Key Points
Ancestral sequence reconstruction identified recombinant FIX variants that possess enhanced activity equivalent and additive to FIX Padua.
AAV-ancestral FIX Padua vectors display enhanced potency over AAV-human FIX Padua vectors in hemophilia B mice.
Abstract
Orthologous proteins contain sequence disparity guided by natural selection. In certain cases, species-specific protein functionality predicts pharmacological enhancement, such as greater specific activity or stability. However, immunological barriers generally preclude use of nonhuman proteins as therapeutics, and difficulty exists in the identification of individual sequence determinants among the overall sequence disparity. Ancestral sequence reconstruction (ASR) represents a platform for the prediction and resurrection of ancient gene and protein sequences. Recently, we demonstrated that ASR can be used as a platform to facilitate the identification of therapeutic protein variants with enhanced properties. Specifically, we identified coagulation factor VIII (FVIII) variants with improved specific activity, biosynthesis, stability, and resistance to anti-human FVIII antibody–based inhibition. In the current study, we resurrected a panel of ancient mammalian coagulation factor IX (FIX) variants with the goal of identifying improved pharmaceutical candidates. One variant (An96) demonstrated 12-fold greater FIX activity production than human FIX. Addition of the R338L Padua substitution further increased An96 activity, suggesting independent but additive mechanisms. after adeno-associated virus 2 (AAV2)/8-FIX gene therapy, 10-fold greater plasma FIX activity was observed in hemophilia B mice administered AAV2/8-An96–Padua as compared with AAV2/8-human FIX–Padua. Furthermore, phenotypic correction conferred by the ancestral variant was confirmed using a saphenous vein bleeding challenge and thromboelastography. Collectively, these findings validate the ASR drug discovery platform as well as identify an ancient FIX candidate for pharmaceutical development.
Introduction
Collectively, deficiencies in coagulation factor VIII (FVIII) or IX (FIX) represent the most common severe bleeding disorder, hemophilia, and are designated A or B, respectively. Their combined prevalence is estimated at 1 in 3333 newborn males.1 Despite dramatic improvements in the standard of care through factor replacement therapy, this option remains limited to a minor fraction of the total hemophilia population because of product cost, compliance with lifelong IV therapy, and antidrug antibody responses, clinically referred to as inhibitors.
Gene therapy represents a potentially transformative therapeutic option, and numerous FVIII and FIX gene therapy product candidates are progressing through clinical development. One common aspect of all clinical gene therapy programs is the inclusion of bioengineered elements, including vector capsid, transcriptional regulatory elements, and/or transgenes to maximize product potency and durability while reducing the risks of vector-related toxicities that remain a challenge to adeno-associated virus (AAV) gene therapy achieving 100% normal FIX activity levels. Therefore, further improvement in vector potency seems necessary to unlock the full potential of liver-directed AAV gene therapy for hemophilia B.
Recently, we demonstrated the utility of ancestral sequence reconstruction (ASR) for the discovery of FVIII variants possessing greater expression, specific activity, and active half-life compared with human FVIII while also exhibiting reduced antigenicity to human FVIII (hFVIII) inhibitors.2 Despite extensive research focused on extant FVIII orthologs and their biochemical differences (horizontal comparisons), limited progress has been made toward understanding structure/function relationships at high resolution, and even less has been made toward the translation of preclinical findings into clinical study and practice. Unlike horizontal study of extant protein orthologs, ASR facilitates vertical analyses through the prediction of ancient gene/protein sequences followed by de novo DNA synthesis and laboratory resurrection in the form of recombinant proteins or gene therapy transgene products. ASR also provides a high-resolution mapping solution by enabling empirical comparisons of ancient proteins predicted within sequential branches on a phylogeny.3 Furthermore, ASR generates protein variants that uniformly possess the intended biomolecular function yet can also display unpredicted or expanded properties. In the current study, ASR was applied to FIX with the goal of harnessing information contained in the ancient and extant vertebrate coagulation systems toward hemophilia B biopharmaceutical development.
Materials and methods
Ancestral FIX sequence inference and plasmid construction
FIX ASR was performed as described previously.2 Fifty-nine extant FIX sequences were aligned using MUSCLE, and an evolutionary tree extending beyond Mammalia was inferred using MrBayes. Ancient mammalian and reptilian sequences were inferred using both DNA and amino acid–based models in PAML (version 4.1). On the basis of the amino acid sequences inferred for An102, An97, An96, An88, An84, An70, An65, and An63, complementary DNA (cDNA) sequences were generated using a liver codon optimization (LCO) algorithm described previously and de novo synthesized by GenScript (Piscataway, NJ) to contain flanking 5' XhoI and 3' NotI restriction sites for subcloning into the mammalian expression vectors ReNeo and pcDNA 3.4. as well as recombinant adeno-associated and lentiviral vector expression plasmids.4 Human FIX-T148 as selected as the wild-type FIX control based on its higher allele frequency and prior functional characterization by our laboratory.5
AnFIX expression analysis
HEK293T/17 and HepG2 cells were transfected with polyethylenimine or TransIT-X2, respectively, in antibiotic-free media. Transiently transfected cells were washed twice with Dulbecco’s phosphate-buffered saline and carefully switched to serum-free media 24 hours after transfection. FIX activity was measured by 1-stage coagulation assay after an additional 24-hour culture period. Stable Expi293F clones were generated, and 18 to 25 FIX-expressing clones per construct were isolated for screening. FIX activity was measured in serum-free media by 1-stage coagulation assay and normalized to cell counts taken at the time of FIX activity determination.
