Key Points
Naïve CB CD8+ T cells undergo exponential expansion and become activated after granulocyte transfusions.
Expansion of CB CD8+ T cells, although transient, eradicates refractory leukemia without GVHD.
Abstract
The action of hematopoietic cell transplantation in controlling leukemia is principally mediated by donor T cells directed against residual recipient malignant cells. However, its utility is limited by graft-versus-host disease (GVHD), where alloreactivity is extended beyond leukemic and marrow cells. In a human/murine chimeric model, we previously showed that the preferential infiltration of cord blood (CB) CD8+ T cells eradicates an Epstein-Barr virus–driven lymphoblastoid tumor without causing xenogeneic GVHD. In the clinic, however, cord blood CD8+ T-cell reconstitution is significantly delayed, and the observation of such a robust antileukemia effect mediated by cord blood CD8+ T cells has not been reported. We describe an observation of very early T-cell expansion in 4 high-risk pediatric leukemia patients receiving third-party, pooled granulocytes after T cell–replete CB transplantation (CBT). The T-cell expansion was transient but robust, including expansion of CD8+ T cells, in contrast to the delayed CD8+ T-cell expansion ordinarily observed after T cell–replete CBT. The CD8+ T cells were polyclonal, rapidly switched to memory phenotype, and had the ability to mediate cytotoxicity. This phenomenon is reproducible, and each patient remains in long-term remission without GVHD. The results suggest that fetal-derived CB CD8+ T cells can be exploited to generate robust antileukemia effects without GVHD.
Introduction
Cord blood (CB) is a preferred donor cell source in patients with refractory malignancy, because it mediates an enhanced antileukemia effect compared with transplantation using adult volunteer donors.1 We previously compared CB with adult peripheral blood T cells in a powerful antigen-presenting tumor model of B-cell lymphoma.2 There was rapid infiltration of CB CD8+ T cells into the tumor, and a significantly higher number of circulating CD8+ T cells were observed in the CB T-cell group compared with the peripheral blood T-cell group. Thus, the tumor model indicated that CB T cells could mediate a potent antitumor cytotoxic CD8+ T-cell response. The cytotoxic CD8+ T cell–biased responses in our tumor model were surprising, because the early adaptive immune system recovery after T cell–replete CB transplantation (CBT) recapitulates fetal ontogeny, with a striking CD4+ T-cell bias.3-6
T-cell/APC interaction is central to the orchestration of a graft-versus-leukemia effect. In the clinic, a dendritic cell–acute myeloid leukemia (DC-AML) fusion vaccine is capable of inducing cytotoxic T-cell responses in patients with AML, and an autologous DC-AML vaccine has shown promising results in adults with AML.7,8 Similarly, DCs could be isolated and expanded from a portion of a CB graft infused on day 0 to manufacture a cord DC-AML fusion vaccine for subsequent infusion.9 Such approaches require access to the original tumor material and significant laboratory expertise, infrastructure, and expense.
We describe the robust induction of an immune response in 4 of 5 consecutive patients undergoing CBT for chemotherapy-refractory acute leukemia. A very early CD8+ T-cell expansion was observed in these patients in response to the administration of a third-party pooled granulocyte product for concomitant serious infection.10 Such an accelerated and amplified CD8+ T-cell expansion after CBT could potentially mediate a reproducible and safe graft-versus-malignancy effect.
Methods
Patient and controls
Five patients underwent T cell–replete CBT for high-risk chemotherapy-refractory or relapsed acute leukemia. All patients received a daily pooled granulocyte product during the peritransplantation period because of serious preexisting infection. The third-party granulocyte unit from the NHS Blood Transfusion Service was a pooled irradiated product derived from 10 blood donations. The specifications of each pooled product were as follows: an average (standard deviation) of 1 (0.3) × 1010 per unit neutrophils, 1.22 (0.37) × 109 per unit monocytes, and 6.72 (0.75) × 109 per unit lymphocytes.11 The cellular content of each individual product was not measured. Patient and transplantation characteristics are summarized in Table 1, and patient details are provided in supplemental methods.
