Key Points
In patients with newly diagnosed AML, incremental improvement in EFS leads to a decrease in use of health care.
The decline in health care use was steep for patients achieving complete remission and held true across age, treatment, and disease risk subgroups.
Abstract
The value of event-free survival (EFS) as an end point in acute myeloid leukemia (AML) trials has been questioned. We hypothesized that rather than a surrogate for overall survival (OS), improvement in EFS may decrease the use of health care. In this retrospective study, we identified 400 patients with AML who were treated on first-line therapy trials and had OS between 2 and 36 months. We captured health care use from diagnosis until death or until the patient was censored at stem cell transplantation (SCT). We used correlation and regression analysis to determine the relation between health care use and EFS. Among patients with newly diagnosed AML, 35% had adverse-risk AML, 48% received intensive chemotherapy, and 28% received hypomethylating agents. The median EFS censored at SCT was 9.7 months. Longer EFS led to a significant decline in health care use regardless of OS. This held true for all observations, including overall health care use (r = −0.45), sum of clinic visits, emergency room visits, hospitalizations, consultations (r = −0.44), sum of invasive procedures, laboratory and imaging studies (r = −0.51), and blood product transfusions (r = −0.19). These correlations were stronger for patients who achieved a complete remission and held true across age, treatment, and disease risk subgroups. In patients with newly diagnosed AML, improvement in EFS correlates with a decrease in all health care use irrespective of OS duration.
Introduction
Acute myeloid leukemia (AML) accounts for 25% of all leukemia in adults, with poor survival of less than 5% at 5 years in older age groups.1,2 Despite significant recent advances, drug development in AML has lagged behind that for other hematologic malignancies because of the complex and heterogeneous biology, aggressive clinical course, and the necessary rigor for AML therapies. Improvement in overall survival (OS) is considered the ultimate reflection of clinical benefit for clinical trials in AML, but it has been an elusive goal for numerous therapies tested across decades. Although event-free survival (EFS) is a frequently reported outcome in AML trials and has several merits, it is not universally accepted as a robust end point and is frequently viewed as a poor surrogate for OS.3-5
EFS offers a direct measure of the ability of the treatment to achieve a response, the durability of the response achieved, and its capacity to prolong life.6 In comparison, OS is impacted by salvage therapies and supportive care, both of which are improving over time and contribute toward OS. OS also may take longer to be determined.7 Only recently has improvement in EFS been considered a factor for regulatory approval of drugs for AML, specifically for gemtuzumab ozogamicin in newly diagnosed adult patients with CD33+ AML.8 Drugs that can improve EFS or get patients into remission or work as a bridge to stem cell transplantation (SCT) may still not receive regulatory approval if they fail to prolong OS (eg, clofarabine). This may have an impact on patient care by limiting therapeutic options and may delay development of novel combination therapies, a necessary approach in most instances for treating patients with AML.
We hypothesized that improved EFS may decrease use of health care. This can potentially offer value to patients and health care systems by minimizing the cost of care and providing patients more time away from health care facilities, which means that patients would be less burdened by the disease and related interventions.
Methods
This was a retrospective cohort and medical record review study. We included adult patients older than 18 years with newly diagnosed AML who started treatment on any clinical trial of first-line therapy at our institution between 2003 and 2013. EFS was defined as time from the start date of study treatment to the time when primary refractory disease was confirmed (ie, the date when failure to achieve a response to induction therapy was determined), relapse, or death. Patients with OS ranging from 2 to 36 months were included. Patients must have had an EFS of ≥2 months, suffered an adverse event, and died by the time of data collection. EFS cutoff of 2 months was chosen because patients typically need 2 cycles of therapy before determining that the induction therapy has failed to achieve a response. Because use of health care may dramatically increase after SCT, EFS was censored at the time of SCT. The 2017 European LeukemiaNet (ELN) guidelines for AML were used for risk stratification of patients.9 Responses to the first-line regimens discussed here included complete remission (CR), CR with incomplete hematologic recovery (CRi), morphologic leukemia-free state (MLFS), and partial remission (PR) per the modified International Working Group criteria for AML.10
Medical records were reviewed to determine the amount of health care use from diagnosis of AML until death or until the patient was censored at time of SCT. This included number of clinic visits, emergency room (ER) visits, hospitalizations, inpatient or outpatient consultations from other services, blood product transfusions (packed red blood cells and platelets), laboratory studies (complete blood count, basic metabolic profile, and blood cultures), imaging studies (chest radiographs, computed tomograms, magnetic resonance imaging, ultrasonograms, echocardiograms, radionuclide angiograms), and invasive procedures (bone marrow aspiration and/or biopsy, any other biopsy, bronchoscopy, gastrointestinal endoscopy, endotracheal intubation, interventional radiology procedures). We carefully reviewed all outside correspondence, including results of laboratory, radiology, and other studies, as well as all clinic notes to minimize underestimation of medical care received at outside institutions. All data abstractors followed uniform procedures for coding data. This helped to minimize inter-rater and intra-rater variability.11
Overall health care use was defined as the sum of all of the previously mentioned data points. Because longer OS will result in numerically higher health care use, to enable comparison between patients with different OS, overall health care use and other data points were divided by the respective patient’s OS duration in months to normalize those data (eg, overall health care use per month of OS, clinic visits per month of OS). To account for the effect of increasing OS on health care use, we used another subanalysis to assess the correlation of health care use per EFS month, with increasing EFS, and in smaller subgroups of patients with a narrow range of OS (eg, 12-18 months or 18-24 months).