Purification of AnFIX variants
Expi293F clones displaying the greatest rate of FIX production were expanded into Erlenmeyer flasks, and FIX was collected in serum-free media clarified by centrifugation at 1000× g for 25 minutes and stored at −20°C until purification. Recombinant FIX was purified using methods similar to those described previously and are presented in the data supplement.6 Elution fractions were analyzed for purity via sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Activity determinations were made using 1-stage coagulation assay, and mass concentrations were measured by A280 with A320 correction using an estimated extinction coefficient of 64 590 M−1 cm−1. Purified FIX preparations were aliquoted and stored at −80°C. Comparisons were made to a commercial recombinant human FIX product (BeneFIX; Takeda).
Thrombin generation assay
Calibrated automated thrombography was performed using a Thrombinoscope (Diagnostica Stago, Inc., Parsippany, NJ). Briefly, 80 μL of warmed FIX-deficient plasma was placed in a 2HB transparent round-bottom 96-well plate. Twenty microliters of thrombin calibrator or PPP-Reagent Low was added to either calibration wells or test wells, respectively. FIX was added at the specified concentration in triplicate wells and the program initiated per manufacturer instructions.
AAV-FIX production and characterization
Recombinant AAV2(ITR)/8(capsid) vectors were manufactured and titrated via quantitative polymerase chain reaction (qPCR) by ViGene (Rockville, MD). Additionally, the AAV2/8-HHS4 vector preps were subjected to size-exclusion chromatography/multiangle light scattering (SEC-MALS) analysis using an Infinity II high-performance liquid chromatography system (Agilent, Santa Clara, CA), 18-angle DAWN (Wyatt Technologies, Santa Barbara, CA), Optilab (Wyatt Technologies), AAV column (Wyatt Technologies), and ASTRA 7.3 software (Wyatt Technologies). This approach has been described previously, and additional information is presented in the data supplement.7
In vivo FIX gene transfer
The AAV2/8 vector design consisted of synthetic liver-directed promoters (either hepatic combinatory bundle [HCB] or HHS4-TTR), a minute virus of mouse intron, a minimal synthetic β-globin polyadenylation sequence, and either the hFIX-148T-LCO, An-96-LCO, or An96-Padua-LCO transgene. Vector sequences are provided in the data supplement. Mice were weighed and randomized. AAV vector was diluted to the desired vector genomes (vg) per kilogram or vector particle (vp) per kilogram dose in 100 μL of sterile DPBS with 0.001% Pluronic F68. Blinded AAV doses were administered to hemophilia B mice age 8 to 12 weeks. Blood plasma was collected biweekly after AAV administration. FIX activity was measured by 1-stage coagulation assay using a standard curve generated from pooled citrated human plasma (FACT). Experimental and control mice were subjected to saphenous vein bleed challenge as previously described or thromboelastography as described in this report.
Statistical analysis
All data analysis, graphing, and statistical calculations were performed using GraphPad Prism 7.04 software (GraphPad Software, San Diego, CA). Comparisons made between AnFIX constructs and hFIX with or without Padua were performed by 1-way analysis of variance. Individual comparisons among the groups were made by post hoc multiple comparison testing (Tukey or Holm-Sidak as specified).
Results
FIX ASR screen
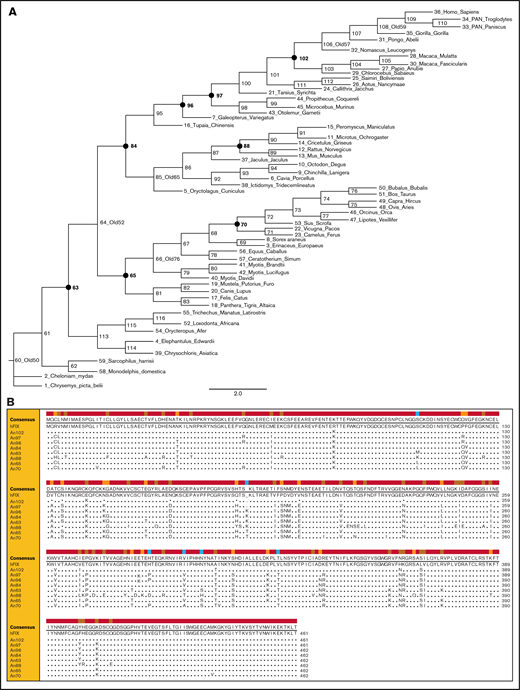
ASR of FIX was performed as previously described to infer a phylogenetic tree beyond the class Mammalia (Figure 1A).2 AnFIX sequences representing 8 nodes sharing 83% to 98% amino acid identity with hFIX were reconstructed using the LCO algorithm described previously and de novo cDNA synthesis.4Figure 1B and supplemental Table 1 illustrate the amino acid sequence variation in the reconstructed AnFIX sequences compared with hFIX.
AnFIXphylogeny and sequences. (A) Phylogeny and ancestral FIX sequences were constructed using extant FIX sequence information. A phylogram of the tree is shown for display purposes. Closed circles and bold numbers denote node designations for reconstructed AnFIX molecules. (B) Amino acid sequence alignment of AnFIX sequences was generated using Clone Manager 9 software. Sequence variations from hFIX are shown in the lower rows.
AnFIXphylogeny and sequences. (A) Phylogeny and ancestral FIX sequences were constructed using extant FIX sequence information. A phylogram of the tree is shown for display purposes. Closed circles and bold numbers denote node designations for reconstructed AnFIX molecules. (B) Amino acid sequence alignment of AnFIX sequences was generated using Clone Manager 9 software. Sequence variations from hFIX are shown in the lower rows.