Patient and transplantation characteristics
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age at transplantation | 7 y 3 mo | 1 y 7 mo | 1 y 10 mo | 1 y 2 mo | 7 y 8 mo |
| Disease | AML refractory to chemotherapy | AML refractory to chemotherapy | AML; relapse after completion of fourth chemotherapy cycle | High-risk infant ALL; on-treatment myeloid relapse | AML refractory to chemotherapy |
| Cytogenetics | Monosomy 7 | t(9;11) | t(9;11) | t(4;11) | t(8;21) |
| Disease status at transplantation | 65% blasts | 90% blasts | Prolonged aplasia after salvage chemotherapy | MRD+(0.65%) | 30% blasts |
| Conditioning | Fludarabine, treosulfan, thiotepa | Fludarabine, busulfan | Treosulfan | Treosulfan, thiotepa | Busulfan, cyclophosphamide |
| HLA matching (A, B, C, DRB) | 8 of 8 | 7 of 8 (DRB1 mismatch) | 8 of 8 | 6 of 8 (HLA-B and -C mismatch) | 7 of 8 (HLA-C mismatch) |
| Other HLA loci (DQB, DPB) | DQB1 match, DPB1 mismatch at 1 allele | DQB1 mismatch at 1 allele, DPB1 mismatch at 1 allele | DQB1 match, DPB1 mismatch for both loci | DQB1 match, DPB1 mismatch at 1 allele | DQB1 mismatch, DPB1 mismatch |
| GVHD prophylaxis | Ciclosporin | Ciclosporin | Ciclosporin | Ciclosporin | Ciclosporin |
| Total nucleated cell dose, ×107/kg | 8.01 | 14.7 | 7.65 | 18.9 | 4.6 |
| CD34+ cell dose, ×105/kg | 1.5 | 3.5 | 5.7 | 6.9 | 1.1 |
| No. of days of granulocytes | 12 (day −6, −5, −4, −1, 0, +1, +2, +3, +8, +9, +10, +11) | 13 (day −10, −9, −6, −5, −3, −2, +2, +3, +4, +8, +9 +10, +14) | 10 (day +1, +2, +3, +4, +5, +8, +9, +10, +11, +12) | 11 (day −2, −1, 0, +1, +2, +5, +6, +7, +8, +9, +12) | 10 (day +0, +1, +2, +3, +4, +7, +8, +9, +10, +11) |
| Reason for granulocytes | Fungal chest infection | Fungal chest infection | Bacteraemia and meningitis | Fungal chest infection | Fungal chest infection |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age at transplantation | 7 y 3 mo | 1 y 7 mo | 1 y 10 mo | 1 y 2 mo | 7 y 8 mo |
| Disease | AML refractory to chemotherapy | AML refractory to chemotherapy | AML; relapse after completion of fourth chemotherapy cycle | High-risk infant ALL; on-treatment myeloid relapse | AML refractory to chemotherapy |
| Cytogenetics | Monosomy 7 | t(9;11) | t(9;11) | t(4;11) | t(8;21) |
| Disease status at transplantation | 65% blasts | 90% blasts | Prolonged aplasia after salvage chemotherapy | MRD+(0.65%) | 30% blasts |
| Conditioning | Fludarabine, treosulfan, thiotepa | Fludarabine, busulfan | Treosulfan | Treosulfan, thiotepa | Busulfan, cyclophosphamide |
| HLA matching (A, B, C, DRB) | 8 of 8 | 7 of 8 (DRB1 mismatch) | 8 of 8 | 6 of 8 (HLA-B and -C mismatch) | 7 of 8 (HLA-C mismatch) |
| Other HLA loci (DQB, DPB) | DQB1 match, DPB1 mismatch at 1 allele | DQB1 mismatch at 1 allele, DPB1 mismatch at 1 allele | DQB1 match, DPB1 mismatch for both loci | DQB1 match, DPB1 mismatch at 1 allele | DQB1 mismatch, DPB1 mismatch |
| GVHD prophylaxis | Ciclosporin | Ciclosporin | Ciclosporin | Ciclosporin | Ciclosporin |
| Total nucleated cell dose, ×107/kg | 8.01 | 14.7 | 7.65 | 18.9 | 4.6 |
| CD34+ cell dose, ×105/kg | 1.5 | 3.5 | 5.7 | 6.9 | 1.1 |
| No. of days of granulocytes | 12 (day −6, −5, −4, −1, 0, +1, +2, +3, +8, +9, +10, +11) | 13 (day −10, −9, −6, −5, −3, −2, +2, +3, +4, +8, +9 +10, +14) | 10 (day +1, +2, +3, +4, +5, +8, +9, +10, +11, +12) | 11 (day −2, −1, 0, +1, +2, +5, +6, +7, +8, +9, +12) | 10 (day +0, +1, +2, +3, +4, +7, +8, +9, +10, +11) |
| Reason for granulocytes | Fungal chest infection | Fungal chest infection | Bacteraemia and meningitis | Fungal chest infection | Fungal chest infection |
Thirty consecutive patients from the historical cohort that underwent systematic immune reconstitution monitoring after T cell–replete CBT without receiving a granulocyte product were controls for comparing the immune constitution. Six patients who underwent immune reconstitution monitoring including CD8+ T-cell memory and effector differentiation after typical T cell–replete CBT without receiving a granulocyte product were controls for comparing the memory and effector differentiation. Twenty consecutive and recently grafted patients who underwent T cell–replete CBT without receiving a granulocyte product were controls for comparing the clinical features of preengraftment syndrome.
Clinical outcomes.
Preengraftment syndrome.
Preengraftment syndrome was diagnosed based on clinical and biochemical features. Maximum temperature, number of days of fever, number of days of oxygen, days to oxygen requirement, and C-reactive protein (CRP) and peak CRP between the index patients and controls were compared.
GVHD.
Acute graft-versus-host disease (GVHD) was classified according to Glucksberg classification, and chronic GVHD was classified according to the National Institutes of Health Consensus Conference severity scoring system.
Relapse.
Minimal residual disease (MRD) monitoring by flow cytometry was performed in all patients. In patients with a fluorescence in situ hybridization (FISH) abnormality or molecular MRD marker, MRD was also monitored by FISH or molecular studies.
T-cell kinetics.
Daily full blood count with differential count was performed for the first 30 days after transplantation. In all patients, the immune reconstitution of CD3, CD4, and CD8 T cells, CD19 B cells, and CD56 natural killer cells was recorded. In patients 3, 4, and 5, daily immune reconstitution analysis was performed during T-cell expansion. Median and interquartile range (IQR) of CD4+ T cells and CD8+ T cells from the historical cohort of 30 patients were plotted and compared with CD4+ and CD8+ T cells in patients 3, 4, and 5.