Informed consent was obtained from all patients before they enrolled on these clinical trials, and patients were treated according to the Declaration of Helsinki in such trials. All therapeutic clinical trials were approved by the institutional review board. The analysis was conducted under an institutional review board–approved retrospective medical record review. Results were summarized by using descriptive statistics and were analyzed by using scatter plots with linear regression. Coefficient of correlation with 95% confidence intervals (CIs) and 2-tailed P values were determined between EFS and health care use. Pearson’s product-moment correlation was used because all patients had died; thus, there were no censored data. P < .05 was considered to be statistically significant. GraphPad Prism 7.03 (GraphPad Software Inc., San Diego, CA) was used for statistical analyses.
Results
There were 400 adult patients between 2003 and 2013 with newly diagnosed AML who met our inclusion criteria: 230 patients (58%) with ELN intermediate-risk disease, 141 patients (35%) with ELN adverse-risk disease, and 29 patients (7%) with ELN favorable-risk disease. Baseline characteristics and outcomes of these patients are provided in Table 1. The median age of the cohort was 65 years (interquartile range [IQR], 55-72 years), 47% of patients received intensive chemotherapy (with or without non-chemotherapy agents), 27% received hypomethylating agents (alone or in combination with non-chemotherapy agents), and 26% received nonintensive chemotherapy (and/or non-chemotherapy agents [supplemental Data]). Sixteen percent of patients received SCTs. Three patients received SCTs before 2 months and thus had EFS of less than 2 months when censored at SCT.
Patient baseline characteristics, treatments, and outcomes
| Characteristic . | All patients (N = 400) . | European LeukemiaNet risk groups . | ||||||
|---|---|---|---|---|---|---|---|---|
| Favorable (n = 29) . | Intermediate (n = 230) . | Adverse (n = 141) . | ||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||||
| Median (IQR) | 65 (55-72) | 53 (48-67) | 55 (55-74) | 65 (56-72) | ||||
| >75 | 74 | 19 | 2 | 7 | 50 | 22 | 22 | 16 |
| Sex | ||||||||
| Male | 225 | 15 | 129 | 81 | ||||
| Female | 175 | 14 | 101 | 60 | ||||
| ECOG performance status | ||||||||
| 0-1 | 334 | 84 | 27 | 93 | 192 | 83 | 115 | 82 |
| ≥2 | 41 | 10 | 2 | 7 | 24 | 10 | 15 | 11 |
| Unknown | 25 | 6 | 0 | 0 | 14 | 6 | 11 | 8 |
| Median peripheral blasts (IQR), % | 6 (0-28) | 22 (6-53) | 30 (0-24) | 6 (1-24) | ||||
| Median bone marrow blasts (IQR), % | 38 (25-64) | 66 (39-74) | 29 (25-61) | 35 (22-64) | ||||
| Mutations | ||||||||
| FLT3-ITD/TKD | 75 | 35821 | 16 | 2857 | 44 | 20821 | 15 | 12213 |
| IDH1/2 | 42 | 16625 | 4 | 1527 | 27 | 8532 | 11 | 6617 |
| First-line regimens | ||||||||
| Intensive chemotherapy only | 114 | 29 | 12 | 41 | 67 | 29 | 35 | 25 |
| With non-chemotherapy* | 74 | 19 | 7 | 24 | 47 | 20 | 20 | 14 |
| Hypomethylating agent only | 58 | 15 | 2 | 7 | 29 | 13 | 27 | 19 |
| With chemotherapy | 6 | 2 | 0 | 0 | 5 | 2 | 1 | 1 |
