Key Points
A trimeric extracellular moiety of APRIL has enhanced binding to BCMA and TACI compared with monomeric APRIL when incorporated into a CAR.
T cells transduced with a trimeric APRIL-based CAR are a promising approach for the treatment of MM.
Abstract
Chimeric antigen receptor (CAR) T cells (CARTs) have shown tremendous potential for the treatment of certain B-cell malignancies, including patients with relapsed/refractory multiple myeloma (MM). Targeting the B-cell maturation antigen (BCMA) has produced the most promising results for CART therapy of MM to date, but not all remissions are sustained. Emergence of BCMA escape variants has been reported under the selective pressure of monospecific anti-BCMA CART treatment. Thus, there is a clinical need for continuous improvement of CART therapies for MM. Here, we show that a novel trimeric APRIL (a proliferation-inducing ligand)–based CAR efficiently targets both BCMA+ and BCMA− MM. Modeled after the natural ligand-receptor pair, APRIL-based CARs allow for bispecific targeting of the MM-associated antigens BCMA and transmembrane activator and CAML interactor (TACI). However, natural ligands as CAR antigen-binding domains may require further engineering to promote optimal binding and multimerization to adequately trigger T-cell activation. We found that using a trimeric rather than a monomeric APRIL format as the antigen-binding domain enhanced binding to BCMA and TACI and CART activity against MM in vitro and in vivo. Dual-specific, trimeric APRIL-based CAR are a promising therapeutic approach for MM with potential for preventing and treating BCMA escape.
Introduction
Multiple myeloma (MM) is defined by the clonal expansion of plasma cells in the bone marrow and accounts for 13% of all hematological malignancies.1,2 Despite advances in the treatment of MM by use of high-dose chemotherapy with autologous stem-cell transplantation and the introduction of novel agents,3,4 patients ultimately relapse with increasingly refractory disease. Chimeric antigen receptor T cells (CARTs) are capable of targeting tumor-associated surface antigens5 and effecting rapid and durable responses in B-cell malignancies.6-12 However, disease resistance and relapse related to loss of antigen expression is a major cause of failure of CD19-directed CART therapy.13-15 In the context of MM, B-cell maturation antigen (BCMA), a member of the tumor necrosis factor receptor (TNFR) superfamily, has emerged as a promising target for immunotherapy.16,17 BCMA expression is restricted to terminally differentiated B cells and plasma cells and promotes survival and proliferation of myeloma cells.16,18-21 Encouraging results have been reported using BCMA redirected CARTs in early phase clinical trials for relapsed/refractory (r/r) MM.22-25 However, there is emerging data on BCMA loss after BCMA CART treatment.24,26 In 1 study, the overall objective response rate was 85%, yet the median progression-free survival was 11.8 months,25 indicating that monospecific targeting of BCMA with CARTs may not be curative therapy for most patients.
Combinatorial antigen recognition approaches may improve efficacy of CART therapy and circumvent antigen escape. We hypothesized that additional targets in MM could overcome BCMA loss. Several targets aside from BCMA have been suggested for adoptive cell therapy of MM.27-31 However, expression patterns that account only for a subset of myeloma cells (CD19), or overlap with normal tissues (CD38, CD138, and CS1) may limit the potential of these approaches. Transmembrane activator and CAML interactor (TACI), like BCMA, is a TNFR superfamily member that is almost solely expressed on plasma cells and found at high levels on most myeloma cells.18 As members of the same TNFR superfamily, BCMA and TACI may have a redundant role in providing plasma cells with survival signals.32 It is not known whether escape variants, selected under the pressure of BCMA-directed therapy, will retain TACI expression, thus preserving this essential prosurvival signal for MM cells. The natural ligand for both BCMA and TACI is a proliferation-inducing ligand (APRIL),33,34 which is produced by myeloid cells in the bone marrow and secreted in a trimeric form.35 Dual-targeting of BCMA and TACI on myeloma cells has successfully been reported by use of an APRIL-based CAR in preclinical models.36 A phase 1 and 2 trial treating r/r MM patients with APRIL-based CARTs has been underway since 2017 (www.clinicaltrial.gov number NCT03287804), but results have not yet been reported. Monoclonal antibodies are typically selected for their high-affinity binding, and most successful antibody-based CARs have binding affinities in the low-nanomolar (10−9 M) range.37 APRIL binds BCMA with high affinity (KD 3.8 × 10−10 M), whereas APRIL binding to TACI is 10-fold lower (KD 3.9 × 10−9 M).38 Most antibodies used to design CARs are derived from murine monoclonal antibodies, which may also decrease their persistence related to host rejection of xenogeneic proteins.