Magnitude of T-cell expansion
The T-cell content in the CB graft of the index patients was not measured. The magnitude of T-cell expansion was therefore calculated using the extrapolated T-cell content in each graft. Median and IQR values of T cells per kilogram from the 30 historical CB grafts were calculated and multiplied by the weight of the index patients to derive the approximate T-cell content in each graft. Total blood volume based on the weight of each index patient was calculated, and thus, total number of circulating T cells during T-cell expansion was calculated to derive the magnitude of T-cell proliferation.
Flow cytometry
Memory and effector differentiation.
In patients 3 and 4, 8-color flow cytometric immunophenotyping was performed to characterize CD45RA+CD27+ naïve T lymphocytes, CD45RA−CD27+ memory T lymphocytes, and CD45RA+CD27− effector T lymphocytes.
T-cell activation.
Eight-color flow cytometric immunophenotyping was also performed to characterize the activation of lymphocytes in patients 3 and 4: CD3+ T lymphocytes, CD3+CD4+ T lymphocytes, CD3+CD8+ T lymphocytes, CD38+ T lymphocytes, and HLA-DR+ T lymphocytes.
T-regulatory cells.
Four-color flow cytometric immunophenotyping was performed to characterize T-regulatory cells in patients 3 and 4: CD3+ T lymphocytes, CD3+CD4+ T lymphocytes, CD3+CD25+ T lymphocytes, and CD127dim T lymphocytes.
Absolute values of circulating CD8+, CD8+CD38+HLA-DR+, and CD4+ T-regulatory cells were used to calculate the activated CD8/T-regulatory cell ratio.
CD107a staining.
Lysosomal-associated membrane protein-1 (CD107a) staining was performed in 2 patients. Cells from patients 3 and 4 were permeabilized and stained as per Wheeler et al.12 Whole-blood samples (5-10 mL) were collected in EDTA-containing vials. One healthy control sample was processed in parallel to each patient sample.
For the CD107a staining, peripheral blood mononuclear cells were separated from whole blood and cultured overnight with interleukin-2 (IL-2). After overnight culture with IL-2, the cells were stimulated with anti-CD3 antibody in the presence of fluorescein isothiocyanate anti-CD107a antibody for 2 hours. The cells were then stained with cell surface markers and analyzed by flow cytometry.
Vbeta repertoire.
Flow cytometric Vbeta repertoire analysis was undertaken with the Beta Mark TCR vβ Repertoire Kit (Beckman Coulter) and CD3, CD4, and CD8 antibodies. Families were plotted on a clonogram to detect any abnormalities, including clonal expansions and reduced repertoire.
Molecular assessment
Spectratype analysis.
Complementarity-determining region-3 (CDR3) T-cell receptor (TCR) spectratyping of patients 3 and 4 was performed as previously described.13 Analysis of the spectratype data was performed using GeneMapper software 5. Complexity scoring was based on counting the number of peaks per subfamily.14 A score of 0 was assigned for complete absence of a subfamily profile, 1 for a subfamily with a single monoclonal peak, 2 for a biclonal profile, and so on up to a maximum score of 8 for a subfamily with ≥8 peaks. The overall complexity score per sample was then calculated as the summation of the individual subfamilies (maximum score, 24 × 8 = 192, with 24 variable region primers).15 A score of ≥142 was taken as normal spectratype as previously described.14
Chimerism monitoring.
Chimerism analysis was performed using short tandem repeats at the peak of T-cell expansion and then on days +30, +60, +90, +180, and +360.
Virology monitoring.
Cytomegalovirus, adenovirus, and Epstein-Barr virus (EBV) in the blood were monitored weekly by polymerase chain reaction test.
Statistical analysis
Unpaired Student t test was used to compare the clinical features of preengraftment syndrome, such as CRP, days to peak CRP after CBT, days to oxygen requirement after CBT, number of days of fever, maximum temperature, and number of days of oxygen. Unpaired Student t test was also used to compare percentage of memory T cells in the index and control patients.
Results
T-cell kinetics
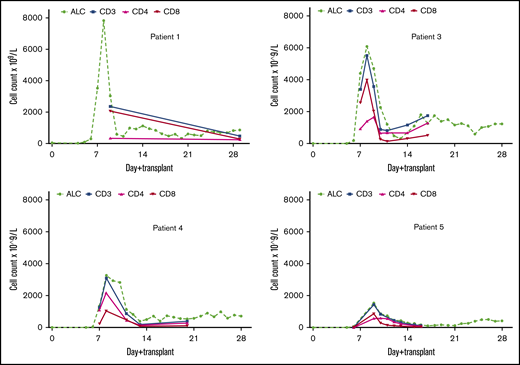
In 4 of 5 patients, we observed early (day +8 or +9) T-cell expansion (1420 to 7820 per microliter), which was both transient and CD8+ biased and which preceded myeloid engraftment (Figure 1; Table 2). The remaining patient died as a result of conditioning-related, multiorgan toxicity early after transplantation. The magnitudes of T-cell expansion in the first, third, fourth, and fifth patients were 123-, 84-, 48-, and 21-fold, respectively (Figure 2A). On comparing the T-cell expansion with the historical controls, we found that such expansion of CD8+ and CD4+ T cells was never observed after CBT, and the control patients were extremely lymphopenic on day 8 after CBT (Figure 2B-C). In all 4 patients, the expanding T cells were 100% donor in origin.