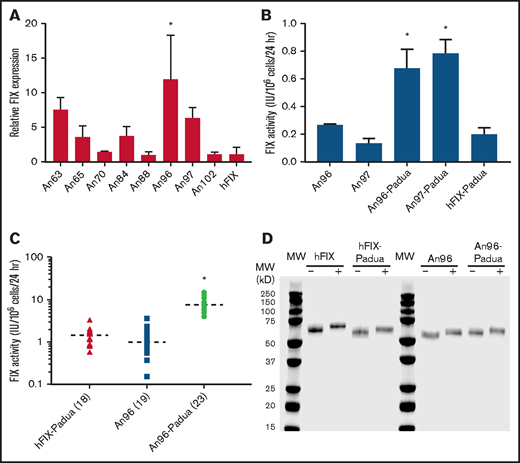
As an initial screen for AnFIX candidates with enhanced properties, the 1-stage coagulation assay was used. This assay allows for the detection of potential variants with more efficient FIX biosynthesis (ie, protein translation and secretion) and/or enhanced specific activity, but it does not distinguish between the 2 possibilities. Inferred ancient cDNA sequences were cloned into a mammalian expression plasmid and transfected into cell lines used for recombinant coagulation factor manufacturing. FIX expression levels, defined in the current study as the FIX activity (IU) accumulation per 24 hours per million cells, were measured after 24 hours in serum-free media by 1-stage coagulation assay. Two of the 8 AnFIX constructs, designated An96 and An97, demonstrated 12- and 7.5-fold greater FIX activity, respectively, than an identically codon-optimized hFIX (Figure 2A).
Recombinant AnFIX expression and characterization. Recombinant AnFIX expression was determined in transient (A-B) and stable (C) mammalian cell expression systems with and without R338L (Padua) mutation (B-C) by measuring FIX activity in the conditioned medium by 1-stage coagulation assay and manually counting the cells present in each well by hemocytometer to obtain a FIX activity (IU) accumulated per million cells per 24 hours (expression value). Error bars indicate sample standard deviation. Closed shapes represent individual clones, with sample size in parentheses and mean as a dashed line. Statistical analysis was performed using a 1-way analysis of variance with post hoc Tukey multiple comparisons test. (D) Highly purified recombinant hFIX, hFIX-Padua, An96, and An96-Padua were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by Coomassie blue staining under reducing (+) and nonreducing (−) conditions after incubation at 95°C for 5 minutes with or without dithiothreitol, respectively. *AnFIX variants with significant expression above the other variants (P < .05).
Recombinant AnFIX expression and characterization. Recombinant AnFIX expression was determined in transient (A-B) and stable (C) mammalian cell expression systems with and without R338L (Padua) mutation (B-C) by measuring FIX activity in the conditioned medium by 1-stage coagulation assay and manually counting the cells present in each well by hemocytometer to obtain a FIX activity (IU) accumulated per million cells per 24 hours (expression value). Error bars indicate sample standard deviation. Closed shapes represent individual clones, with sample size in parentheses and mean as a dashed line. Statistical analysis was performed using a 1-way analysis of variance with post hoc Tukey multiple comparisons test. (D) Highly purified recombinant hFIX, hFIX-Padua, An96, and An96-Padua were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by Coomassie blue staining under reducing (+) and nonreducing (−) conditions after incubation at 95°C for 5 minutes with or without dithiothreitol, respectively. *AnFIX variants with significant expression above the other variants (P < .05).
Samelson-Jones et al8 recently reported detailed biochemical studies on recombinant hFIX-Padua showing that interaction with activated FVIII (FVIIIa) is critical to specific activity enhancement, which is not observed when FVIIIa is replaced with the bispecific mimetic Hemlibra. On the basis of the earlier described benefits observed in clinical gene therapy trials incorporating R338L and the finding that inferred AnFIX sequences do not possess a substitution at R338, R338L (Padua) was incorporated into An96 and An97 to determine if the relevant mechanisms conferring increased activity to An96 and An97 overlapped, inhibited, or synergized with the seven- to eightfold increase in the FVIIIa cofactor–dependent specific activity of hFIX-Padua.8,9 An96-Padua and An97-R338L displayed FIX expression levels up to fourfold higher than hFIX R338L, indicative of the presence of functional residues independent of and additive to the known specific activity benefit conferred by R338L (Figure 2B). Given that there are only 2 amino acid differences between the mature An96 and An97 variants and An96 trended toward higher expression in the initial screen (Figure 2A), we conservatively selected An96 and An96-Padua as the lead candidates for further study.
Generation and characterization of clonal cell lines stably expressing recombinant proteins represents a more rigorous and reliable method than transient transfection.2,8-15 Stable HEK293 cell lines expressing hFIX-Padua, An96, and An96-Padua were generated, and a collection of 18 to 23 clones each were assessed for FIX production. An96-Padua demonstrated fivefold greater mean productivity compared with hFIX-Padua and An96 without the Padua mutation, supporting the independent and additive enhancement of An96 substitutions with R338L (Figure 2C). Commercial enzyme-linked immunosorbent assay detection of An96 was limited and did not generate a parallel concentration-signal curve to that obtained with hFIX (supplemental Figure 1). Therefore, it was not possible to estimate the specific activity of the AnFIX variants in transfection experiments to establish whether the increased activity resulted from improved biosynthesis/secretion or specific activity enhancement.
Biochemical and pharmacological studies of An96 FIX
Conditioned media from the top expressing hFIX-Padua, An96, and An96-Padua HEK293 stable clones were harvested for recombinant FIX purification. In total, 17 543 IU of An96-Padua FIX activity was harvested in 2.86 L of conditioned media (6.13 IU/mL). Mixed-mode and anion exchange chromatography were employed sequentially to purify recombinant An96 and An96-Padua to near homogeneity as evidenced by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Table 1; Figure 2D; data supplement). The specific activity of recombinant An96-Padua (6450 IU/mg) was ∼32 times greater than that of commercial recombinant hFIX (200 IU/mg), 3.2-fold greater than that of hFIX-Padua (2013 IU/mg), and 3.1-fold greater than that of An96 (2090 IU/mg) by 1-stage coagulation assay. The observation that the specific activity of An96 and that of hFIX-Padua are similar, while that of An96-Padua is an additional 3.1- to 3.2-fold higher, suggests that the primary mechanism of increased An96 productivity is enhanced specific activity and not increased biosynthesis/secretion. Enhanced specific activity of An96-Padua was further demonstrated using calibrated automated thrombography (Figure 3A-E; data supplement). Respective An96-Padua, An96, hFIX-Padua, and recombinant hFIX thrombograms also support the procoagulant activity relationship of An96-Padua > An96 ≈ hFIX-Padua > hFIX. No differences were observed in the activation kinetics of An96-Padua compared with hFIX-Padua upon treatment with FXIa (supplemental Figure 2).