We designed CARs to target human BCMA and TACI with a human APRIL-based binding moiety, hypothesizing that preserving its trimeric conformation would yield improved binding and efficacy against MM cells and that the use of human sequences would decrease immunogenicity. We took 2 approaches to engineering CARs with a trimeric APRIL structure: either employing a trimerizing 4-1BB transmembrane domain or multimerizing the extracellular moiety of the APRIL binding domain. By testing these different configurations and characterizing their binding affinities and function in bulk and at the single-cell level, we found that CARs using a trimeric APRIL binding domain (TriPRIL) have enhanced activity against both BCMA+ and BCMA− MM lines in vitro and in vivo in xenograft models, as well as potent activity against primary patient-derived myeloma cells.
Methods
CART manufacturing
Primary human T cells were purified (cat. #15061; Stem Cell Technologies) from anonymous healthy donor leukopaks purchased from the Massachusetts General Hospital (MGH) blood bank under an institutional review board–approved protocol and cryopreserved. For expansions and subsequent assays, T cells were thawed and cultured in RPMI medium (10% fetal bovine serum and 1% penicillin-streptomycin), complemented with 20 IU/mL recombinant human interleukin-2 (rhIL-2) at a cell concentration of 0.5-2 × 106/mL. T cells were activated with anti-CD3/CD28 Dynabeads (Life Technologies) at a 1:3 ratio and transduced with lentiviral vector 24 hours later (multiplicity of infection, 5-10). In addition, donor-matched activated, but untransduced T cells (UTDs) were expanded to serve as a negative control in subsequent assays. At days 10 to 14 of culture, CAR expression was measured and normalized by adding UTDs before cryopreservation. For in vitro and in vivo experiments, CARTs were used immediately after thawing.
In vivo studies
All animal experiments were conducted under an Institutional Animal Care and Use Committee–approved protocol. For xenograft studies, NOD/SCID/γ-chain−/− mice (NSG; Jackson Laboratories) were injected IV with 1 × 106 MM.1S or MM.1S BCMA-KO cells transduced to express CBG-luc. After confirmation of tumor engraftment by bioluminescent imaging (BLI) 14 days later, 2 × 106 CARTs or UTDs (normalized to the same number of total T cells) were injected via tail vein. Tumor burden was monitored by BLI and peripheral blood was collected and examined for CART persistence by flow cytometry. BLI was performed on an Ami spectral imaging apparatus and analyzed using IDL software, version 4.3.1. Mice were euthanized as specified in the experimental protocol, either when finishing the experiment or when meeting Institutional Animal Care and Use Committee predefined end points.
Further methods are available in supplemental Data.
Results
BCMA and TACI are expressed on MM patient plasma cells
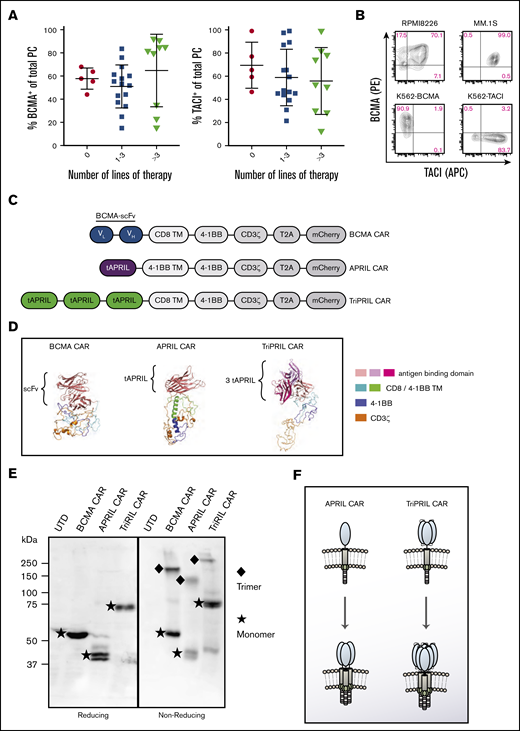
We assessed the surface expression of BCMA and TACI on plasma cells obtained from fresh bone marrow aspirates of 29 MM patients (gating strategy; supplemental Figure 1). Because of the limited number of plasma cells and the restrictions of our clinically validated assay, we could not simultaneously stain for BCMA and TACI. We found that most plasma cells were positive for BCMA and TACI with similar distribution patterns. In the most heavily pretreated patients (>3 previous lines of therapy), BCMA expression tended to separate into either highly positive (≥80%) or slightly positive (20%-30%), whereas TACI expression did not change as much during the course of treatment (Figure 1A). Of note, prior BCMA-directed treatment did not account for the lower BCMA expression in more heavily treated patients, as only 1 patient in this cohort had previously received BCMA-directed therapy and did not have low BCMA expression. The frequent expression of both markers, detected by us as well as others,17,18,36 led us to pursue simultaneous targeting of BCMA and TACI. Mirroring the expression patterns of BCMA and TACI in myeloma patients, the MM cell lines RPMI8226 and MM.1S both express BCMA and TACI on their surface. In addition, we generated K562 artificial antigen-presenting cells expressing either BCMA (K562-BCMA) or TACI (K562-TACI; Figure 1B) to facilitate testing of novel CAR designs that target these antigens individually.