Kinetics of lymphocyte reconstitution in patients 1, 3, 4 and 5. A rapid expansion of T cells was observed very early (between days +7 and +9) after transplantation. It is noteworthy that a very early CD8+ T-cell expansion was observed, in contrast to the significantly delayed CD8+ T-cell reconstitution after typical T cell–replete CBT. ALC, absolute lymphocyte count.
Kinetics of lymphocyte reconstitution in patients 1, 3, 4 and 5. A rapid expansion of T cells was observed very early (between days +7 and +9) after transplantation. It is noteworthy that a very early CD8+ T-cell expansion was observed, in contrast to the significantly delayed CD8+ T-cell reconstitution after typical T cell–replete CBT. ALC, absolute lymphocyte count.
T-cell kinetics
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Peak lymphocyte per µL | 7820 (day +8) | 600 (day 14) | 6100 (day +8) | 3258 (day +8) | 1420 (day +9) |
| Trough lymphocyte per µL | 460 (day +11) | 100 (day 18) | 310 (day +13) | 390 (day +13) | 410 (day +12) |
| Peak CD3 count per µL | Not done; 2364 (day +9) | NA | 5520 (day +8) | 3082 (day +8) | 1420 (day +9) |
| Peak CD4 count per µL | Not done; 252 (day +9) | NA | 1656 (day +9) | 2179 (day +8) | 840 (day +9) |
| Peak CD8 count per µL | Not done; 2052 (day +9) | NA | 3983 (day +8) | 1021 (day +8) | 584 (day +10) |
| CD8/CD4 ratio | 5.86 (day +9) | NA | 2.84 (day +8) | 0.46 (day +8) | 0.69 (day +9) |
| 1.1 (day +28) | 0.43 (day+13) | 1.02 (day+12) | 4.9 (day+11) | ||
| Magnitude of expansion | 123-fold | NA | 84-fold | 48-fold | 21-fold |
| Chimerism during T-cell expansion | 100% donor (day +9) | NA | 100% donor (day +8) | 100% donor (day +8) | 100% donor (day +9) |
| Neutrophils >0.5 × 103/µL | Day +19 | Day +12 | Day +14 | Day +12 | Day +27 |
| Platelets >20 × 103/µL | Day +42 | Transfusion dependent | Day +58 | Day +34 | Day +46 |
| Cessation of ciclosporin | Day +33 | NA | Day +33 | Day +76 | Day +44 |
| CMV/ADV/EBV reactivation | None | None | None | None | None |
| Survival | Remains in remission 40 mo posttransplantation | Died of venoocclusive disease and multiorgan failure on day +24 | Remains in remission 31 mo posttransplantation | Remains in remission 20 mo posttransplantation | Remains in remission 14 mo posttransplantation |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Peak lymphocyte per µL | 7820 (day +8) | 600 (day 14) | 6100 (day +8) | 3258 (day +8) | 1420 (day +9) |
| Trough lymphocyte per µL | 460 (day +11) | 100 (day 18) | 310 (day +13) | 390 (day +13) | 410 (day +12) |
| Peak CD3 count per µL | Not done; 2364 (day +9) | NA | 5520 (day +8) | 3082 (day +8) | 1420 (day +9) |
| Peak CD4 count per µL | Not done; 252 (day +9) | NA | 1656 (day +9) | 2179 (day +8) | 840 (day +9) |
| Peak CD8 count per µL | Not done; 2052 (day +9) | NA | 3983 (day +8) | 1021 (day +8) | 584 (day +10) |
| CD8/CD4 ratio | 5.86 (day +9) | NA | 2.84 (day +8) | 0.46 (day +8) | 0.69 (day +9) |
| 1.1 (day +28) | 0.43 (day+13) | 1.02 (day+12) | 4.9 (day+11) | ||
| Magnitude of expansion | 123-fold | NA | 84-fold | 48-fold | 21-fold |
| Chimerism during T-cell expansion | 100% donor (day +9) | NA | 100% donor (day +8) | 100% donor (day +8) | 100% donor (day +9) |
| Neutrophils >0.5 × 103/µL | Day +19 | Day +12 | Day +14 | Day +12 | Day +27 |
| Platelets >20 × 103/µL | Day +42 | Transfusion dependent | Day +58 | Day +34 | Day +46 |
| Cessation of ciclosporin | Day +33 | NA | Day +33 | Day +76 | Day +44 |
| CMV/ADV/EBV reactivation | None | None | None | None | None |
| Survival | Remains in remission 40 mo posttransplantation | Died of venoocclusive disease and multiorgan failure on day +24 | Remains in remission 31 mo posttransplantation | Remains in remission 20 mo posttransplantation | Remains in remission 14 mo posttransplantation |
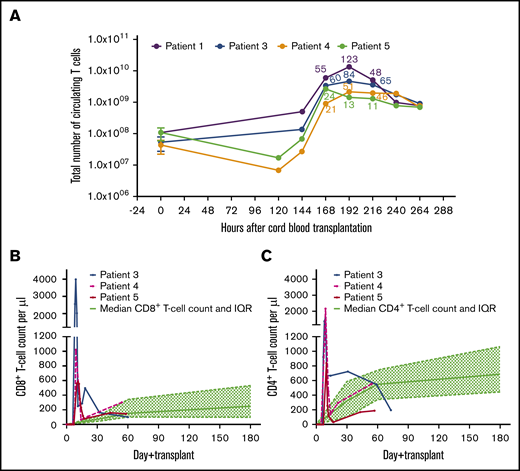
Magnitude of expansion of T cells in patients 1, 3, 4, and 5. (A) Total number of T cells carried with the CB graft of each index patient was extrapolated from 30 historical CB grafts. Median and IQR of T-cell content from the historical CB grafts were multiplied by weight of the index patient to derive approximate number of T cells transferred at the time of CBT. Total blood volume based on weight of the patient was calculated, and thus, total number of circulating T cells during expansion and magnitude of T-cell proliferation were calculated. Magnitude of T-cell expansion after 168, 192, and 216 hours for each patient is labeled in respective color. (B) Absolute cord blood CD8+ T-cell count in patients 3, 4, and 5 was measured daily during the early unprecedented expansion. CD8+ T-cell expansion is compared with median CD8+ T-cell count (and IQR) of control patients (n = 30) from the historical cohort. (C) Absolute cord blood CD4+ T-cell count in patients 3, 4, and 5 was measured daily during the early expansion. CD4+ T-cell expansion is compared with median CD4+ T-cell count (and IQR) of control patients (n = 30) from the historical cohort.