| With non-chemotherapy | 45 | 11 | 2 | 7 | 24 | 10 | 19 | 13 |
| Nonintensive chemotherapy | 82 | 21 | 5 | 17 | 46 | 20 | 31 | 22 |
| And/or non-chemotherapy | 21 | 5 | 1 | 3 | 12 | 5 | 8 | 6 |
| Targeted therapies | ||||||||
| FLT3 inhibitor | 19 | 7520 | 4 | 1625 | 10 | 4416 | 5 | 1527 |
| IDH1/2 inhibitor | 0 | 420 | 0 | 40 | 0 | 270 | 0 | 110 |
| Allogeneic SCT | 63 | 16 | 8 | 28 | 31 | 13 | 24 | 17 |
| Outcomes | ||||||||
| Overall response rate† | 336 | 84 | 29 | 100 | 194 | 84 | 113 | 80 |
| CR | 286 | 72 | 26 | 90 | 164 | 71 | 96 | 68 |
| CRi/MLFS | 45 | 11 | 2 | 7 | 27 | 12 | 16 | 11 |
| PR | 5 | 1 | 1 | 3 | 3 | 1 | 1 | 1 |
| Relapse | 278 | 83 | 21 | 72 | 160 | 83 | 96 | 85 |
| Median months of EFS censored at SCT | 9.7 | 11.7 | 10.8 | 8.0 | ||||
| Median months of OS censored at SCT | 17.6 | 18.8 | 19.2 | 14.5 | ||||
| Characteristic . | All patients (N = 400) . | European LeukemiaNet risk groups . | ||||||
|---|---|---|---|---|---|---|---|---|
| Favorable (n = 29) . | Intermediate (n = 230) . | Adverse (n = 141) . | ||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||||
| Median (IQR) | 65 (55-72) | 53 (48-67) | 55 (55-74) | 65 (56-72) | ||||
| >75 | 74 | 19 | 2 | 7 | 50 | 22 | 22 | 16 |
| Sex | ||||||||
| Male | 225 | 15 | 129 | 81 | ||||
| Female | 175 | 14 | 101 | 60 | ||||
| ECOG performance status | ||||||||
| 0-1 | 334 | 84 | 27 | 93 | 192 | 83 | 115 | 82 |
| ≥2 | 41 | 10 | 2 | 7 | 24 | 10 | 15 | 11 |
| Unknown | 25 | 6 | 0 | 0 | 14 | 6 | 11 | 8 |
| Median peripheral blasts (IQR), % | 6 (0-28) | 22 (6-53) | 30 (0-24) | 6 (1-24) | ||||
| Median bone marrow blasts (IQR), % | 38 (25-64) | 66 (39-74) | 29 (25-61) | 35 (22-64) | ||||
| Mutations | ||||||||
| FLT3-ITD/TKD | 75 | 35821 | 16 | 2857 | 44 | 20821 | 15 | 12213 |
| IDH1/2 | 42 | 16625 | 4 | 1527 | 27 | 8532 | 11 | 6617 |
| First-line regimens | ||||||||
| Intensive chemotherapy only | 114 | 29 | 12 | 41 | 67 | 29 | 35 | 25 |
| With non-chemotherapy* | 74 | 19 | 7 | 24 | 47 | 20 | 20 | 14 |
| Hypomethylating agent only | 58 | 15 | 2 | 7 | 29 | 13 | 27 | 19 |
| With chemotherapy | 6 | 2 | 0 | 0 | 5 | 2 | 1 | 1 |
| With non-chemotherapy | 45 | 11 | 2 | 7 | 24 | 10 | 19 | 13 |
| Nonintensive chemotherapy | 82 | 21 | 5 | 17 | 46 | 20 | 31 | 22 |
| And/or non-chemotherapy | 21 | 5 | 1 | 3 | 12 | 5 | 8 | 6 |
| Targeted therapies | ||||||||
| FLT3 inhibitor | 19 | 7520 | 4 | 1625 | 10 | 4416 | 5 | 1527 |
| IDH1/2 inhibitor | 0 | 420 | 0 | 40 | 0 | 270 | 0 | 110 |
| Allogeneic SCT | 63 | 16 | 8 | 28 | 31 | 13 | 24 | 17 |
| Outcomes | ||||||||
| Overall response rate† | 336 | 84 | 29 | 100 | 194 | 84 | 113 | 80 |
| CR | 286 | 72 | 26 | 90 | 164 | 71 | 96 | 68 |
| CRi/MLFS | 45 | 11 | 2 | 7 | 27 | 12 | 16 | 11 |
| PR | 5 | 1 | 1 | 3 | 3 | 1 | 1 | 1 |
| Relapse | 278 | 83 | 21 | 72 | 160 | 83 | 96 | 85 |
| Median months of EFS censored at SCT | 9.7 | 11.7 | 10.8 | 8.0 | ||||
| Median months of OS censored at SCT | 17.6 | 18.8 | 19.2 | 14.5 | ||||
ECOG, Eastern Cooperative Oncology Group; ITD, internal tandem duplication; TKD, tyrosine kinase domain.