BCMA and TACI expression in MM and design of APRIL-based CARs. (A) Bone marrow aspirates obtained from patients with MM were stained and gated for plasma cell markers and analyzed for expression of BCMA and TACI. The percent of BCMA+ and TACI+ cells per total plasma cells is depicted (median ± SD). Results are grouped according to the number of lines of therapy the patients had received (n = 29). (B) Level of expression of BCMA and TACI on human MM cell lines, RPMI8226 and MM.1S, and K562 cells transduced to express either BCMA or TACI. (C) Construct designs for BCMA, APRIL, and TriPRIL CARs. (D) Predicted tertiary structure of BCMA CAR, APRIL CAR, and TriPRIL CAR modeled using the Phyre2 platform and visualized in PyMOL. (E) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis performed on whole-cell lysates from Jurkat-CD3ζ KO cells expressing the indicated CARs under reducing and nonreducing conditions. For detection of the CARs western blots were probed with an anti-CD3ζ antibody. Under nonreducing conditions, CAR bands were detected at the estimated monomer and trimer sizes. (F) Schematic drawing illustrating the multimerization pattern of APRIL and TriPRIL CARs. t, truncated; TM, transmembrane domain.
BCMA and TACI expression in MM and design of APRIL-based CARs. (A) Bone marrow aspirates obtained from patients with MM were stained and gated for plasma cell markers and analyzed for expression of BCMA and TACI. The percent of BCMA+ and TACI+ cells per total plasma cells is depicted (median ± SD). Results are grouped according to the number of lines of therapy the patients had received (n = 29). (B) Level of expression of BCMA and TACI on human MM cell lines, RPMI8226 and MM.1S, and K562 cells transduced to express either BCMA or TACI. (C) Construct designs for BCMA, APRIL, and TriPRIL CARs. (D) Predicted tertiary structure of BCMA CAR, APRIL CAR, and TriPRIL CAR modeled using the Phyre2 platform and visualized in PyMOL. (E) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis performed on whole-cell lysates from Jurkat-CD3ζ KO cells expressing the indicated CARs under reducing and nonreducing conditions. For detection of the CARs western blots were probed with an anti-CD3ζ antibody. Under nonreducing conditions, CAR bands were detected at the estimated monomer and trimer sizes. (F) Schematic drawing illustrating the multimerization pattern of APRIL and TriPRIL CARs. t, truncated; TM, transmembrane domain.
Design of APRIL-based CARs
To design CARs that target both BCMA and TACI, we leveraged their natural ligand APRIL to serve as the antigen-binding domain.38-40 To avoid potential off-target interactions, a truncated version of APRIL, lacking the furin protease cleavage and the proteoglycan-binding sites, was employed.41 APRIL is a TNF family member, and BCMA and TACI are TNFR family members. Since TNF-TNFR interactions occur with trimeric forms of the molecules,42,43 we hypothesized that binding and signaling of APRIL-based CARs would be enhanced by facilitating trimerization. We took 2 engineering approaches to achieve this objective. First, to induce trimerization of the entire CAR, we substituted the homodimerizing domains of the CD8 hinge and transmembrane domain44,45 with the extracellular and transmembrane domain of 4-1BB, which is a trimerizing TNFR superfamily member. Because the characteristics of a 4-1BB transmembrane domain are lesser known, we initially designed 2 APRIL monomer CARs that were identical, except for the transmembrane domain, which was either CD8 or 4-1BB (APRIL-CD8-TM CAR and APRIL-4-1BB-TM CAR; supplemental Figure 2A). The APRIL-4-1BB-TM CAR showed equal or superior effector function against a panel of BCMA and/or TACI+ target cells in comparison with the APRIL-CD8-TM CAR (supplemental Figure 2B) and was chosen for the remainder of this study. Truncated APRIL (tAPRIL) was synthesized as a monomer fused to a 4-1BB transmembrane and intracellular domain in tandem with the CD3ζ intracellular domain (APRIL CAR). Second, to induce multimerization of the APRIL binding domain, 3 tAPRIL monomers connected by linkers (TriPRIL CAR) were synthesized and fused to a CD8 transmembrane domain, a 4-1BB intracellular domain, and a CD3ζ intracellular domain in tandem. In parallel, a scFv-based anti-BCMA CAR (BCMA CAR) with a CD8 transmembrane domain and 4-1BB and CD3ζ intracellular domains, reflecting the constructs that are currently in advanced clinical trials, was synthesized to be used as a control (Figure 1C). We modeled the predicted tertiary structure of the CAR constructs using Phyre2 (Figure 1D).46 In addition, to study multimerization patterns, all 3 CAR constructs were expressed in Jurkat cells that lacked expression of the endogenous CD3ζ chain. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed under reducing and nonreducing conditions and the western blots were probed with an anti-CD3ζ antibody. Reducing conditions confirmed expression of the CARs at the expected sizes. The APRIL CAR was present in 2 different forms, likely corresponding to differential glycosylation. Nonreducing conditions demonstrated that all 3 CARs (BCMA, APRIL, and TriPRIL), including those with the CD8 transmembrane domain, spontaneously formed trimers (Figure 1E-F).