Magnitude of expansion of T cells in patients 1, 3, 4, and 5. (A) Total number of T cells carried with the CB graft of each index patient was extrapolated from 30 historical CB grafts. Median and IQR of T-cell content from the historical CB grafts were multiplied by weight of the index patient to derive approximate number of T cells transferred at the time of CBT. Total blood volume based on weight of the patient was calculated, and thus, total number of circulating T cells during expansion and magnitude of T-cell proliferation were calculated. Magnitude of T-cell expansion after 168, 192, and 216 hours for each patient is labeled in respective color. (B) Absolute cord blood CD8+ T-cell count in patients 3, 4, and 5 was measured daily during the early unprecedented expansion. CD8+ T-cell expansion is compared with median CD8+ T-cell count (and IQR) of control patients (n = 30) from the historical cohort. (C) Absolute cord blood CD4+ T-cell count in patients 3, 4, and 5 was measured daily during the early expansion. CD4+ T-cell expansion is compared with median CD4+ T-cell count (and IQR) of control patients (n = 30) from the historical cohort.
Preengraftment syndrome
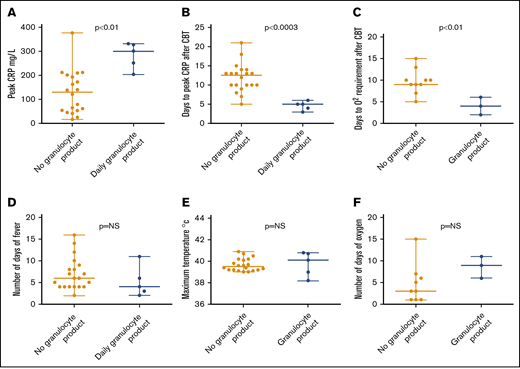
The peak CRP levels were higher in patients receiving granulocytes than in the control group (median, 301 vs 129.5 mg/L; P < .001; Figure 3). CRP levels also peaked earlier in patients receiving granulocytes than in the control group (median, 5 vs 12.5 days; P < .0003; Figure 3). Oxygen was required earlier in patients receiving granulocytes than in the control group (median, 4 vs 9 days; P < .01; Figure 3). Three patients in the granulocyte therapy group vs 9 patients in the control group required oxygen during preengraftment syndrome. One patient in each group was oxygen dependent before CBT.
Characteristics of preengraftment syndrome in patients with and without granulocyte therapy. (A) Higher levels of CRP were seen in patients who received granulocyte product vs those who did not (P < .001). (B) CRP peaked early in patients who received granulocyte product vs those who did not (P < .0003). (C) Oxygen was required early in patients who received granulocyte product vs those who did not (P < .01). Clinical features such as number of days of fever (D), maximum temperature (E), and number of days of oxygen (F) were not statistically different between 2 groups. NS, not significant.
Characteristics of preengraftment syndrome in patients with and without granulocyte therapy. (A) Higher levels of CRP were seen in patients who received granulocyte product vs those who did not (P < .001). (B) CRP peaked early in patients who received granulocyte product vs those who did not (P < .0003). (C) Oxygen was required early in patients who received granulocyte product vs those who did not (P < .01). Clinical features such as number of days of fever (D), maximum temperature (E), and number of days of oxygen (F) were not statistically different between 2 groups. NS, not significant.
There was no statistically significant difference in the clinical features of preengraftment syndrome, including number of days of fever, maximum temperature, and number of days on oxygen (Figure 3). Although early onset of preengraftment syndrome and biochemically severe preengraftment syndrome were observed in those receiving granulocyte therapy, the severity of clinical features of preengraftment syndrome was similar in the 2 groups. All 4 patients with T-cell expansion were treated with steroids. Details of their clinical features and treatment are shown in Table 3 and supplemental Table 1.