Non-chemotherapy agents included targeted therapies, tyrosine kinase inhibitors, immunotherapies, and other agents.
Overall response rate, CR + CRi + MLFS + PR.
A response (CR, CRi, MLFS, PR) was achieved in 87% of all patients, and 84% patients relapsed (Table 1). Patients receiving intensive chemotherapy–based regimens achieved a response (CR, CRi, MLFS, PR) after a median of 1 cycle (IQR, 1-1 cycles) and completed a median of 4 total cycles (IQR, 2-5 cycles). Patients receiving hypomethylating agent–based regimens achieved a response after a median of 3 cycles (IQR, 2-4 cycles) and completed a median of 9 total cycles (IQR, 6-13 cycles). Patients receiving nonintensive chemotherapy–based regimens achieved a response after a median of 1 cycle (IQR, 1-2 cycles) and completed a median of 4 total cycles (IQR, 2-8 cycles). Patients receiving non-chemotherapy agents achieved a response after a median of 4 cycles (IQR, 1-6 cycles) and competed a median of 8 total cycles (IQR, 4-9 cycles). The median EFS censored at SCT was 9.7 months, and the median OS censored at SCT was 17.6 months (supplemental Figure 1).
Decline in use of health care per OS month
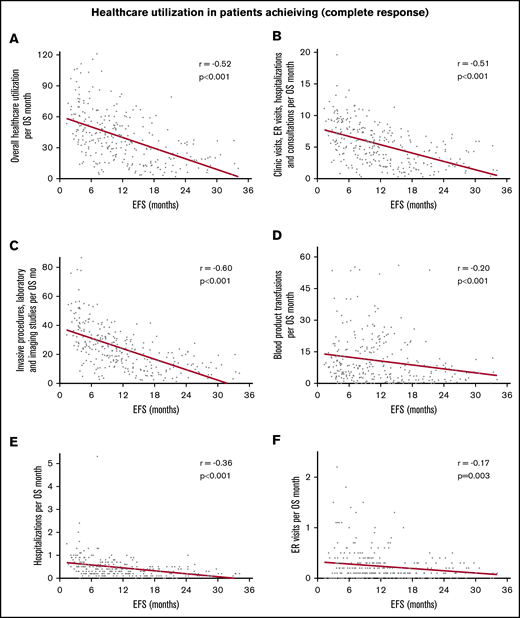
In the entire cohort, there was steady decline in use of health care with increasing EFS (Table 2). The strength of these correlations was moderate to weak but all were highly statistically significant. With increasing EFS, there was a steady decline in overall use of health care per month of OS (r = −0.45; 95% CI, −0.53 to −0.37; P < .0001; Figure 1A), combined clinic visits, ER visits, hospitalizations, and consultations per month of OS (r = −0.44; 95% CI, −0.51 to −0.35; P < .0001; Figure 1B), combined invasive procedures, imaging studies, and laboratory studies per month of OS (r = −0.51; 95% CI, −0.58 to −0.43; P < .0001; Figure 1C), and blood product transfusions per month of OS (r = −0.19; 95% CI, −0.28 to −0.09; P < .0001; Figure 1D). Patients who achieved a CR had more pronounced decline in the use of health care with prolonged EFS (r ranging from −0.17 to −0.60; Table 2; Figure 2). There were very few patients with CRi or MLFS (n = 45) for statistical analysis. Similar decline in overall use of health care per month of OS with increasing EFS was seen among patients in ELN 2017 risk groups, younger and older patients, and those receiving intensive chemotherapy, hypomethylating agents, and nonintensive chemotherapy, with or without other agents (Table 2; Figure 3).
