Key Points
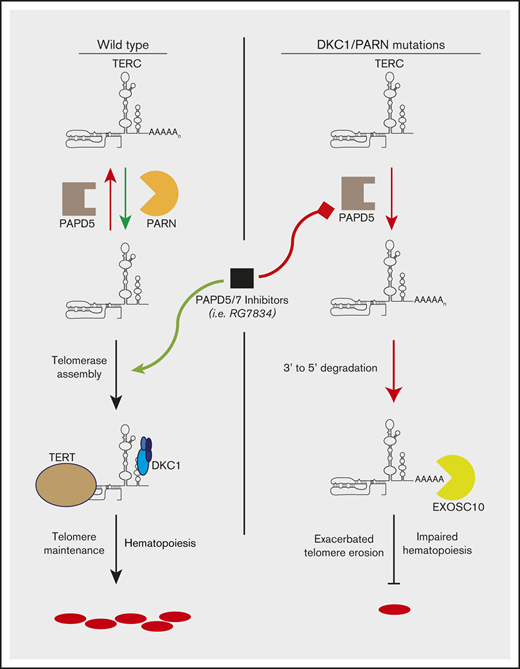
Chemical inhibition of PAPD5/7 restores TERC levels, reduces TERC 3′-end oligoadenylation, and improves telomerase function in DC mutants.
Treatment with RG7834 restores definitive hematopoietic development from DKC1 mutant human embryonic stem cells.
Abstract
Dyskeratosis congenita (DC) is a pediatric bone marrow failure syndrome caused by germline mutations in telomere biology genes. Mutations in DKC1 (the most commonly mutated gene in DC), the 3′ region of TERC, and poly(A)-specific ribonuclease (PARN) cause reduced levels of the telomerase RNA component (TERC) by reducing its stability and accelerating TERC degradation. We have previously shown that depleting wild-type DKC1 levels by RNA interference or expression of the disease-associated A353V mutation in the DKC1 gene leads to decay of TERC, modulated by 3′-end oligoadenylation by noncanonical poly(A) polymerase 5 (PAPD5) followed by 3′ to 5′ degradation by EXOSC10. Furthermore, the constitutive genetic silencing of PAPD5 is sufficient to rescue TERC levels, restore telomerase function, and elongate telomeres in DKC1_A353V mutant human embryonic stem cells (hESCs). Here, we tested a novel PAPD5/7 inhibitor (RG7834), which was originally discovered in screens against hepatitis B viral loads in hepatic cells. We found that treatment with RG7834 rescues TERC levels, restores correct telomerase localization in DKC1 and PARN-depleted cells, and is sufficient to elongate telomeres in DKC1_A353V hESCs. Finally, treatment with RG7834 significantly improved definitive hematopoietic potential from DKC1_A353V hESCs, indicating that the chemical inhibition of PAPD5 is a potential therapy for patients with DC and reduced TERC levels.
Introduction
Dyskeratosis congenita (DC) is characterized by a classic triad of dysplastic nails, lacy reticular pigmentation of the upper chest and neck, and oral leukoplakia.1 Patients with DC present at early age with short telomeres, and bone marrow failure represents the major cause of morbidity.2 Treatment is challenging and tailored to the individual.3 Hematopoietic stem cell transplantation remains the only curative treatment of bone marrow failure in these patients but historically has had poor long-term efficacy.4 Novel alternatives for clinical management are urgently needed.
Mutations in patients with DC are found in different genes involved in telomere protection or maintenance.5,6 Four of these genes impair the function of the telomerase RNA component TERC leading to reduced telomerase activity, including mutations in TERC itself, poly(A)-specific ribonuclease (PARN),7-9 NAF1,10 and DKC1,11,12 which represents the most commonly mutated gene in DC (X-linked inheritance). Mutations in PARN and DKC1 reduce TERC stability and cause increased TERC degradation by the exosome component EXOSC10.13,14 We and others have shown that the degradation of TERC by the exosome can be inhibited by reducing the 3′-end oligoadenylation of TERC through the modulation of PAPD5 [noncanonical poly(A) polymerase 5] levels.13-17 Utilizing the targeted differentiation of human embryonic stem cells (hESCs), we showed that the genetic silencing of PAPD5 is able to improve the hematopoietic potential of DC cells,18 thereby rescuing the major phenotype observed in this disease. This finding opens the possibility that the chemical inhibition of PAPD5 by a safe, efficient, orally available compound could represent a novel alternative to be pursued in the clinical management of patients with DC and mutations that impair TERC biology.