Purification of recombinant An96-Padua
| Sample . | Volume, mL . | A280* . | Total A280 . | Activity, IU/mL . | IU . | IU/A280 . | Yield, % . | Fold purification . |
|---|---|---|---|---|---|---|---|---|
| Harvest | 2860 | 4.307 | 12 318 | 6.13 | 17 543 | 1.42 | 100 | 1 |
| Multimodal chromatography peak | 100 | 0.240 | 24 | 134.7 | 13 471 | 561 | 76 | 395 |
| Anion exchange chromatography peak | 10 | 0.768 | 7.68 | 748.5 | 7484 | 975 | 43 | 687 |
| Sample . | Volume, mL . | A280* . | Total A280 . | Activity, IU/mL . | IU . | IU/A280 . | Yield, % . | Fold purification . |
|---|---|---|---|---|---|---|---|---|
| Harvest | 2860 | 4.307 | 12 318 | 6.13 | 17 543 | 1.42 | 100 | 1 |
| Multimodal chromatography peak | 100 | 0.240 | 24 | 134.7 | 13 471 | 561 | 76 | 395 |
| Anion exchange chromatography peak | 10 | 0.768 | 7.68 | 748.5 | 7484 | 975 | 43 | 687 |
*A280 measurements are A320 corrected.
Thrombin generation analysis. Calibrated automated thrombography was performed using varying concentrations of recombinant hFIX (blue squares and lines) or An96-Padua (red circles and lines) spiked into hemophilia B plasma spanning the severe hemophilia B boundary (∼1%) to 100% normal pooled plasma hFIX range (0.05-5 μg/mL). Concentration-response curves of endogenous thrombin potential (ETP) (C), peak thrombin level (D), and lag time (E) for recombinant An96-Padua and hFIX are depicted.
Thrombin generation analysis. Calibrated automated thrombography was performed using varying concentrations of recombinant hFIX (blue squares and lines) or An96-Padua (red circles and lines) spiked into hemophilia B plasma spanning the severe hemophilia B boundary (∼1%) to 100% normal pooled plasma hFIX range (0.05-5 μg/mL). Concentration-response curves of endogenous thrombin potential (ETP) (C), peak thrombin level (D), and lag time (E) for recombinant An96-Padua and hFIX are depicted.
Next, we sought to determine if the enhanced in vitro properties of An96 and An96-Padua also translated to in vivo activity enhancement in the setting of recombinant FIX infusion into hemophilia B mice. The up-and-down staircase method combined with a saphenous vein bleed challenge was used to estimate 50% effective dose.16-19 Consistent with and even exceeding the in vitro findings, An96-Padua demonstrated an 50% effective dose of 4.8 U/kg, or 740 ng/kg, compared with 8.7 U/kg, or 44 μg/kg, for both hFIX and hFIX-Padua (supplemental Figure 4). Given that hemophilia B mice were dosed using equivalent FIX activity and not protein concentration, these results convey a 60- and sixfold potency enhancement (on a mass basis) over hFIX and hFIX-Padua, respectively, for An96-Padua in an in vivo model of hemophilia B.
AAV-AnFIX gene therapy
On the basis of the improved activity of recombinant An96-Padua in coagulation assays and murine infusion studies, An96-Padua was investigated in the setting of liver-directed AAV gene therapy. Two sets of AAV2/8 vectors were constructed (Figure 4A). The first contained a minimal liver-specific synthetic 146bp promoter, termed HCB, while the second contained a larger more potent promoter termed HHS4.4,5 Vectors encoding the LCO-An96, LCO-An96-Padua, or LCO-hFIX-Padua transgene driven by either promoter were produced in HEK293 cells after transient triple-plasmid transfection and purified using iodixanol gradient ultracentrifugation-based separation and concentration. Vector titers were obtained by qPCR of DNA obtained from the final vector preparations (Table 2). Additionally, physical characterization of the HHS4 promoter–containing vectors was performed using SEC-MALS analysis. As shown in Table 2, the total AAV vector concentrations varied from (3.37 ± 0.28) × 1012 to (2.11 ± 0.01) × 1013 vp/mL. The ratio of full/total capsids ranged from 0.86 ± 0.05 to 0.99 ± 0.34. The molar mass of each vector, capsid, and nucleic acid genome is reported in Table 3. On the basis of these findings, the qPCR titer data provided by the manufacturer seemed to overestimate the full capsid titer by 3.4- to 3.7-fold.