Details of preengraftment syndrome
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Days of fever >38.5°C | 6 | 2 | 3 | 4 | 11 |
| Oxygen requirement | Day +4 to +15 | Required oxygen before transplantation; ventilated on day +2 for early venoocclusive disease and multiorgan failure | Day +6 to +12 | Day +2 to +11 | No |
| Peak CRP, mg/L | 332 | 328 | 251 | 301 | 204 |
| Days to peak CRP | 3 | 4 | 5 | 5 | 6 |
| Steroids for preengraftment syndrome | Day +5 to +21 for preengraftment syndrome | None | Day +0 to +20 as GVHD prophylaxis | Day +7 to +14 for preengraftment syndrome | Day +4 to +39 for preengraftment syndrome |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Days of fever >38.5°C | 6 | 2 | 3 | 4 | 11 |
| Oxygen requirement | Day +4 to +15 | Required oxygen before transplantation; ventilated on day +2 for early venoocclusive disease and multiorgan failure | Day +6 to +12 | Day +2 to +11 | No |
| Peak CRP, mg/L | 332 | 328 | 251 | 301 | 204 |
| Days to peak CRP | 3 | 4 | 5 | 5 | 6 |
| Steroids for preengraftment syndrome | Day +5 to +21 for preengraftment syndrome | None | Day +0 to +20 as GVHD prophylaxis | Day +7 to +14 for preengraftment syndrome | Day +4 to +39 for preengraftment syndrome |
Immunological assessment of T cells
T-cell diversity.
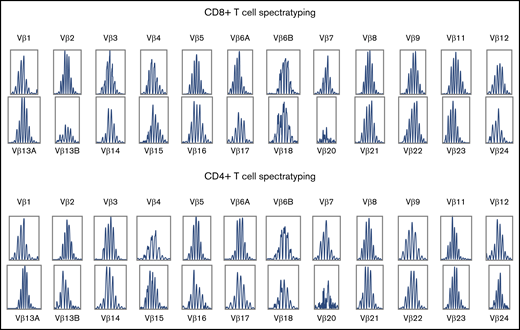
Quantification of CD8+ and CD4+ T-cell receptor diversity by flow cytometry and spectratyping confirmed polyclonal TCR repertoire. The CD8+ T-cell complexity scores of patients 3 and 4 were 172 and 187, respectively, and the CD4+ T-cell complexity scores of these patients were 157 and 187, respectively. Spectratyping of patient 3 is shown in Figure 4, and the flow cytometric Vbeta repertoire of patient 3 is shown as percentage of CD8+ and CD4+ T cells in supplemental Figure 3.
TCR spectratyping of CD8+ and CD4+ T cells during T-cell expansion in patient 3.
TCR spectratyping of CD8+ and CD4+ T cells during T-cell expansion in patient 3.
CD8+ T-cell differentiation.
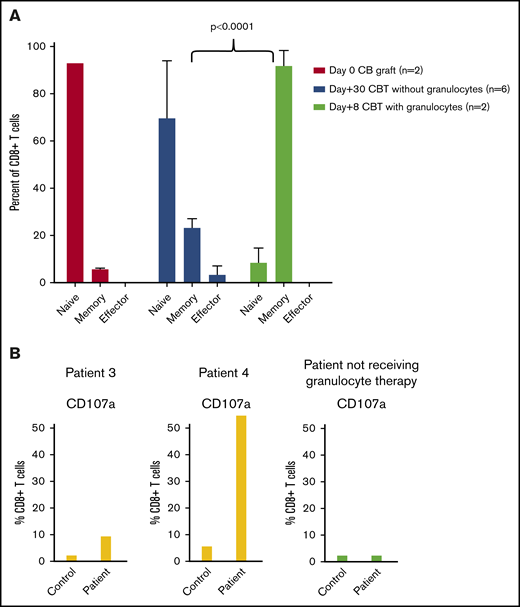
A majority (ie, >90%) of CB T cells are naive. During T-cell expansion, we found that >85% of CD8+ T cells had switched to memory phenotype. The switch to memory phenotype was significantly higher in patients receiving granulocyte transfusions compared with those undergoing typical T cell–replete CBT (Figure 5A; P < .0001).
CD8+ T-cell differentiation and cytotoxicity in patients with and without granulocyte therapy. (A) Rapid switch from naïve to memory phenotype was observed during expansion of CD8+ T cells in CBT patients receiving granulocytes (n = 2; patients 3 and 4). In contrast, only 6% to 27% of CB CD8+ T cells switched to memory phenotype at 30 days after transplantation in those not receiving granulocytes. (B) CD8+ T cells during early expansion acquired cytotoxic function (CD107a) on anti-CD3 stimulation. CD107a was significantly higher in index patients compared with the adult control. In contrast, a patient who underwent CBT without granulocyte transfusion had (low) CD107a, similar to the control.
CD8+ T-cell differentiation and cytotoxicity in patients with and without granulocyte therapy. (A) Rapid switch from naïve to memory phenotype was observed during expansion of CD8+ T cells in CBT patients receiving granulocytes (n = 2; patients 3 and 4). In contrast, only 6% to 27% of CB CD8+ T cells switched to memory phenotype at 30 days after transplantation in those not receiving granulocytes. (B) CD8+ T cells during early expansion acquired cytotoxic function (CD107a) on anti-CD3 stimulation. CD107a was significantly higher in index patients compared with the adult control. In contrast, a patient who underwent CBT without granulocyte transfusion had (low) CD107a, similar to the control.
CD8+ T-cell activation.
We measured activated CD8+ T cells and CD4+ T-regulatory cells in patients 3 and 4 during T-cell expansion and after day +20. During early T-cell expansion, 54.8% and 43.2% of CD8+ T cells were CD38+HLADR+ in patients 3 and 4, respectively. After day +20, 7.4% and 8.1% of CD8+ T cells had CD38+HLADR+ phenotype in patients 3 and 4, respectively (Table 4). Therefore, there was significant reduction in activated CD8+ T cells after day +20.