Correlation between increasing EFS and use of health care
| Use of health care per mo of OS . | Coefficient of correlation . | P . | |
|---|---|---|---|
| r . | 95% CI . | ||
| All patients | |||
| Overall use of health care | −0.45 | −0.53 to −0.37 | <.001 |
| Clinic visits | −0.38 | −0.46 to −0.29 | <.001 |
| ER visits | −0.15 | −0.24 to −0.05 | .004 |
| Hospital admissions | −0.34 | −0.43 to −0.25 | <.001 |
| Consultations from other services | −0.39 | −0.47 to −0.30 | <.001 |
| Blood product transfusions | −0.18 | −0.28 to −0.09 | <.001 |
| Laboratory studies | −0.51 | −0.58 to −0.44 | <.001 |
| Imaging studies | −0.38 | −0.46 to −0.29 | <.001 |
| Invasive procedures | −0.49 | −0.56 to −0.41 | <.001 |
| Patients with CR (n = 286) | |||
| Overall use of health care | −0.52 | −0.60 to −0.43 | <.001 |
| Combined clinic visits, ER visits, hospitalizations, and consultations | −0.51 | −0.59 to −0.42 | <.001 |
| Combined invasive procedures, imaging studies, and laboratory studies | −0.60 | −0.67 to −0.52 | <.001 |
| Blood product transfusions | −0.20 | −0.31 to −0.09 | <.001 |
| Hospitalizations | −0.36 | −0.46 to −0.25 | <.001 |
| ER visits | −0.17 | −0.28 to −0.06 | .003 |
| Overall use of health care in ELN risk groups | |||
| Favorable (n = 29) | −0.36 | −0.64 to 0.01 | .06 |
| Intermediate (n = 230) | −0.40 | −0.50 to −0.29 | <.001 |
| Adverse (n = 141) | −0.55 | −0.66 to −0.42 | <.001 |
| Overall use health care according to age, y | |||
| <65 (n = 193) | −0.43 | −0.54 to −0.31 | <.001 |
| ≥65 (n = 207) | −0.41 | −0.52 to −0.29 | <.001 |
| Overall use of health care according to therapy | |||
| Intensive chemotherapy with or without other agents (n = 188)* | −0.47 | −0.57 to −0.34 | <.001 |
| HMA with or without other agents or chemotherapy (n = 109)† | −0.47 | −0.61 to −0.20 | <.001 |
| NIC with or without other agents (n = 93)* | −0.43 | −0.58 to −0.24 | <.001 |
| Use of health care per mo of OS . | Coefficient of correlation . | P . | |
|---|---|---|---|
| r . | 95% CI . | ||
| All patients | |||
| Overall use of health care | −0.45 | −0.53 to −0.37 | <.001 |
| Clinic visits | −0.38 | −0.46 to −0.29 | <.001 |
| ER visits | −0.15 | −0.24 to −0.05 | .004 |
| Hospital admissions | −0.34 | −0.43 to −0.25 | <.001 |
| Consultations from other services | −0.39 | −0.47 to −0.30 | <.001 |
| Blood product transfusions | −0.18 | −0.28 to −0.09 | <.001 |
| Laboratory studies | −0.51 | −0.58 to −0.44 | <.001 |
| Imaging studies | −0.38 | −0.46 to −0.29 | <.001 |
| Invasive procedures | −0.49 | −0.56 to −0.41 | <.001 |
| Patients with CR (n = 286) | |||
| Overall use of health care | −0.52 | −0.60 to −0.43 | <.001 |
| Combined clinic visits, ER visits, hospitalizations, and consultations | −0.51 | −0.59 to −0.42 | <.001 |
| Combined invasive procedures, imaging studies, and laboratory studies | −0.60 | −0.67 to −0.52 | <.001 |
| Blood product transfusions | −0.20 | −0.31 to −0.09 | <.001 |
| Hospitalizations | −0.36 | −0.46 to −0.25 | <.001 |
| ER visits | −0.17 | −0.28 to −0.06 | .003 |
| Overall use of health care in ELN risk groups | |||
| Favorable (n = 29) | −0.36 | −0.64 to 0.01 | .06 |
| Intermediate (n = 230) | −0.40 | −0.50 to −0.29 | <.001 |
| Adverse (n = 141) | −0.55 | −0.66 to −0.42 | <.001 |
| Overall use health care according to age, y | |||
| <65 (n = 193) | −0.43 | −0.54 to −0.31 | <.001 |
| ≥65 (n = 207) | −0.41 | −0.52 to −0.29 | <.001 |
| Overall use of health care according to therapy | |||
| Intensive chemotherapy with or without other agents (n = 188)* | −0.47 | −0.57 to −0.34 | <.001 |
| HMA with or without other agents or chemotherapy (n = 109)† | −0.47 | −0.61 to −0.20 | <.001 |
| NIC with or without other agents (n = 93)* | −0.43 | −0.58 to −0.24 | <.001 |
ELN, European LeukemiaNet 2017; HMA, hypomethylating agent; NIC, nonintensive chemotherapy.
Other agents included non-chemotherapy, targeted therapy, and immunotherapy.
Other agents included non-chemotherapy, targeted therapy, immunotherapy, and low-dose chemotherapy.
Correlation between increasing EFS and decreasing use of health care for all patients. Overall use of health care per month of OS (A), combined clinic visits, ER visits, hospitalizations, and consultations per month of OS (B), combined invasive procedures, imaging, and laboratory studies per month of OS (C), and blood product transfusions per month of OS (D). Overall use of health care consisted of the sum of the number of clinic visits, ER visits, hospital admissions, consultations, blood product transfusions, laboratory studies, imaging studies, and invasive procedures. Blood product transfusions included packed red blood cell and platelet transfusions.