AAV-AnFIX gene therapy. (A) AAV vectors incorporating AAV2 inverted terminal repeats, either the HCB (146 nt) or HHS4 (294 nt) promoter, a minute virus of mice (MVM) intron (92 nt), a FIX transgene, and a synthetic β-globin polyadenylation signal are depicted. The predicted HCB- and HHS4-containing ssDNA AAV genome sizes are 2003 and 2151 nt, respectively. (B) Hemophilia B mice were injected IV with 5 × 1012 vg/kg AAV2/8 encoding An96 Padua-LCO (closed circles) or hFIX-Padua (open triangles) driven by the highly compact, liver-directed HCB promoter (n = 6 per group). Plasma was collected biweekly for 12 weeks, and FIX activity was determined by 1-stage clotting assay. (C) Hemophilia B mice were injected IV with a 37-fold lower dose (1.4 × 1011 vp/kg) of AAV2/8-HHS4-An96-Padua (closed circles), AAV2/8-HHS4-An96 (closed squares), or AAV2/8-HHS4-hFIX-Padua (open triangles; n = 4-6 per group). Plasma was collected biweekly for 12 weeks, and FIX activity was determined by 1-stage clotting assay (C) or chromogenic assay (Rox Factor IX; Diapharma, West Chester, OH) (D). (E) AAV vector copy number was determined from liver tissue genomic DNA using qPCR. No significant differences were observed among the groups (1-way analysis of variance [ANOVA]; P = .2). (F) Saphenous vein bleeding challenge was performed on mice from each group (from left to right, open diamonds represent wild-type C57Bl/6; closed diamonds, untreated hemophilia B; open triangles, hFIX-Padua transgene; closed circles, An96-Padua transgene; closed squares, An96 transgene). The average time to clot for each mouse was measured over 30 minutes. Comparisons among the groups were made by 1-way ANOVA and post hoc Holm-Sidak testing. All groups were significantly different to the untreated hemophilia B (negative control) group (P < .05). (G) Dose-response curves were generated by administration of log10 doses of AAV2/8-HHS4-An96-Padua at 1.4 × 109 (circles), 1.4 × 1010 (squares), or 1.4 × 1011 vp/kg (triangles) to hemophilia B mice (n = 5-7 per group). Plasma FIX activity was measured by 1-stage clotting assay at biweekly intervals.
AAV-AnFIX gene therapy. (A) AAV vectors incorporating AAV2 inverted terminal repeats, either the HCB (146 nt) or HHS4 (294 nt) promoter, a minute virus of mice (MVM) intron (92 nt), a FIX transgene, and a synthetic β-globin polyadenylation signal are depicted. The predicted HCB- and HHS4-containing ssDNA AAV genome sizes are 2003 and 2151 nt, respectively. (B) Hemophilia B mice were injected IV with 5 × 1012 vg/kg AAV2/8 encoding An96 Padua-LCO (closed circles) or hFIX-Padua (open triangles) driven by the highly compact, liver-directed HCB promoter (n = 6 per group). Plasma was collected biweekly for 12 weeks, and FIX activity was determined by 1-stage clotting assay. (C) Hemophilia B mice were injected IV with a 37-fold lower dose (1.4 × 1011 vp/kg) of AAV2/8-HHS4-An96-Padua (closed circles), AAV2/8-HHS4-An96 (closed squares), or AAV2/8-HHS4-hFIX-Padua (open triangles; n = 4-6 per group). Plasma was collected biweekly for 12 weeks, and FIX activity was determined by 1-stage clotting assay (C) or chromogenic assay (Rox Factor IX; Diapharma, West Chester, OH) (D). (E) AAV vector copy number was determined from liver tissue genomic DNA using qPCR. No significant differences were observed among the groups (1-way analysis of variance [ANOVA]; P = .2). (F) Saphenous vein bleeding challenge was performed on mice from each group (from left to right, open diamonds represent wild-type C57Bl/6; closed diamonds, untreated hemophilia B; open triangles, hFIX-Padua transgene; closed circles, An96-Padua transgene; closed squares, An96 transgene). The average time to clot for each mouse was measured over 30 minutes. Comparisons among the groups were made by 1-way ANOVA and post hoc Holm-Sidak testing. All groups were significantly different to the untreated hemophilia B (negative control) group (P < .05). (G) Dose-response curves were generated by administration of log10 doses of AAV2/8-HHS4-An96-Padua at 1.4 × 109 (circles), 1.4 × 1010 (squares), or 1.4 × 1011 vp/kg (triangles) to hemophilia B mice (n = 5-7 per group). Plasma FIX activity was measured by 1-stage clotting assay at biweekly intervals.
AAV vector quality attributes
| AAV sample . | qPCR titer, vg/mL . | Total AAV concentration, Cp/mL . | Capsid occupancy SEC-MALS, vg/Cp . | Capsid occupancy qPCR, vg/Cp . |
|---|---|---|---|---|
| hFIX-Padua | 7.74 × 1013 | 2.1 ± 0.1 × 1013 | 0.86 ± 0.05 | 3.67 |
| An96 | 2.52 × 1013 | 7.5 ± 0.5 × 1012 | 0.86 ± 0.03 | 3.36 |
| An96-Padua | 1.15 × 1013 | 3.4 ± 0.3 × 1012 | 0.99 ± 0.34 | 3.41 |
| AAV sample . | qPCR titer, vg/mL . | Total AAV concentration, Cp/mL . | Capsid occupancy SEC-MALS, vg/Cp . | Capsid occupancy qPCR, vg/Cp . |
|---|---|---|---|---|
| hFIX-Padua | 7.74 × 1013 | 2.1 ± 0.1 × 1013 | 0.86 ± 0.05 | 3.67 |
| An96 | 2.52 × 1013 | 7.5 ± 0.5 × 1012 | 0.86 ± 0.03 | 3.36 |
| An96-Padua | 1.15 × 1013 | 3.4 ± 0.3 × 1012 | 0.99 ± 0.34 | 3.41 |
Values are mean and standard deviation of 2 injections except An96-Padua (n = 1 because of lower titer and limited sample availability).
Cp, capsid particle.
AAV vector molar mass and composition
| AAV sample . | Vector Mw, MDa . | Capsid Mw, MDa . | Nucleic acid Mw, kDa . |
|---|---|---|---|
| hFIX-Padua | 4.53 ± 0.04 | 3.86 ± 0.01 | 666 ± 36 |
| An96 | 4.42 ± 0.08 | 3.81 ± 0.05 | 609 ± 32 |
| An96-Padua | 4.62 ± 0.08 | 3.74 ± 0.05 | 914 ± 390 |
| AAV sample . | Vector Mw, MDa . | Capsid Mw, MDa . | Nucleic acid Mw, kDa . |
|---|---|---|---|
| hFIX-Padua | 4.53 ± 0.04 | 3.86 ± 0.01 | 666 ± 36 |
| An96 | 4.42 ± 0.08 | 3.81 ± 0.05 | 609 ± 32 |
| An96-Padua | 4.62 ± 0.08 | 3.74 ± 0.05 | 914 ± 390 |
Values are mean and standard deviation of 2 injections except An96-Padua (n = 1 because of lower titer and limited sample availability).