Qualitative analysis of CD8+ T cells
| . | Patient 3 . | Patient 4 . |
|---|---|---|
| Activated CD8+T cells during T-cell expansion | ||
| CD8+CD38+DR+ | 54.8% (day +8) | 43.2% (day +8) |
| Activated CD8+T cells after trough of T-cell expansion | ||
| CD8+CD38+DR+ | 7.4% (day +23) | 8.1% (day +20) |
| Activated CD8+T-cell/T-regulatory cell ratio during T-cell expansion | ||
| Activated CD8+ T-cell/T-regulatory cell ratio | 10.9 (day +8) | 4.2 (day +8) |
| Activated CD8+T-cell/T-regulatory cell ratio after trough of T-cell expansion | ||
| Activated CD8+ T-cell/T-regulatory cell ratio | 0.42 (day +23) | 0.69 (day +20) |
| Cytotoxic degranulation assay during T-cell expansion | ||
| CD107a, patient/control | 9.4/2.3 (day +8) | 54.8/5.8 (day +8) |
| . | Patient 3 . | Patient 4 . |
|---|---|---|
| Activated CD8+T cells during T-cell expansion | ||
| CD8+CD38+DR+ | 54.8% (day +8) | 43.2% (day +8) |
| Activated CD8+T cells after trough of T-cell expansion | ||
| CD8+CD38+DR+ | 7.4% (day +23) | 8.1% (day +20) |
| Activated CD8+T-cell/T-regulatory cell ratio during T-cell expansion | ||
| Activated CD8+ T-cell/T-regulatory cell ratio | 10.9 (day +8) | 4.2 (day +8) |
| Activated CD8+T-cell/T-regulatory cell ratio after trough of T-cell expansion | ||
| Activated CD8+ T-cell/T-regulatory cell ratio | 0.42 (day +23) | 0.69 (day +20) |
| Cytotoxic degranulation assay during T-cell expansion | ||
| CD107a, patient/control | 9.4/2.3 (day +8) | 54.8/5.8 (day +8) |
We also calculated the ratio of activated CD8+ T-cells/CD4+ T-regulatory cells. A strikingly positive ratio of activated CD8+ T-cells/CD4+ T-regulatory cells (ratio, 10.9 and 4.2 in patients 3 and 4, respectively) was observed at the peak of expansion (Table 4). This indicates that the immune system during the peak of T-cell expansion is biased toward immune activation. The activated immune system switched toward regulation within days (ratio, 0.42 and 0.69 in patients 3 and 4, respectively; Table 4).
CD8+ T-cell cytotoxicity.
We also measured CD107a expression in rapidly proliferating CD8+ T cells to determine the cytotoxic potential. CD107a expression in CD8+ T cells from patients 3 and 4 was significantly higher than the control samples from normal adults (P < .03; Figure 5B). In another 5-month-old patient who underwent CBT for AML without granulocyte transfusions, we measured CD107a expression on day +14 when T-cell count reached >500/µL. CD107a expression in this patient was similar to the control samples from normal adults (Figure 5B). This suggests the enhanced cytotoxic potential of the T cells in the granulocyte-infused patients after CBT.
Outcomes
Viral reactivation and GVHD.
None of the patients developed cytomegalovirus, adenovirus, or EBV reactivation. There was no significant acute GVHD in any patient. Immune suppression was stopped early in all 4 children, and there was no emergence of chronic GVHD.
Survival.
Five consecutive patients underwent CBT with granulocyte therapy for fungal or bacterial infection. Invasive fungal and bacterial infections contribute to the nonrelapse mortality in patients undergoing CBT because of delayed neutrophil engraftment. Early initiation of granulocyte transfusions improves outcomes in patients with neutrophil dysfunction.16 We therefore transfused a pooled granulocyte product to all CBT patients with bacterial or fungal infection. Four of 5 patients who underwent CBT for chemorefractory leukemia and received granulocyte therapy for concomitant infection continued in remission after a median of 26 months (range, 14-40 months) after CBT (Table 2). The remaining patient died as a result of multiorgan failure caused by severe venoocclusive disease.
Discussion
We describe accelerated, amplified, and transient CB T-cell expansion mediated by third-party granulocyte therapy. CD4+ T cell–biased immune reconstitution with a relatively delayed CD8+ T-cell recovery is typically observed after T cell–replete CBT, and CD8+ T-cell numbers such as those reported here during granulocyte administration are not usually seen, even after recovery of thymopoiesis.3 This CD8+ T-cell expansion is analogous to the intratumoral infiltration of the CB CD8+ T cells that mediate an antitumor response in the EBV-driven lymphoblastoid tumor model.2 Early, transient CD4+ T-cell expansion was also observed in these patients, and it is likely that these CD4+ T cells contribute to and facilitate the graft-versus-leukemia effect.17
All 4 patients who achieved remission after T-cell expansion had high-risk relapsed or refractory myeloid disease. This suggests that the T-cell expansion in patients treated with the third-party granulocyte product could mediate an enhanced graft-versus-leukemia effect.