TriPRIL CARTs show enhanced binding and effector function compared with APRIL CARTs
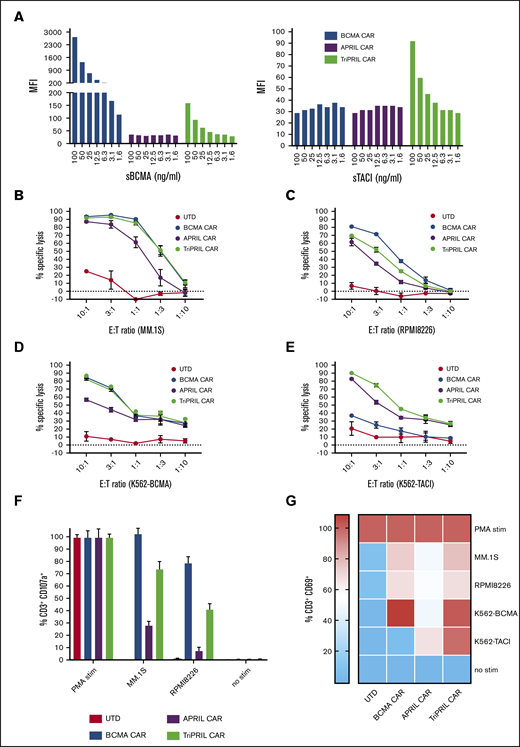
We proceeded to characterize the binding and functional properties of these CARs when transduced into primary human T cells (CARTs). First, we tested the binding affinity of our CARs to antigen by measuring the mean fluorescence intensity of CARTs incubated with labeled soluble BCMA (sBCMA) and TACI (sTACI) over a range of concentrations. We found that BCMA CAR bound strongly to sBCMA but poorly to sTACI, consistent with the described binding characteristics of the antibody from which the scFv was derived.17 The APRIL CAR showed low binding capacity to both sBCMA and sTACI, whereas the TriPRIL CAR bound to both antigens, albeit to a lower extent to sBCMA than the BCMA CAR (Figure 2A). Next, we evaluated the cytotoxic potential of the CARTs against a panel of BCMA+ and/or TACI+ target cells. MM.1S (Figure 2B) and RPMI8226 (Figure 2C) MM cell lines were lysed at high efficiency by BCMA and TriPRIL CARTs, whereas specific lysis mediated by APRIL CARTs was lower. For target cells expressing BCMA only, a similar pattern was observed: efficient lysis by BCMA and TriPRIL CARTs, with weaker lysis by APRIL CARTs (Figure 2D). In contrast, solely APRIL and TriPRIL CARTs were able to lyse TACI only expressing target cells, though APRIL-mediated lysis was still weaker than TriPRIL-mediated lysis (Figure 2E).
Binding affinity and activation of MM CARTs in response to BCMA and TACI target antigens. (A) CARTs were incubated with fluorophore-conjugated sBCMA and sTACI for 45 minutes at 4°C. Plots indicate mean fluorescence intensity of mCherry-gated CARTs bound to BCMA or TACI at the indicated concentrations. (B-E) Luciferase-based cytotoxicity assays of CARTs against a panel of targets cocultured at various E:T ratios, as indicated on the x-axis. Luciferase activity of targets was measured after 8 hours (MM.1S, RPMI8226) or 16 hours (K562). Data points indicate the mean ± standard error of the mean (SEM) of triplicates from a representative of 3 normal donors. Degranulation (F) and activation (G) of UTDs and BCMA, APRIL, and TriPRIL CARTs stimulated with BCMA- and/or TACI-expressing target cells at a 1:1 E:T ratio. Percentage of CD3+ or CD3+mCherry+ cells, respectively, that express CD107a and CD69, accordingly, was measured by flow cytometry and is displayed relative to the positive control (phorbol 12-myristate 13-acetate/ionomycin). Bars and heat map show means ± SEM of 3 normal donors.
Binding affinity and activation of MM CARTs in response to BCMA and TACI target antigens. (A) CARTs were incubated with fluorophore-conjugated sBCMA and sTACI for 45 minutes at 4°C. Plots indicate mean fluorescence intensity of mCherry-gated CARTs bound to BCMA or TACI at the indicated concentrations. (B-E) Luciferase-based cytotoxicity assays of CARTs against a panel of targets cocultured at various E:T ratios, as indicated on the x-axis. Luciferase activity of targets was measured after 8 hours (MM.1S, RPMI8226) or 16 hours (K562). Data points indicate the mean ± standard error of the mean (SEM) of triplicates from a representative of 3 normal donors. Degranulation (F) and activation (G) of UTDs and BCMA, APRIL, and TriPRIL CARTs stimulated with BCMA- and/or TACI-expressing target cells at a 1:1 E:T ratio. Percentage of CD3+ or CD3+mCherry+ cells, respectively, that express CD107a and CD69, accordingly, was measured by flow cytometry and is displayed relative to the positive control (phorbol 12-myristate 13-acetate/ionomycin). Bars and heat map show means ± SEM of 3 normal donors.