Identical AAV2/8 vectors encoding the HCB promoter driving either an LCO-An96-Padua or LCO-hFIX-Padua transgene were administered to hemophilia B mice at a dose of 5 × 1012 vg/kg based on qPCR titer, because SEC-MALS data were not available (n = 6 mice per group; Figure 4A). Analysis of plasma FIX activity over the 12 weeks postadministration demonstrated fourfold greater mean FIX activity in mice administered AAV-An96-Padua compared with mice administered AAV-hFIX-Padua (Figure 4B). The minimal HCB promoter was designed for an AAV-FVIII platform, where vector genome size was a major constraint. Because AAV-FIX vectors do not have the same size limitation, a larger, stronger HHS4 promoter was incorporated in place of HCB. Using a significantly lower dose of 1.4 × 1011 vp/kg based on SEC-MALS (or ∼5 × 1011 vg/kg via qPCR), AAV2/8-HHS4-An96-Padua-LCO–treated mice displayed FIX activity levels ∼10-fold higher than AAV-An96-LCO– or AAV-hFIX-Padua-LCO–treated hemophilia B mice (n = 4-6 mice per group) over the course of the 12-week study (Figure 4C). The latter 2 vectors produced similar plasma FIX activity levels by 1-stage clotting assay, again demonstrating that An96 sequences confer an enhancement similar to, but independent of, R338L. Previous studies documented a 1.8-fold discrepancy between the 1-stage clotting assay and chromogenic assay results for hFIX-Padua, with the 1-stage clotting assay producing the higher result.20 Consistent with this observation, plasma samples from animals treated with AAV-hFIX-Padua displayed >50% lower FIX activity across all timepoints when measured using a chromogenic assay (Figure 4D). In contrast, AAV-An96 and AAV-An96-Padua samples produced more consistent results, although slightly lower activity was observed for An96-Padua using the chromogenic assay. These data again support the conclusion of a differential enhancement mechanism for An96 as compared with R338L. However, because of the lack of parallelism between dose-response curves for An96 in a parallel fashion to using a commercial enzyme-linked immunosorbent assay, it is not possible to discriminate between greater An96/An96-Padua production from the genetically modified cells or enhanced specific activity or the possibility of a combination of both.
Consistent with previous results using similar doses of AAV2/8 vectors in mice, liver vector copy number per diploid genome equivalent was <1, and no significant differences were observed between the groups (Figure 4E). Before euthanasia, a subset of mice from each group were subjected to the saphenous vein bleeding challenge to assess the level of phenotypic correction. Each of the vectors conferred significant phenotypic correction that was indistinguishable from wild-type C57Bl/6 mice (Figure 4F). Given that the 1.4 × 1011 vp/kg dose resulted in supraphysiologic FIX activity levels, a decreasing dose-response study was performed to further characterize vector potency and estimate the minimal effective dose. AAV2/8-HHS4-An96-Padua-LCO vector was administered at doses ranging down to 1.4 × 109 vp/kg (n = 5-7 mice per group). A clear dose response was observed with mice displaying durable FIX activity levels in the ranges of 10, 2, and 0.2 IU/mL for the 1.4 × 1011, 1.4 × 1010, and 1.4 × 109 vp/kg groups, respectively, over the 12-week study period (Figure 4G). The extended durability of plasma FIX activity in all current AAV-FIX hemophilia B mouse groups suggest the absence of immune responses to the transduced cells or transgene products. TEG analysis of AAV2/8-An96-Padua–treated mice was performed to provide additional evidence of hemostatic correction (supplemental Figure 5). Other than the TEG angle in the 1.4 × 109 vp/kg AAV-HHS4-An96-Padua group, no other TEG parameters measured for any of the treatment groups were significantly different from those obtained for positive control C57Bl/6 mice. These data demonstrate that low levels of An96-Padua promote stable clot formation comparable to that in wild-type control mice.