Herein, we show that treatment of PARN- or DKC1-depleted cells with RG7834, a novel small molecule inhibitor of PAPD5 and PAPD7,19,20 leads to increased TERC levels and correct telomerase RNA subcellular localization. Moreover, RG7834 treatment significantly decreased the 3′-end oligoadenylation of TERC in DKC1_A353V hESCs, leading to increased TERC levels in these cells. The chemical inhibition of PAPD5/7 was sufficient to elongate telomeres in DKC1_A353V hESCs and to significantly improve the definitive hematopoietic potential of these cells. Combined, our results indicate that the chemical modulation of PAPD5 activity could serve as a potential novel therapy for patients with DC.
Methods
Wild-type (WT) (WA01) and DKC1_A353V mutant hESCs were maintained as previously described.21 RG7834 was acquired from MedKoo Biosciences (Morrisville, NC) and diluted in dimethyl sulfoxide (DMSO) (MilliporeSigma, Burlington, MA). Detailed methods and experimental procedures are described in the supplemental Methods.
Results and discussion
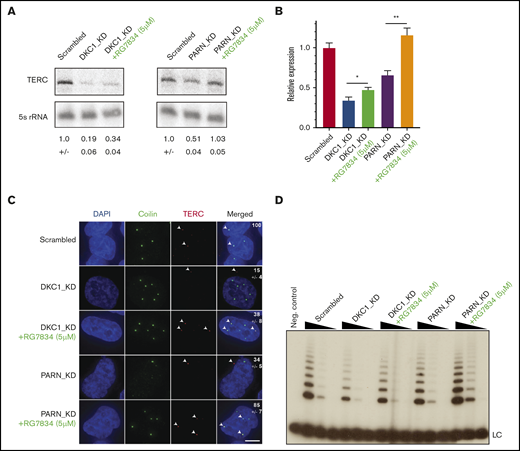
To investigate whether RG7834 treatment phenocopies PAPD5 knockdown, we depleted DKC1 in HeLa cells (supplemental Figure 1A-B) and treated them with either DMSO (control) or 5 μM of RG7834 for 2 days. Similar to previous results,13 knockdown of DKC1 led to a significant reduction in TERC levels (Figure 1A-B). Importantly, treatment with RG7834 led to a significant increase in TERC levels in DKC1 knockdown cells, indicating that RG7834 is able to increase TERC levels in DKC1-deficient cells. Because the depletion of PARN also leads to TERC degradation mediated by 3′ end adenylation by PAPD5,13 we silenced PARN in HeLa cells and treated them with DMSO or 5 μM of RG7834 for 2 days. We found that PARN knockdown leads to a significant reduction in TERC levels, which is completely rescued by RG7834 treatment. Thus, similar to the genetic silencing of PAPD5, treatment with RG7834 increased TERC levels in both PARN- and DKC1-deficient cells, without affecting levels of either DKC1 or PARN in individual knockdown cells. Finally, RG7834 treatment did not affect TERC levels in HeLa cells that were not subject to silencing of either DKC1 or PARN (supplemental Figure 1C).
RG7834 treatment rescues TERC levels and localization in DKC1- and PARN-depleted cells. (A) Representative northern blots for TERC levels in HeLa cells under indicated conditions. Numbers below panels: average ± standard deviation for 3 biological replicates. (B) Quantification of TERC by quantitative reverse transcription polymerase chain reaction in HeLa cells under the indicated conditions (n = 3; biological replicates). Values are expressed in relation to scrambled control. (C) Representative images for 4′,6-diamidino-2-phenylindole (DAPI) (nucleus), coilin (cajal body), TERC, and merge of individual channels obtained from HeLa cells transfected with indicated siRNAs and treated with RG7834. White arrows indicate TERC localization within the cell. Scale bar, 5 μm. Numbers in image panels: Quantification of fraction of cells with TERC colocalized to cajal bodies from at least 30 independent cells and 3 replicates. Cells with at least one TERC focus colocalized with coilin were counted. (D) Telomerase activity by telomere repeat amplification in HeLa cells transfected with indicated siRNAs and treated with 5 μM of RG7834 or DMSO. Range of protein concentrations represent fourfold serial dilutions. *P < .05; **P < .01. LC, loading control.