Additional measures of antigen-mediated T-cell activation include degranulation that results in surface expression of CD107a, and upregulation of the surface marker CD69. We noted robust degranulation of BCMA and TriPRIL CARTs when cocultured with MM target cells, whereas the APRIL CARTs degranulated only weakly (Figure 2F). Similarly, CD69 expression was evident on BCMA CARTs after coculture with BCMA+ target cells, whereas target cells expressing only TACI did not result in CD69 expression. APRIL CARTs upregulated CD69 most in response to TACI (K562-TACI), but only weakly in response to MM cell lines or BCMA (K562-BCMA). In contrast, TriPRIL CARTs activated robustly in response to cells expressing either BCMA, TACI, or both (Figure 2G). The degree of both CD107a and CD69 expression as a measure of T-cell activation correlated with target antigen expression (K562-transduced cells > MM.1S > RPMI8226), consistent with published data for CARs targeting other antigens.47-50
Long-term proliferation and polyfunctionality of BCMA, APRIL, and TriPRIL CARTs
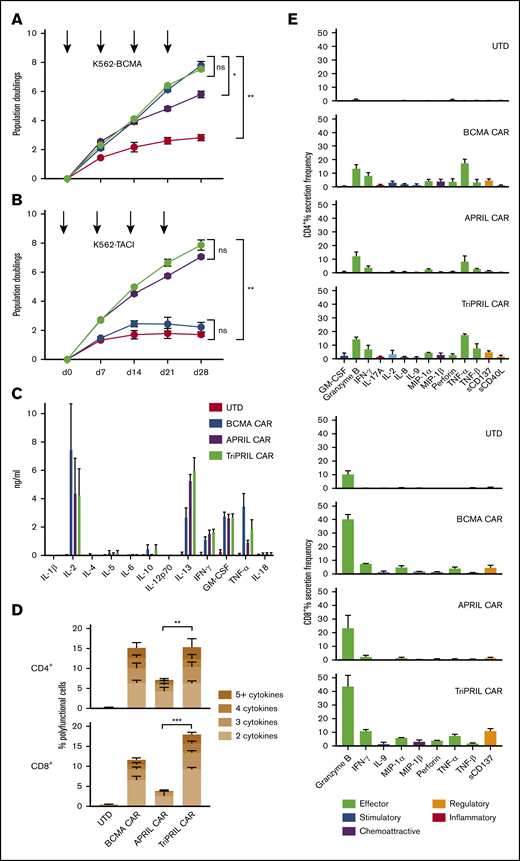
In clinical trials, CART persistence, proliferation, and polyfunctionality have each been correlated with clinical outcomes.8,10,51,52 We performed long-term growth cultures to test the proliferative capacity of our CARTs in response to stimulation with HLA− artificial antigen-presenting cells expressing either BCMA or TACI. Weekly stimulation with K562-BCMA resulted in logarithmic growth of BCMA and TriPRIL CARTs over 4 weeks; APRIL CARTs grew logarithmically after 2 stimulations, but then tapered (P < .05; Figure 3A). In contrast, repeated K562-TACI stimulation induced logarithmic growth only of APRIL and TriPRIL CARTs, with no significant difference between them (P ≥ .05; Figure 3B). BCMA CARTs did not expand more than the control UTDs when stimulated with K562-TACI (P ≥ .05; Figure 3B). Thus, responsiveness to BCMA stimulation constituted the main difference between APRIL and TriPRIL CART function, whereas responsiveness to TACI was the discriminating factor between BCMA and TriPRIL CARTs.
Long-term proliferation and cytokine production of MM CARTs. (A-B) Growth curves of thawed UTDs and BCMA, APRIL, and TriPRIL CARTs in vitro during repeated antigen stimulation (arrows) with irradiated K562 cells expressing either BCMA (A) or TACI (B). Results are displayed as mean population doublings ± SEM of 3 normal donors. (C) Levels of cytokines in supernatants of UTDs, and BCMA, APRIL, and TriPRIL CARTs after overnight coculture with human MM.1S myeloma cells at a 1:1 E:T ratio. Cytokines were measured by 12-plex Luminex assay in technical duplicates. Bars show the mean ± SEM of 3 normal donors. (D-E) Single-cell cytokine profiles of UTDs and BCMA, APRIL, and TriPRIL CARTs stimulated with BCMA- and TACI-expressing target cells captured by 32-plex antibody barcoded chip. Percentage of polyfunctional (≥2 cytokines) CD4+ and CD8+ T cells (D) and protein secretion of CD4+ and CD8+ T cells (E) across functional groups (effector, stimulatory, chemoattractive, regulatory, and inflammatory, as indicated). Bars show the mean ± SEM of 3 normal donors. ns, P ≥ .05; *P < .05 **P < .01; ***P < .001 by 2-way analysis of variance (ANOVA).