Discussion
An emerging concept in the development of protein drugs and gene therapies is the requirement for bioengineering of the native protein and nucleic acid sequences to improve therapeutic performance. For example, virtually all gene therapies are codon optimized to improve production of the respective transgene products. This engineering is performed without a priori knowledge of the respective codons or other RNA sequences that are limiting expression or a weak understanding of the mechanism of action.21 However, the striking improvements observed upon empirical testing serve as evidence that many human cDNA and messenger RNA sequences have not evolved to maximize protein output.4,22,23 This can be rationalized by the common requirement for endogenous proteins to maintain a concentration and specific activity window of functionality where there exists sufficient activity to avoid deficiency and not enough to cause pathogenic effects. Coagulation FVIII and FIX are examples of this principle. Deficiencies <50% of the normal levels (∼1 and 90 nM, respectively) result in bleeding severity that inversely correlates with their concentration. On the other end of the spectrum, levels >150% of normal increase the lifetime risk of thrombotic events, which may serve as a selective disadvantage, as has been suggested previously by us and others.2,8,24
When proteins and genes are taken out of their endogenous biosynthetic context and used as pharmaceuticals, the parameters established by nature may not be optimal. In gene therapy, vector potency is the key optimization parameter. Although significant progress has been made toward making more efficient gene transfer vectors, clinical AAV gene therapy often requires vector doses equal to or vastly exceeding the total number of cells in the human body to achieve efficacy. Some dose levels seem near or even exceed the maximum tolerated dose based on reported adverse events.25 Therefore, optimization of the internal vector components is advantageous. Recently, we reported nucleic acid design strategies aimed at optimizing transgene expression specifically for the target cell type of interest. For example, LCO generated significant improvements in the expression of hFIX as well as several FVIII variants, including an AnFVIII.2,4 The second arm of internal vector bioengineering, optimization of the transgene product sequence, has been the slowest to advance into clinical testing. Two main challenges exist to this approach. The first relates to immunogenicity risks associated with incorporation of nonhuman protein sequences. The development of antiprotein drug antibodies, referred to as inhibitors in the case of FVIII and FIX, has been observed clinically in many settings, including monoclonal antibody therapy, coagulation factor deficiencies, and inborn errors of metabolism. In some cases, this is not completely unexpected because of the use of xenoproteins (eg, murine monoclonal antibodies) or the naïve immune status of the patient in the case of null mutations (ie, cross-reactive material negative). However, in the setting of gene therapy, where the therapeutic protein arises from within the body, the immunogenicity risks are less clear, and no predictive preclinical models have been established. To date, no inhibitors have been observed in >100 hemophilia A and B patients who have received liver-directed AAV gene therapy in at least a dozen independent clinical trials. However, all patients had extensive prior exposure to FVIII and FIX replacement products, without a history of inhibitor development. It is important to recognize that in a majority of these cases, the gene therapy product was both codon optimized as well as protein sequence optimized to include B domain deletion in the case of FVIII or the Padua mutation in the case of FIX. In the current study, no AAV-An96-Padua or AAV-human FIX-Padua vector–treated animals were observed to have undergone a rapid decrease in plasma FIX activity indicative of an inhibitory humoral immune response to the bioengineered transgene product. However, the clinical immunogenicity risks of ASR variants such as An96-Padua remain to be established and likely will require clinical testing in the relevant delivery settings to understand product-specific risks (eg, protein infusion or gene therapy).
A second challenge to protein sequence engineering is the limited, high-resolution structure/function information available and the lack of effective methods for in silico prediction of functional protein enhancements. Instead, protein engineering methods have relied heavily on high-throughput screening after random mutagenesis or directed evolution. Although these techniques have led to major improvements in commercial biotechnology products, they have yet to penetrate protein therapeutics and gene therapies, presumably because of the structural and mechanism-of-action complexities mentioned previously. As in the case of the Padua mutation, an effective strategy has been to use information from nature. Similarly, we and others have developed bioengineered therapeutic candidates using information obtained from interspecies protein diversity as well as inferred ancient protein sequences.2,26-28 Employing ASR, FVIII variants with improved biosynthesis, specific activity, and stability were identified without any information other than extant FVIII sequences found in publicly available databases. The power of this approach relies on the ability to rapidly span an evolutionary space where protein functionality is uniformly retained and often enhanced, presumably to thrive under physiological, sociological, and environmental pressures.
ASR was applied to FIX with the goal of identifying FIX variants that can improve the potency of AAV-FIX gene therapy vectors. In the initial screen of 8 AnFIX variants inferred from nodes corresponding to early placental mammals, supraprimates, primates, rodents, carnivores, and ungulates, 2 AnFIX variants (An96 and An97), inferred as the last common ancestors of humans and lemurs, were discovered to possess 10-fold enhanced FIX activity beyond that achieved with hFIX-Padua while retaining >90% sequence identity. These nodes are not too distant but do not overlap with the AnFVIII node that possessed the greatest activity enhancement, An84 (previously designated An53 by Zakas et al2). Comparison of the AnFIX sequences with other reported bioengineered hFIX variants reveals that novel functional residues are likely to be identified. For example, none of the residues addressed by Quade-Lyssy et al29 are altered in An96, and only 1 matching substitution is shared with the TripleL variant described by Kao et al30 (V86A). Of note, An96 does possesses an alternative substitution, E277K, compared with the E277A in the TripleL variant described by Kao et al. On the basis of the specific activity estimates provided for the Triple and TripleL variants (2258 and 4309 IU/mg), An96 and An96-Padua display higher specific activity, suggesting a role for additional substituted residues present in the ancestral variants.
In the previous AnFVIII studies as well as the current AnFIX study, these inferred ancient variants were studied in the context of human plasma, purified human or animal coagulation factors, or in vivo murine hemophilia models. Therefore, interspecies compatibility of the interacting clotting factors was essential and consistently observed in both studies. Although, it has not been investigated yet, it will be of interest to study the activity of the FIX An96 variant in the presence of the FVIII An84 variant, which may help define the mechanisms of enhancement. The complexity of the FVIII and FIX proteins as well as the substrate, FX, and the requirement for their tripartite, macromolecular interaction on a phospholipid membrane interaction has contributed to the difficulty in determining high-resolution structure/function relationships. It is possible that ASR may enable significant advancements in this longstanding area of basic research investigation. However, as a direct approach to protein drug and gene therapy optimization, the FIX ASR data reported in the current study validate this powerful approach.
One aspect of AAV gene therapy that makes intervector comparisons uncertain is the lack of universal AAV standards and methods for assessing vector titer and quality.31 Even within individual clinical programs using identical vector designs but different manufacturing lots, striking outcome variability has been observed. In both preclinical and clinical studies, AAV vectors are administered on a vg/kg dose basis using vector titers (vg/mL) determined using qPCR or digital droplet PCR. Other vector characteristics such as particle size, molecular weight, and empty/full AAV genome-containing capsids typically are not reported despite the potential impact on performance. As an attempt to address this critical issue in the current study, we performed SEC-MALS analysis on the AAV2/8-HHS4-FIX vectors. SEC-MALS provides a rapid, automated, label-free, nondestructive method to measure the 3 primary quality attributes of AAV vectors (ie, molar mass, composition, and concentration). All 3 AAV-HHS4-FIX vector preparations displayed similar physical characteristics. SEC-MALS also revealed that vector titers seemed to be overestimated several-fold by qPCR (Table 3). Because of the limited vector samples available in this study, it will be important to verify these results using multiple vector designs and independent manufacturing runs in future studies.