RG7834 treatment rescues TERC levels and localization in DKC1- and PARN-depleted cells. (A) Representative northern blots for TERC levels in HeLa cells under indicated conditions. Numbers below panels: average ± standard deviation for 3 biological replicates. (B) Quantification of TERC by quantitative reverse transcription polymerase chain reaction in HeLa cells under the indicated conditions (n = 3; biological replicates). Values are expressed in relation to scrambled control. (C) Representative images for 4′,6-diamidino-2-phenylindole (DAPI) (nucleus), coilin (cajal body), TERC, and merge of individual channels obtained from HeLa cells transfected with indicated siRNAs and treated with RG7834. White arrows indicate TERC localization within the cell. Scale bar, 5 μm. Numbers in image panels: Quantification of fraction of cells with TERC colocalized to cajal bodies from at least 30 independent cells and 3 replicates. Cells with at least one TERC focus colocalized with coilin were counted. (D) Telomerase activity by telomere repeat amplification in HeLa cells transfected with indicated siRNAs and treated with 5 μM of RG7834 or DMSO. Range of protein concentrations represent fourfold serial dilutions. *P < .05; **P < .01. LC, loading control.
We have previously shown that in DKC1- or PARN-depleted cells, TERC accumulates in cytoplasmic puncta called “cyTER bodies,”13 instead of its normal localization to cajal bodies in the nucleus.22,23 To investigate whether RG7834 treatment rescued TERC localization to cajal bodies in the nucleus, we treated DKC1- or PARN-depleted cells with RG7834 for 2 days. We found that in WT control cells, TERC localized to cajal bodies, suggesting normal accumulation and trafficking of the telomerase RNA component (Figure 1C). However, in control DKC1 knockdown cells, only ∼15% of cells showed TERC localization to cajal bodies, with a significant number of cells exhibiting TERC accumulation in cyTER bodies. Upon treatment with RG7834, ∼38% of cells had TERC localized to cajal bodies, which is consistent with the 1.5× increase in TERC levels in these cells. RG7834 treatment also rescued TERC localization to cajal bodies in PARN-depleted cells. In PARN knockdown cells, ∼34% of cells had TERC in cajal bodies, with TERC also mostly being localized to cyTER bodies in these cells. However, upon RG7834 treatment, ∼85% of cells had TERC localized to cajal bodies. Finally, treatment with RG7834 was also able to increase telomerase activity in DKC1- and PARN-depleted cells (detected by using 2-step telomere repeat amplification assays) (Figure 1D; supplemental Figure 1D), which is consistent with the increase in TERC levels (Figure 1A-B). No toxicity was observed in RG7834-treated cells for the duration of these experiments. Taken together, these results suggest that the chemical inhibition of RG7834 is effective at increasing TERC levels, correcting TERC localization to cajal bodies, and increasing telomerase activity in DKC1- or PARN-depleted cells, indicating restored telomerase function in these conditions.
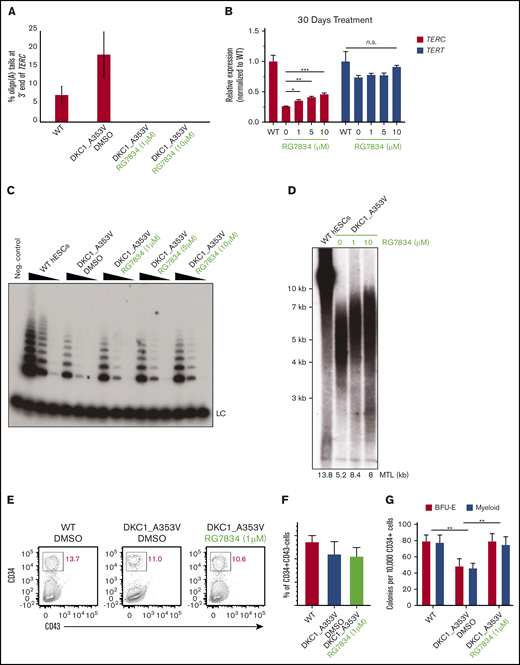
We next investigated whether RG7834 exhibits a similar effect on TERC levels and function in a physiologically relevant model of DC. We have previously generated (through CRISPR/Cas9 gene editing) DKC1_A353V mutant hESCs, which exhibit reduced TERC levels and telomerase activity, progressive telomere shortening, and impaired definitive hematopoietic specification.21 Treatment of DKC1_A353V hESCs with RG7834 caused a >15-fold reduction in the 3′-end oligoadenylation of TERC, indicating efficient PAPD5 inhibition in these cells (Figure 2A). Inhibition of 3′ end oligoadenylation led to a significant increase in TERC levels in DKC1_A353V hESCs (4 days of treatment) (supplemental Figure 2A), which was sustained up to 30 days upon continuous treatment with different concentrations of RG7834 (Figure 2B). Although the levels of the telomerase reverse transcriptase (TERT) remained unchanged during this period, the increased TERC levels found in DKC1_A353V hESCs treated with RG7834 caused a noticeable rise in telomerase activity in DKC1_A353V hESCS treated with different concentrations of the PAPD5/7 inhibitor (Figure 2C; quantification in supplemental Figure 2B).