Long-term proliferation and cytokine production of MM CARTs. (A-B) Growth curves of thawed UTDs and BCMA, APRIL, and TriPRIL CARTs in vitro during repeated antigen stimulation (arrows) with irradiated K562 cells expressing either BCMA (A) or TACI (B). Results are displayed as mean population doublings ± SEM of 3 normal donors. (C) Levels of cytokines in supernatants of UTDs, and BCMA, APRIL, and TriPRIL CARTs after overnight coculture with human MM.1S myeloma cells at a 1:1 E:T ratio. Cytokines were measured by 12-plex Luminex assay in technical duplicates. Bars show the mean ± SEM of 3 normal donors. (D-E) Single-cell cytokine profiles of UTDs and BCMA, APRIL, and TriPRIL CARTs stimulated with BCMA- and TACI-expressing target cells captured by 32-plex antibody barcoded chip. Percentage of polyfunctional (≥2 cytokines) CD4+ and CD8+ T cells (D) and protein secretion of CD4+ and CD8+ T cells (E) across functional groups (effector, stimulatory, chemoattractive, regulatory, and inflammatory, as indicated). Bars show the mean ± SEM of 3 normal donors. ns, P ≥ .05; *P < .05 **P < .01; ***P < .001 by 2-way analysis of variance (ANOVA).
Analysis of supernatants from coculture of CARTs with MM.1S targets demonstrated antigen-specific production of Th1-type cytokines like IL-2, interferon γ, granulocyte-macrophage colony-stimulating factor, and TNF-α (Figure 3C), in line with the cytokine profile of other CART designs bearing 4-1BB costimulation.53,54 More recently, polyfunctional cytokine production at the single-cell level has emerged as a correlative function of CART products that successfully induce clinical responses in lymphoma patients.52 We measured the percentage of polyfunctional (≥2 cytokines) CD4+ and CD8+ T cells in each of CAR designs upon BCMA and TACI stimulation, using a 32-plex single-cell cytokine assay. The CD4+ subset of BCMA and TriPRIL CARTs both consisted of 10% to 24% of polyfunctional cells, whereas the percentage polyfunctional APRIL CARTs was significantly lower (5%-10%; P < .01). Differences in the CD8+ subset were more pronounced: the TriPRIL CART product contained significantly more polyfunctional cells than the BCMA and the APRIL CART products (16%-21% vs 8%-15% and 0%-6%; P < .001; Figure 3D). Single-cell analysis demonstrated that both CD4+ and CD8+ T cells predominantly secreted effector-type cytokines, with similar profiles but distinct frequencies among the CAR constructs (Figure 3E). In addition, we calculated the polyfunctional strength index (PSI) as a measure of the combined potency of polyfunctional T cells for each CART product.55,56 TriPRIL CARTs had the highest PSI, whereas APRIL CARTs showed the lowest PSI increase among the 3 CAR constructs, in both CD4+ and CD8+ CARTs, compared with UTDs (supplemental Figure 3A). Representation of the data set as polyfunctional heat maps further revealed polyfunctional cell subsets with distinct protein combinations (supplemental Figure 3B).
