes with sufficient power. A general 7+3–based treatment approach evidently is far from ideal for identifying potential benefits of a new drug for a particular subtype; in this study, in an exploratory and hypothesis-generating analysis, lenalidomide showed indications for survival and relapse benefits in SRSF2-mutant AML. Whether these positive effects are true would require further study.
Our study lacked statistical power regarding a robust evaluation of lenalidomide for maintenance, although most of the randomly assigned patients were able to receive at least 5 cycles of lenalidomide. Only 88 (42%) of 212 patients who had received cycle 3 or auto-SCT and had remained in CR/CRi entered this part of the study. The reason for the relatively small numbers of patients enrolled relates to the strict inclusion criteria that excluded many patients for reasons of intercurrent relapse, refusal of treatment, and not fulfilling the condition of absolute neutrophil count ≥1.5 × 109/L and platelet count ≥75 × 109/L (supplemental Figure 1A-B).
In a general sense, it is notable that this study, with an 84% CR/CRi rate after no more than 2 induction cycles and only 1 risk-adapted consolidation treatment (cycle 3, auto-SCT, or allo-SCT), showed favorable EFS (44% at 4 years) and OS (54% at 4 years) and median OS estimates of 61 months compared with historical studies.2 The EFS and OS results, for instance, are distinctly better than those of our most recent previous study of similar size and with a similar median age of 55 years (EFS 36% and OS 43% at 4 years) which, being at variance with this study, had not applied MRD guidance for postremission treatment assignment and had fewer patients allocated to auto-SCT (8% vs 22% in this study).2 MRD is especially clinically relevant for informing treatment choice in intermediate-risk AML. Thus, the similar treatment outcomes of patients in the intermediate prognostic AML spectrum with positive MRD and negative MRD (of whom many were consolidated with auto-SCT with avoidance of allo-SCT) is reassuring about the value of MRD-adapted treatment choice (RFS: 50% MRD-positive vs 52% MRD-negative at 4 years; HR, 1.18; 95% CI, 0.65-2.14; P = .59; OS: 64% MRD-positive vs 69% MRD-negative at 4 years; HR, 1.31; 95% CI, 0.64-2.69; P = .46).
Finally, this large study allowed for additional clinically relevant observations. First, detailed centralized molecular diagnostics and MRD monitoring in an international multicenter setting confirmed the prognostic value of MRD status in CR/CRi for survival (RFS and OS), although it seems that in this case, the risk-adjusted treatment may have reduced its quantitative impact. Second, the era of studies in molecular/biologic nonstratified AML has ended. Our study is likely one of the last to be conducted in FLT3-mutated AML that applied 7+3–based remission induction therapy without an FLT3 inhibitor. It is informative to note that in this risk-adapted study, there was absolutely no indication of a negative prognostic impact of FLT3-ITD on outcome for either high or low mutational FLT3-ITD burden.
To request data, please e-mail Bob Löwenberg at b.lowenberg@erasmusmc.nl.
Acknowledgments
The authors thank The Dutch Cancer Foundation for financial support and the local institutional data managers as well as the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) Data Center Trial teams responsible for the central data management for their eminent support, and Jeroen Knijnenburg (Leiden), Pino Poddighe (Amsterdam), Clemens Mellink (Amsterdam), and Simone Snijder (Utrecht) for cytogenetic review.
This investigator-sponsored trial was supported by Celgene (currently Bristol Myers Squibb), and lenalidomide was provided free of charge.
Authorship
Contribution: The study was conceived and designed by the Leukemia Working Group of the Dutch-Belgian Hemato-Oncology Cooperative Group/Swiss Group for Clinical Cancer Research (HOVON/SAKK) Cooperative consortium, which included all of the authors; P.J.M.V. performed the molecular analyses; J.C. performed immunodiagnostic and MFC-MRD analyses; B.B. and L.M. coordinated the cytogenetic analyses; B.L., T.P., J.M., B.J.B., O.S., E.V., L.G., L.W.T., M.J.-L., M.v.M.K., M.-C.V., W.J.F.M.v.d.V., C.G., D.D., O.d.W., J.W.J.v.E., M.B., S.K.K., A.G., P.E.W., H.V., M.G., T.S., D.v.L.-V., I.M., D.A.B., M.H., M.-C.J.C.L., T.F., J.K., J.C., K.P., G.J., P.M., M.H., B.T.G., J.J.W.M.J., G.H., J.P., C.H.M.J.v.E., M.G.M., Y.F., and G.J.O. recruited patients; P.G. conducted the statistical analysis; B.L., P.G., and G.J.O. reviewed the data analysis and interpretation; B.L. and P.G. produced the first version of the manuscript which was circulated for comments to the other authors; and all authors contributed to the conduct of the trial and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bob Löwenberg, Department of Hematology, Erasmus University Medical Center, Faculty Building, Room Ee1314, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: b.lowenberg@erasmusmc.nl.
References
Author notes
The full-text version of this article contains a data supplement.

![EFS and OS following lenalidomide remission induction therapy vs control induction treatment. EFS (A) and OS (B) for patients receiving remission induction therapy (control group vs lenalidomide [Lena] therapy group). Patients were randomly assigned for their first and second induction cycles of combination chemotherapy without additional lenalidomide (control treatment) or with lenalidomide at 15 mg on days 1 to 21 of both cycles. F, failure (or event).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/4/10.1182_bloodadvances.2020003855/1/m_advancesadv2020003855f2.png?Expires=1769121411&Signature=y-AD58oK7HGtvT5lfuChBeGbqq3OoX2bSuqTyMYJx8JPWaMHUgNFI4p-7KKtnDGSgFyrK4W38D4nCCEk74TCMUlZ0VZeyljWDjwFvqKLoCSuT5weIqT76fT97jhepz0PT72GdAyvoGYvcCYsyseJQvXbj4ZfdWVpCVyo5xs~~vzg7YqMYDVEKRoIyHbFdgclgxkTUfJIhuD3L0fz5QOTGHPYc8w6E62vBNAsgYYi5ozOIsoDN3rUfXPgyy~fcO53sgAwSJ15zX68TWTYpzJRaSJUE9mOp4SGg~fvCznYUIDEXVawS~DJl09owUf4smlz1hOM8bwBxrodXZ9xU8ixVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)