Correlation between increasing EFS and decreasing use of health care for all patients. Overall use of health care per month of OS (A), combined clinic visits, ER visits, hospitalizations, and consultations per month of OS (B), combined invasive procedures, imaging, and laboratory studies per month of OS (C), and blood product transfusions per month of OS (D). Overall use of health care consisted of the sum of the number of clinic visits, ER visits, hospital admissions, consultations, blood product transfusions, laboratory studies, imaging studies, and invasive procedures. Blood product transfusions included packed red blood cell and platelet transfusions.
Correlation between increasing EFS and decreasing use of health care in 286 patients achieving a complete response. Correlation with overall use of health care per month of OS (A), combined clinic visits, ER visits, hospitalizations, and consultations per month of OS (B), combined invasive procedures, imaging, and laboratory studies per month of OS (C), blood product transfusions per month of OS (D), hospitalizations per month (E), and ER visits per month (F).
Correlation between increasing EFS and decreasing use of health care in 286 patients achieving a complete response. Correlation with overall use of health care per month of OS (A), combined clinic visits, ER visits, hospitalizations, and consultations per month of OS (B), combined invasive procedures, imaging, and laboratory studies per month of OS (C), blood product transfusions per month of OS (D), hospitalizations per month (E), and ER visits per month (F).
Correlation between increasing EFS and decreasing overall use of health care per month of OS. Among patients with ELN intermediate-risk AML (A), ELN adverse-risk AML (B), age <65 years (C), age ≥65 years (D), receiving intensive chemotherapy with or without other non-chemotherapy agents (E), and receiving hypomethylating agents with or without other non-chemotherapy agents (F).
Correlation between increasing EFS and decreasing overall use of health care per month of OS. Among patients with ELN intermediate-risk AML (A), ELN adverse-risk AML (B), age <65 years (C), age ≥65 years (D), receiving intensive chemotherapy with or without other non-chemotherapy agents (E), and receiving hypomethylating agents with or without other non-chemotherapy agents (F).
Decline in use of health care per EFS month
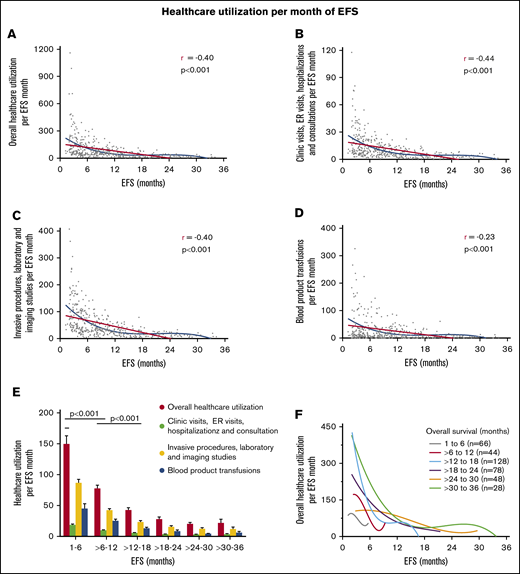
With increasing EFS, there was a nonlinear decline in overall use of health care per month of EFS, combined clinic visits, ER visits, hospitalizations, and consultations per month of EFS, combined invasive procedures, imaging studies, and laboratory studies per month of EFS, and blood product transfusions per month of EFS (Figure 4). Use of health care per month of EFS was higher among patients with shorter duration of EFS (1-6 months and 6-12 months) compared with those who had longer EFS of more than 12 months, and this was highly statistically significant for all health care use parameters (P < .0001; Figure 4E). The steady decline in overall use of health care with increasing EFS occurred irrespective of OS duration, as shown in patients with narrower groups of OS (Figure 4F).
Linear correlation and nonlinear fit between increasing EFS and decreasing use of health care per month of EFS for all patients. Overall use of health care per month of EFS (A), combined clinic visits, ER visits, hospitalizations, and consultations per month of EFS (B), combined invasive procedures, imaging, and laboratory studies per month of EFS (C), and blood product transfusions per month of EFS (D). (E) Use of health care in patients grouped according to increasing EFS. The decline in use of health care between patients with EFS of 1 to 6 months, >6 to 12 months, and >12 to 18 months was highly statistically significant. (F) Trend of overall use of health care with increasing EFS in patients grouped according to narrow OS ranges. Overall use of health care consisted of the sum of the number of clinic visits, ER visits, hospital admissions, consultations, blood product transfusions, laboratory studies, imaging studies, and invasive procedures. Blood product transfusions included packed red blood cell and platelet transfusions.