TriPRIL CARTs clear MM tumors in a xenograft mouse model
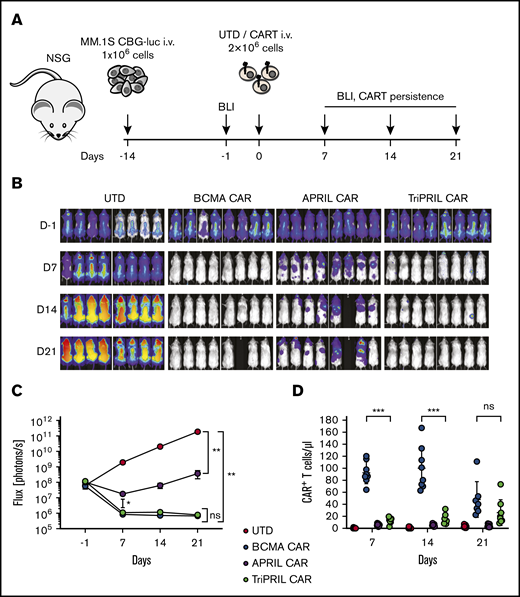
Next, we tested the antitumor efficiency of our CARTs in a xenograft mouse model of MM. NSG mice were IV injected with luciferized MM.1S myeloma cells, and engraftment was confirmed 1 day before T-cell injection by BLI. On day 0, mice were injected with a single IV dose of normalized BCMA, APRIL, or TriPRIL CARTs or UTDs. Tumor burden was monitored weekly by BLI, and CART persistence was measured in peripheral blood weekly by flow cytometry (Figure 4A). Although tumor burden continuously progressed in the UTD-treated group, all CART-treated mice showed antitumor responses. In this high-tumor-burden model, BCMA and TriPRIL CARTs were able to eradicate the tumors, whereas APRIL CARTs only led to a stabilization of tumor burden (Figure 4B). Treatment response in all 3 groups receiving CARTs was statistically significant in relation to the UTD control group (P < .01). There was no significant difference between BCMA- and TriPRIL CART-treated animals (P ≥ .05) in terms of tumor response (Figure 4C). In the peripheral blood, BCMA CARTs showed a rapid increase and then contraction at day 14 after CART administration. In contrast, the TriPRIL CARTs underwent slower expansion kinetics with the cell number still increasing on day 21 after CART administration. UTD and APRIL CARTs did not show measurable expansion in the blood at the analyzed time points (Figure 4D). Together, these data indicate that our APRIL CAR was not optimally functional against MM cells bearing BCMA or TACI. Further studies were focused on comparing TriPRIL CARTs to BCMA CARTs and, in particular, their responsiveness to MM with loss of BCMA.
TriPRIL CARTs specifically eradicate MM cells in vivo. Antitumor efficiency of UTDs and BCMA, APRIL, and TriPRIL CARTs was assessed in a xenograft model of MM. (A) Experimental design: NSG mice were injected with 1 × 106 MM.1S myeloma cells and tumor burden was monitored by BLI over time. After tumor engraftment and randomization, the mice were treated with a single dose of UTDs or BCMA, APRIL, or TriPRIL CARTs from the same donors normalized to the same number of total T cells. (B) Representative BLI of myeloma xenografts over time. (C) Quantification of flux (photons per second) in the 4 experimental groups at the indicated time points. (D) Persistence of CARTs (CD3+mCherry+) measured in the peripheral blood by flow cytometry. Data points indicate the means ± SEM of 2 normal donors with 4 mice per each group. **P < .01 by 2-way ANOVA.
TriPRIL CARTs specifically eradicate MM cells in vivo. Antitumor efficiency of UTDs and BCMA, APRIL, and TriPRIL CARTs was assessed in a xenograft model of MM. (A) Experimental design: NSG mice were injected with 1 × 106 MM.1S myeloma cells and tumor burden was monitored by BLI over time. After tumor engraftment and randomization, the mice were treated with a single dose of UTDs or BCMA, APRIL, or TriPRIL CARTs from the same donors normalized to the same number of total T cells. (B) Representative BLI of myeloma xenografts over time. (C) Quantification of flux (photons per second) in the 4 experimental groups at the indicated time points. (D) Persistence of CARTs (CD3+mCherry+) measured in the peripheral blood by flow cytometry. Data points indicate the means ± SEM of 2 normal donors with 4 mice per each group. **P < .01 by 2-way ANOVA.
Generation and characterization of BCMA− myeloma cells
To model heterogenous antigen expression and antigen escape in MM, we used CRISPR-Cas9 gene editing to knock out BCMA from MM.1S myeloma cells. We confirmed lack of BCMA expression in knockout (KO) cell lines made with different guide RNAs and observed that the level of TACI expression was not affected, indicating independent expression of these 2 molecules (supplemental Figure 4A). Parental and BCMA-KO MM.1S cells showed identical growth kinetics in vitro (supplemental Figure 4B). The in vitro cytotoxicity of TriPRIL CARTs against BCMA-KO MM.1S was undiminished when compared with the parental MM.1S cells (P ≥ .05), whereas the in vitro potency of BCMA CARTs was significantly reduced (P < .001; supplemental Figure 4C).