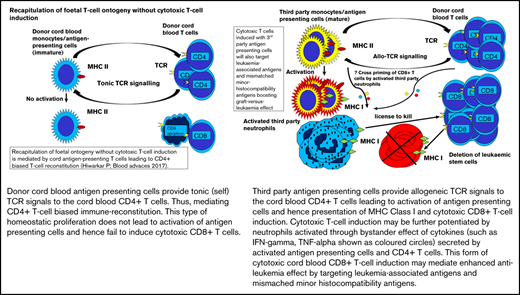
Each pooled granulocyte product contains neutrophils and mononuclear cells, such as lymphocytes and monocytes, as described in "Methods."11 B cells and monocytes constitutively express major histocompatibility complex (MHC) class 2.18 Neutrophils express MHC class 1, and in preclinical models, efficient cross-priming of naïve T cells with neutrophils has been demonstrated.19-24 A majority of fetal T cells are naïve.25 Therefore, the serendipitous T-cell response is most likely a response to the granulocyte product, and CD8+ T cell–biased expansion may be potentiated by cross-priming of naïve CD8+ T cells with the huge MHC class 1 load presented by the third-party mismatched neutrophils.
Interestingly, >95% of CD8+ T cells had acquired a memory phenotype by day +8, suggesting rapid cycling of naïve CB CD8+ T cells. The rapidly cycling T cells were significantly skewed toward immune activation, and activated CD8+ T cells precipitously declined, shifting the balance toward regulation.26 A significant percentage (≥10%) of CB CD8+ T cells were CD107a+ after anti-CD3 stimulation, further confirming their ability to mediate a cytotoxic response. Fetal and adult T cells are distinct T-cell lineages.27 Evidence from murine neonates points toward rapid gain of effector function mediated by short-lived CD8+ T cells.28,29 It is therefore likely that the very early but transient CD8+ T-cell response observed in these patients represents a fetal T-cell characteristic. In the absence of allogeneic, leukemic, or viral antigens, short-lived fetal-derived CD8+ T cells probably undergo apoptosis without expansion.30
An effect of CD8+ T-cell content in the CB graft on overall survival in adults with hematological malignancies has also been reported.31 Evidence from double-unit CBT suggests that CB CD8+ T cells can mediate an allogeneic response. Gutman et al32 provided compelling evidence about the role of CD8+ T cells in mediating the rejection of the losing graft. Similarly, Milano et al33 showed a correlation between infused CD3+CD8+ T cells and single-unit dominance. The dominance of the single unit was decided in the first 2 weeks after CBT, suggesting that naïve CD8+ T cells infused with the CB graft can mount an accelerated alloreactive response. However, the T-cell expansion after double CBT is not as robust as that seen in the patients reported here.
Despite the expansion of CB T cells in the reported patients, there was no evidence of GVHD, and immune suppression was stopped early after transplantation in all of them, including in mismatched grafts. We speculate that any GVHD was abrogated by the transient nature of the immunogenic response. This speculation is consistent with previous observations of the low incidence of chronic GVHD after CBT.34
We have demonstrated the importance of human CB CD8+ T-cell allogeneic responses in mediating antitumor responses in an in vivo murine model.2 The model confirmed that naïve CB CD8+ T cells could be rapidly educated to mediate an enhanced antitumor effect without xenogeneic GVHD. However, appropriate immunogenic conditions to enhance antigen presentation are required. Projects to enhance immunogenicity, such as generation of CB DC-AML fusion vaccines, are under way.9 This report supports a role of third-party antigen-presenting cells in enhancing the antileukemia effect mediated by CB T cells without increasing the risk of GVHD.
T-cell expansion was observed in 4 of 5 CBT patients receiving granulocyte therapy. Absence of T-cell expansion in 1 patient could be attributed to venoocclusive disease and multiorgan failure. The observation requires replication with large patient numbers to assess safety, replicability, and efficacy. Although no patients developed serious adverse events after granulocyte transfusion, there is a risk of alloimmunization or transfusion-related acute lung injury (TRALI) in patients receiving granulocyte transfusions.10 However, the incidence of TRALI has been significantly reduced by suspending granulocytes in plasma from male donors, and the incidence of immunological complications has been reduced by replacing a substantial proportion of plasma with the additive solution.11
In summary, this report provides proof of concept for enhancing the immunogenicity of fetal-derived CD8+ T cells to prevent disease relapse in high-risk myeloid leukemia. Whether this approach could also mediate an enhanced antileukemia effect against a refractory lymphoid malignancy remains to be tested. This is a simple platform that is readily deliverable in transplantation units, and it might be made more specific to leukemia-associated antigen and mismatched minor histocompatibility antigens by using antigen-presenting cells derived from the parents of a transplant recipient rather than from a pooled product. In the era of specialized gene-modified cells, such as chimeric antigen receptor T cells, which require significant infrastructure and expertise, amplifying T-cell antileukemia responses by enhancing antigen presentation after CBT could provide a simple and cost-effective platform for the treatment of refractory hematological malignancies.
Send data sharing requests via e-mail to the corresponding author, Prashant Hiwarkar (phiwarkar@nhs.net).
Authorship
Contribution: P.H. interpreted the observations, designed research, analyzed the data, and performed statistical analysis; R.W. made the observation; S.A., K.G., and K.P. performed laboratory tests; P.H., R.W., and D.B. managed the patients; R.N. collected the data on preengraftment syndrome; P.H. and R.W. wrote the manuscript; and all authors reviewed and provided comments on the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prashant Hiwarkar, Department of Haematology and Bone Marrow Transplantation, Royal Manchester Children’s Hospital, Upper Brook St, Manchester M13 9WL, United Kingdom; e-mail: phiwarkar@nhs.net.
References
Author notes
The full-text version of this article contains a data supplement.