Linear correlation and nonlinear fit between increasing EFS and decreasing use of health care per month of EFS for all patients. Overall use of health care per month of EFS (A), combined clinic visits, ER visits, hospitalizations, and consultations per month of EFS (B), combined invasive procedures, imaging, and laboratory studies per month of EFS (C), and blood product transfusions per month of EFS (D). (E) Use of health care in patients grouped according to increasing EFS. The decline in use of health care between patients with EFS of 1 to 6 months, >6 to 12 months, and >12 to 18 months was highly statistically significant. (F) Trend of overall use of health care with increasing EFS in patients grouped according to narrow OS ranges. Overall use of health care consisted of the sum of the number of clinic visits, ER visits, hospital admissions, consultations, blood product transfusions, laboratory studies, imaging studies, and invasive procedures. Blood product transfusions included packed red blood cell and platelet transfusions.
Discussion
The clinical benefit of a prolonged EFS has been a matter of debate. Efforts to establish EFS as a surrogate for OS have yielded mixed results, casting doubt on the value of EFS.3-5,12,13 In this analysis, instead of establishing surrogacy, we focused on determining whether improved EFS can offer value through decreasing use of health care as a measure of benefit to patients. Our results show that increasing EFS leads to a steady decline in all health care use irrespective of OS, and the decline was preserved in patients with narrower subsets of OS and EFS. The correlation was stronger for patients achieving a CR. These observations held true for all health care parameters tested, across all ELN risk groups, across age groups, and across different types of first-line therapies. We also included only patients who had had an adverse event and who had died, thus minimizing an imbalance created as a result of disproportionately high use of health care in some patients at the end of their life compared with patients who are alive in remission.
Some correlations were weaker but still highly significant. For example, the slope of decline in transfusion requirements is more modest than that for other parameters assessed. Multiple factors determine the use of transfusions for a given patient. Patients in remission may frequently continue therapy (eg, hypomethylating agents) until disease progression, and this may contribute to the need for transfusions in some patients and may result in the slower decline in transfusions with longer EFS. A similar weak but significant correlation was seen for ER visits and hospitalizations. This may be a result of the high incidence of relapse of 83% in this cohort, and patients with relapsed AML continue to need more medical care. Patients achieving a CR tend to require fewer hospitalizations and ER visits, and this was demonstrated by a more pronounced decline in hospitalizations and ER visits that was close to 0 for such patients. Health care use per EFS month according to smaller OS subgroups (Figure 4F) showed the tail end of only a few curves turning upward. This was likely the result of a few outlying patients near the end of those curves with high use of health care who influenced the shape of those nonlinear cubic polynomial curves.
These findings pose new questions for health care economics and regulatory agencies. From a pharmaco-economic perspective, whether the cost savings from the decrease in use of health care can offset the cost of a given drug approved on the basis of EFS benefit is beyond the scope of this work. The sole study evaluating gemtuzumab ozogamicin, which was approved primarily on the basis of EFS benefit, found no increase in cost compared with another comparable regimen.14,15 For patients, the aggregate benefit of requiring fewer transfusions, clinic and hospital visits, laboratory tests and procedures, and other interventions is, in our opinion, of great value. Unfortunately, because this is a retrospective analysis, we did not directly measure quality of life to demonstrate such correlation. Recent trends toward developing oral and outpatient therapies may further help decrease the use of health care for patients with AML.
This was a retrospective analysis with all inherent limitations thereof. Management of AML has evolved over the time period of this study, and many molecular risk stratification strategies were not available for patients from earlier time points. Hence, many patients, particularly in the intermediate-risk group, might have been classified into different risk groups if current standard testing results had been available. This alone would probably have a minor impact on the overall results, if there were any impact at all. Our analysis did not consider how comorbidities and other potential confounders affected health care use that may have played a role, possibly a significant one, in the use of health care. Many patients travel to our institution to receive a portion of their medical care and receive the rest of it closer to their homes. Hence, the data on use of health care may not be fully reflective of all medical care received, and despite our best efforts, there may have been an underestimation of some of these data points for some patients. Because all patients were enrolled in clinical trials, data collection was prospective, rigorous, and monitored in near real-time, minimizing such gaps. Only 10% of patients had performance status of 2 or higher. Whether these observations apply to such patients requires additional confirmation. In addition, the treatments used were heterogeneous, and the EFS effect of different therapies may vary. The newer modalities of therapy such as targeted agents and monoclonal antibodies are underrepresented considering today’s standards. What impact, if any, these therapies may have on the parameters analyzed in this article should be further investigated. OS and EFS are unlikely to have a linear effect on the decline in the use of health care. Although our report provides an intuitive analysis of this effect, more complex statistical methods may better delineate the true nature of such nonlinear correlation. Finally, it is important to highlight that any correlations observed in our analysis do not imply causation, and further studies are required to better understand the causes of these correlations.