RG7834 treatment rescues telomerase activity, increases telomere length, and improves hematopoietic specification in DKC1_A353V mutant hESCs. (A) Quantification of oligo(A) reads at the 3′ end (UGC) of TERC in indicated conditions (average ± standard deviation from 2 independent replicates). TERC reads with more than 2 As at the 3′ end are considered oligoadenylated. (B) Quantification of TERC and TERT by quantitative reverse transcription polymerase chain reaction in DKC1_A353V hESCs treated with DMSO or different concentrations of RG7834 for 30 days (n = 3; biological replicates). (C) Telomerase activity by telomere repeat amplification in DKC1_A353V hESCs treated with DMSO or different concentrations of RG7834. Range of protein concentrations represent fourfold serial dilutions. (D) Telomere length analysis by telomere restriction fragment analysis of DKC1_A353V hESCs treated with DMSO or different concentrations of RG7834 for 90 days. (E) Representative flow cytometric analysis of CD34 and CD43 expression on day 8 of definitive hematopoietic differentiation, following CHIR99021 and SB-431542 treatment in DKC1_A353V cells treated with DMSO or different concentrations of RG7834. (F) Quantification of CD34+CD43– population obtained from day 8 differentiation cultures treated with CHIR99021 and SB-431542, as in panel E. (G) CFC potential of definitive hematopoietic progenitors in WT and DKC1_A353V cells treated with DMSO or 1μM of RG7834 (n = 3; biological replicates). Statistical significance was determined by using one- or two-way analysis of variance following a Bonferroni multiple comparison posttest. *P < .05; **P < .01; ***P < .001. BFU-E, burst forming unit-erythroid; CFC, colony forming cell; MTL, mean telomere length; n.s., not significant.
RG7834 treatment rescues telomerase activity, increases telomere length, and improves hematopoietic specification in DKC1_A353V mutant hESCs. (A) Quantification of oligo(A) reads at the 3′ end (UGC) of TERC in indicated conditions (average ± standard deviation from 2 independent replicates). TERC reads with more than 2 As at the 3′ end are considered oligoadenylated. (B) Quantification of TERC and TERT by quantitative reverse transcription polymerase chain reaction in DKC1_A353V hESCs treated with DMSO or different concentrations of RG7834 for 30 days (n = 3; biological replicates). (C) Telomerase activity by telomere repeat amplification in DKC1_A353V hESCs treated with DMSO or different concentrations of RG7834. Range of protein concentrations represent fourfold serial dilutions. (D) Telomere length analysis by telomere restriction fragment analysis of DKC1_A353V hESCs treated with DMSO or different concentrations of RG7834 for 90 days. (E) Representative flow cytometric analysis of CD34 and CD43 expression on day 8 of definitive hematopoietic differentiation, following CHIR99021 and SB-431542 treatment in DKC1_A353V cells treated with DMSO or different concentrations of RG7834. (F) Quantification of CD34+CD43– population obtained from day 8 differentiation cultures treated with CHIR99021 and SB-431542, as in panel E. (G) CFC potential of definitive hematopoietic progenitors in WT and DKC1_A353V cells treated with DMSO or 1μM of RG7834 (n = 3; biological replicates). Statistical significance was determined by using one- or two-way analysis of variance following a Bonferroni multiple comparison posttest. *P < .05; **P < .01; ***P < .001. BFU-E, burst forming unit-erythroid; CFC, colony forming cell; MTL, mean telomere length; n.s., not significant.
The sustained treatment (up to 3 months) with RG7834 also led to improved telomere maintenance, analyzed by telomere restriction fragment analysis, in DKC1_A353V hESCs (Figure 2D). Together, these data suggest that RG7834 treatment rescued TERC levels, prevented its 3′ end oligoadenylation, increased telomerase activity, and improved telomere homeostasis in DKC1_A353V hESCs.
Finally, we investigated if treatment with RG7834 was sufficient to reduce DNA damage signaling arising from eroded telomeres, a hallmark of DC. We observed that γH2AX levels were reduced in DKC1_A353V cells treated with different concentrations of RG7834 compared with DMSO-treated cells (supplemental Figure 3A). Importantly, we detected no toxicity associated with RG7834 treatment at the concentrations indicated, during the entire duration of the experiments performed (supplemental Figure 3B-C). In addition, whole-genome RNA-sequencing analysis showed that treatment with 1 μM of RG7834 did not lead to significant changes in gene expression in DKC1_A353V mutant hESCs compared with DMSO-treated cells (supplemental Figure 3D), suggesting that RG7834 treatment affects specific RNAs in hESCs and rules out toxicity associated with genome-wide gene expression perturbations. Combined, these data indicate that similar to the genetic silencing of PAPD5, treatment with RG7834 is able to rescue the major biochemical phenotypes observed in DC models.