The primary goal of the current study was to identify FIX variants that can improve the potency of clinical AAV-FIX gene therapy vectors. Clinical data suggest that additional improvements beyond hFIX-Padua will be required to achieve complete correction (ie, 100% normal FIX activity levels) at vector doses that can avoid liver inflammation and demonstrate long-term safety. Furthermore, the ability to lower the viral dose through potency optimization will translate to lower manufacturing cost per dose and greater product supply. As the AAV gene therapy field moves toward multiple administrations of vector products, the cumulative vector dose may be an important safety aspect as well. Collectively, our data demonstrate ASR to be a successful strategy to improve recombinant FIX and AAV-FIX performance, which should be directly translatable to other clinical protein drug and gene therapy development endeavors.
Acknowledgments
This work was supported by grants HL137128 (H.T.S., C.B.D.) and U54 HL141981 (P.L.) from the National Heart, Lung and Blood Institute, National Institutes of Health (NIH); grant R01AR069137 (E.A.G.) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH; and grant RGP0041 (E.A.G.) from the Human Frontier Science Program.
Authorship
Contribution: H.T.S., E.A.G., and C.B.D. conceived the project. P.L., H.T.S., and C.B.D. designed the experiments and analyzed the data; K.A.K., C.W.C., C.E.R., A.F., J.M.S., E.T.P., G.D., F.S., R.M.S., A.P., and M.C. performed experiments and analyzed the data; K.A.K. and C.B.D. drafted the manuscript; and K.A.K., P.L., E.A.G., H.T.S., and C.B.D. edited the manuscript.
Conflict-of-interest disclosure: C.E.R., H.T.S., E.A.G., and C.B.D. are inventors on a patent application describing the ancestral FIX technology filed by Emory University, Children’s Healthcare of Atlanta, and Georgia Institute of Technology. H.T.S. and C.B.D. are inventors on a patent for liver-directed codon-optimization and promoter technology filed by Emory University and Children’s Healthcare of Atlanta. H.T.S. and C.B.D. are cofounders of Expression Therapeutics and own equity in the company. Expression Therapeutics has obtained licenses for the ancestral FIX, liver codon optimized FIX, and synthetic liver-directed promoter intellectual property. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. The remaining authors declare no competing financial interests.
Correspondence: Christopher B. Doering, Aflac Cancer and Blood Disorders Center, Emory University, 2015 Uppergate Drive NE Rm 450, Atlanta, GA 30322; e-mail: cdoerin@emory.edu.
References
Author notes
Please e-mail the corresponding author for additional data requests at cdoerin@emory.edu.
The full-text version of this article contains a data supplement.


![AAV-AnFIX gene therapy. (A) AAV vectors incorporating AAV2 inverted terminal repeats, either the HCB (146 nt) or HHS4 (294 nt) promoter, a minute virus of mice (MVM) intron (92 nt), a FIX transgene, and a synthetic β-globin polyadenylation signal are depicted. The predicted HCB- and HHS4-containing ssDNA AAV genome sizes are 2003 and 2151 nt, respectively. (B) Hemophilia B mice were injected IV with 5 × 1012 vg/kg AAV2/8 encoding An96 Padua-LCO (closed circles) or hFIX-Padua (open triangles) driven by the highly compact, liver-directed HCB promoter (n = 6 per group). Plasma was collected biweekly for 12 weeks, and FIX activity was determined by 1-stage clotting assay. (C) Hemophilia B mice were injected IV with a 37-fold lower dose (1.4 × 1011 vp/kg) of AAV2/8-HHS4-An96-Padua (closed circles), AAV2/8-HHS4-An96 (closed squares), or AAV2/8-HHS4-hFIX-Padua (open triangles; n = 4-6 per group). Plasma was collected biweekly for 12 weeks, and FIX activity was determined by 1-stage clotting assay (C) or chromogenic assay (Rox Factor IX; Diapharma, West Chester, OH) (D). (E) AAV vector copy number was determined from liver tissue genomic DNA using qPCR. No significant differences were observed among the groups (1-way analysis of variance [ANOVA]; P = .2). (F) Saphenous vein bleeding challenge was performed on mice from each group (from left to right, open diamonds represent wild-type C57Bl/6; closed diamonds, untreated hemophilia B; open triangles, hFIX-Padua transgene; closed circles, An96-Padua transgene; closed squares, An96 transgene). The average time to clot for each mouse was measured over 30 minutes. Comparisons among the groups were made by 1-way ANOVA and post hoc Holm-Sidak testing. All groups were significantly different to the untreated hemophilia B (negative control) group (P < .05). (G) Dose-response curves were generated by administration of log10 doses of AAV2/8-HHS4-An96-Padua at 1.4 × 109 (circles), 1.4 × 1010 (squares), or 1.4 × 1011 vp/kg (triangles) to hemophilia B mice (n = 5-7 per group). Plasma FIX activity was measured by 1-stage clotting assay at biweekly intervals.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/17/10.1182_bloodadvances.2021004742/6/m_advancesadv2021004742f4.png?Expires=1769079670&Signature=Q5lWvQNqiAHPAlpI5-hm1LOwCbZdjtX41Yejf7j-qkhcMkiBSm5efl7T3IKl~ZK50Z~7TsilPLmKBxHtSAcRha7Q5cnTwkixJTuR3O6SpB4rdDJny0sKD3KmzUed6rm-W-sOzWFS7uCvpqc~1o9QF1ahSOQNyu6i9Pan1yU8eaPNqM2GLkkzE-QWUcfSK0XwMsZn4zWuhbJZ19ZoBNrg692x0ncLcdiP3O8Ii7UmXHSMsWTIi--3581qkabEog1sbibUhlps~64giVzX63lc8gpq0ELn9r0rxOIHoqYfqO3I2kHuSr359c8iVXEnjaUdliiE2yELUbRzuKcyKORmlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)