In conclusion, in patients with newly diagnosed AML, incremental improvement in EFS is associated with a decrease in the use of health care regardless of OS duration. These findings add further credibility to using EFS as an end point in AML trials.
Presented in part at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017, and published in part as an online abstract in the proceedings of 2019 American Society of Clinical Oncology Annual Meeting, Chicago, IL, 31 May-4 June 2019.
For data sharing requests, please contact Jorge E. Cortes (jorge.cortes@augusta.edu).
Acknowledgments
The authors thank the participating patients, their families, and the members of the study management teams.
This study was supported in part by the Shannon Timmins Endowed Fellowship in Leukemia Research awarded to A.M., and MD Anderson Cancer Center Support Grant CA016672 from the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: J.E.C. and A.M. conceived and designed the study; J.E.C. and H.M.K. provided administrative support; H.M.K., G.G.-M., M.Y.K., S.V., M.A., G.B., T.M.K., N.G.D., N.P., C.D.D., W.G.W., S.M.K., F.R., and J.E.C. designed the clinical trials and provided study materials and patients; A.M., V.P., H.O.A., R.V.G., C.B., M.V., and S.R.P. collected and assembled data; A.M. and J.E.C. analyzed and interpreted data and helped write the manuscript; H.M.K., V.P., H.O.A., R.V.G., C.B., M.V., G.G.-M., M.Y.K., S.V., M.A., G.B., T.M.K., N.G.D., N.P., C.D.D., S.R.P., W.G.W., S.M.K., F.R., and J.E.C. critically reviewed the manuscript for important intellectual content; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: A.M. received research funding from Celgene. H.M.K. received honoraria and research funding from Pfizer, ARIAD, Bristol-Myers Squibb, and Amgen, honoraria from Orsenix, Immunogen, Actinium, and AbbVie, and research funding from Novartis and Astex. M.Y.K. received research funding from Stemline Therapeutics. S.V. received research funding from Genentech, Lilly Oncology, NS Pharma, CTI BioPharma, Incyte, Seattle Genetics, Blueprint Medicines, Celgene, Pfizer, Roche, AstraZeneca, Bristol-Myers Squibb, Promedior, Galena BioPharma, and Gilead. M.A. received research funding from AstraZeneca. H.M.K. received honoraria and research funding from Pfizer, Bristol-Myers Squibb, ARIAD, and Amgen, honoraria from Orsenix, Immunogen, Actinium, and AbbVie, and research funding from Novartis and Astex. T.M.K. has served as a consultant for and received research funding from Amgen, Jazz Pharmaceuticals, and Pfizer, served as a consultant for Takeda, Novartis, and AbbVie, and received research funding from Celgene and Bristol-Myers Squibb. N.G.D. served as a consultant for and received research funding from Incyte, Daiichi-Sankyo, ImmunoGen, Karyopharm, Bristol-Myers Squibb, Pfizer, and Novartis, served as a consultant for Sunesis, Alexion, and Otsuka, and has received research funding from Pfizer, Novartis, Kiromic, and ARIAD. N.P. served as a consultant for and received honoraria from Celgene, and has served as a consultant for and received honoraria and research funding from Stemline. C.D.D. served as a consultant and adviser for AbbVie and Agios, as an adviser for Bayer, Celgene, Medimmune, and Karyopharm, and received research funding from Plexxikon, Novartis, Affymetrix, Samus, Cellectis, SagerStrong Foundation, AbbVie, and Daiichi Sankyo. W.G.W. received research funding from AbbVie and Genentech. F.R. has received honoraria and research funding from and served on the Speakers Bureau for Amgen, has received honoraria from Jazz Pharmaceuticals, Sunesis, and Orsenix, has received research funding from AbbVie, Xencor, Bristol-Myers Squibb, and Seattle Genetics, has received honoraria and research funding from Macrogenix, and has served as a consultant for and received honoraria from Astellas Pharmaceuticals. J.E.C. has received research funding from Amphivena, Arog, Astellas Pharma, Bristol-Myers Squibb, Celgene, Daiichi-Sankyo, Immunogen, Jazz Pharmaceuticals, Merus, Novartis, Pfizer, Sun Pharma, Takeda, Teva, and Rafael, and has served as a consultant for Astellas Pharma, BiolineRx, Biopath Holdings, Bristol-Myers Squibb, Daiichi-Sankyo, Novartis, Pfizer, Takeda, and Jazz Pharmaceuticals (all to the institution). The remaining authors declare no competing financial interests.
Correspondence: Jorge E. Cortes, Georgia Cancer Center, 1410 Laney Walker Blvd, CN-2222, Augusta, GA 30912; e-mail: jorge.cortes@augusta.edu.
References
Author notes
The full-text version of this article contains a data supplement.