Finally, because bone marrow failure is the leading cause of death in DC, we wanted to analyze the consequences of RG7834 treatment in DKC1_A353V cells during definitive hematopoietic differentiation. There are no animal models that faithfully recapitulate the hematopoietic defects observed in patients harboring pathogenic mutations in DKC1 or PARN; we therefore followed established protocols of hESC differentiation into hematopoietic lineages. These protocols recapitulate, in vitro, the major aspects of blood development in vivo,24 a strategy that we and others have shown to accurately model key aspects of DC.18,21,25,26 A schematic of our protocol is depicted in supplemental Figure 4. Our data show that although CD34+CD43– early hematopoietic progenitors (day 8 of differentiation) (Figure 2E-F) were similar in all samples, definitive hematopoietic colony potential analysis (day 28 of differentiation) revealed that treatment with different concentrations of RG7834 significantly increased the hematopoietic potential of DKC1_A353V cells (Figure 2G). These observations provide compelling evidence that chemical inhibition of PAPD5, in addition to rescuing telomerase function, is sufficient to increase definitive, multilineage, hematopoietic potential in DKC1_A353V mutants.
Our data provide functional evidence that a small molecule inhibitor of PAPD5/7 significantly increases TERC levels, localization, and function in DKC1- and PARN-deficient cells. We show that RG7834 reduces the 3′-end oligoadenylation of TERC, increases TERC levels and telomerase activity, and elongates telomeres in PARN- or DKC1-deficient cells. RG7834 treatment was sufficient to restore the in vitro definitive hematopoietic development of DKC1_A353V hESCs, similarly to what we have recently observed with the genetic silencing of PAPD5.18 Although these experiments represent the first nongenetic rescue of hematopoietic development from hESCs in DKC1 mutant cells, it has recently been shown that PAPD5 inhibitors are able to promote telomere restoration in different patient-derived DC samples in vitro and in vivo, through xenotransplantation assays27 ; these results provide further support for the future use of this technology in the clinic. In addition, the studies presented here indicate that small increases in TERC levels (less than twofold) are sufficient to increase telomerase activity and improve hematopoietic output in DKC1 mutant cells. Future experiments performed in cells harboring mutations in other genes that impair TERC levels/function (including TERC itself, NHP2, NOP10, and ZCCHC8) are necessary for determining the scope and range of effectiveness of PAPD5 inhibition for DC treatment. In addition, the efficiency of PAPD5 inhibition to ameliorate other phenotypes that are commonly associated with telomere shortening, such as pulmonary fibrosis and liver disease, must be assessed, as these conditions cause substantial morbidity and mortality and represent an important unmet need for these patients. Nonetheless, the experiments presented here indicate that the chemical inhibition of PAPD5 by RG7834 or other specific small molecule inhibitors can be a promising therapeutic approach for the treatment of DC or other telomere biology syndromes caused by mutations that reduce TERC levels.
The sequencing data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE150596). Any further questions should be directed to the corresponding authors (Roy Parker; e-mail: roy.parker@colorado.edu and Luis Francisco Zirnberger Batista; e-mail: lbatista@wustl.edu).
Acknowledgments
R.P. and S.S. are supported by the Howard Hughes Medical Institute. H.-C.J. and L.F.Z.B. are supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (1R01HL137793), and grants from the Center for Regenerative Medicine at Washington University in St. Louis, the Siteman Cancer Center, and the American Cancer Society.
Authorship
Contribution: S.S., H.-C.J., L.F.Z.B., and R.P. designed the experiments and analyzed the data; S.S. and H.-C.J. performed the experiments; C.M.S provided critical input and reagents; and S.S., H.-C.J., L.F.Z.B., and R.P. wrote the manuscript.
Conflict-of-interest disclosure: R.P. is a consultant for Third Rock Ventures. The remaining authors declare no competing financial interests.
Correspondence: Luis Francisco Zirnberger Batista, Hematology Division, Washington University School of Medicine, Campus Box 8125, 660 South Euclid Ave, St. Louis, MO 63110; e-mail: lbatista@wustl.edu; or Roy Parker, JSC Biotech Building, 596 UCB, Boulder, CO 80309; e-mail: roy.parker@colorado.edu.
References
Author notes
S.S. and H.-C.J. contributed equally to this work.
The full-text version of this article contains a data supplement.