TriPRIL CARTs eradicate BCMA− MM cells in vivo
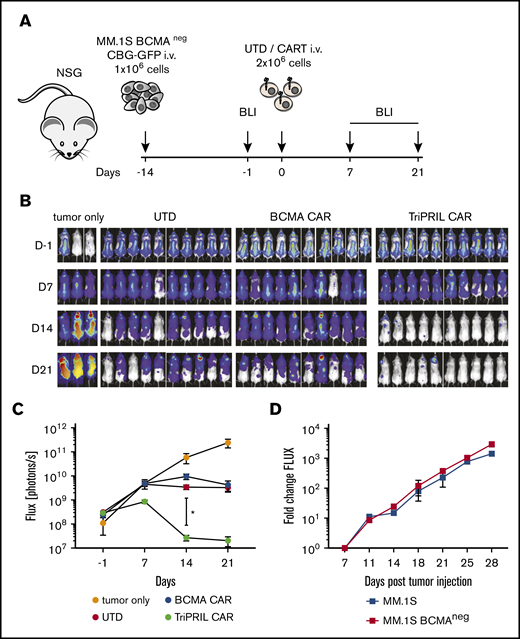
We proceeded to test the in vivo efficacy of TriPRIL CARTs in our newly established BCMA− MM model. NSG mice were injected with BCMA-KO luciferized MM.1S cells and, after confirmation of tumor engraftment, on day 0 mice were injected with a single IV dose of normalized BCMA CARTs, TriPRIL CARTs, or UTD cells (Figure 5A). In addition, a group of mice was left untreated to assess tumorigenicity in the absence of BCMA and to control for allogeneic rejection, which occurs frequently with MM models and limits the evaluable duration of in vivo experiments. By day 21, only the TriPRIL CART–treated mice had cleared the tumors (Figure 5B), consistent with the antigen-specific–mediated responses induced by CARTs. Quantification of tumor burden on day 14 showed a statistically significant difference between groups receiving TriPRIL CARTs and those receiving BCMA CARTs or UTD control cells (P < .05; Figure 5C). At later time points, all mice that had received T cells showed tumor regression, whereas disease burden in the tumor-only group continuously progressed, indicating a non–antigen-specific allogeneic reaction against the tumor through the endogenous T-cell receptor. We repeatedly observed allogeneic responses of T cells against MM.1S BCMA-KO cells, but not against parental MM.1S cells. This discrepancy could not be accounted for by differences in the tumorigenicity or growth kinetics of the MM1.S BCMA-KO vs MM1.S parental cells in NSG mice (Figure 5D). We speculate that allogeneic responses were caused by preexisting T-cell reactivity toward processed and presented SpCas9 protein that the MM1.S BCMA-KO cells harbored, as has recently been reported to occur with T cells from most people.57,58
TriPRIL CARTs specifically eradicate BCMA−MM cells in vivo. Antitumor activity of UTD, BCMA, and TriPRIL CARTs in a xenograft model of BCMA− MM. (A) Experimental design indicating tumor cells, timeline, doses, and imaging time points. Mice were randomized after tumor engraftment and before injection with a single dose of T cells from the same donors that had been normalized to the same number of total T cells. (B) Representative BLI of myeloma xenografts over time. (C) Quantification of flux (photons per second) in the 4 groups at the indicated time points. *P < .05 by 2-way ANOVA at day 14, before the onset of allogeneic responses. Data points show means ± SEM of 2 normal donors with 5 mice per each group. (D) In vivo comparison of tumor growth kinetics of parental MM.1S and MM.1S BCMA KO cells. NSG mice were injected with 1 × 106 tumor cells, and tumor burden was monitored by weekly bioluminescence imaging. Data points indicate means ± SEM of flux fold change compared with baseline (day 7); n = 5 mice per group.
TriPRIL CARTs specifically eradicate BCMA−MM cells in vivo. Antitumor activity of UTD, BCMA, and TriPRIL CARTs in a xenograft model of BCMA− MM. (A) Experimental design indicating tumor cells, timeline, doses, and imaging time points. Mice were randomized after tumor engraftment and before injection with a single dose of T cells from the same donors that had been normalized to the same number of total T cells. (B) Representative BLI of myeloma xenografts over time. (C) Quantification of flux (photons per second) in the 4 groups at the indicated time points. *P < .05 by 2-way ANOVA at day 14, before the onset of allogeneic responses. Data points show means ± SEM of 2 normal donors with 5 mice per each group. (D) In vivo comparison of tumor growth kinetics of parental MM.1S and MM.1S BCMA KO cells. NSG mice were injected with 1 × 106 tumor cells, and tumor burden was monitored by weekly bioluminescence imaging. Data points indicate means ± SEM of flux fold change compared with baseline (day 7); n = 5 mice per group.
TriPRIL CART functions in the presence of soluble competitive ligands and against primary myeloma cells
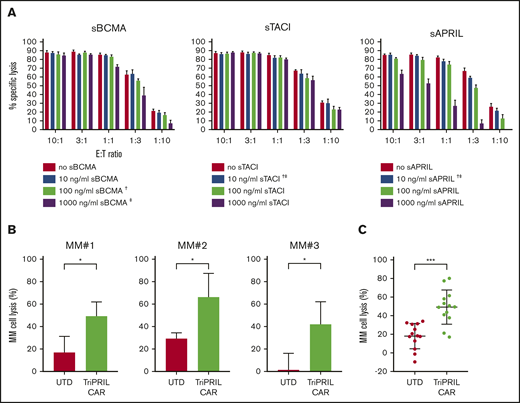
Supraphysiologic concentrations of sBCMA, sTACI, and sAPRIL have been reported in the bone marrow and peripheral blood of MM patients.36,59 We investigated whether sBCMA, sTACI, and sAPRIL block TriPRIL CART lysis against MM cells that express membrane-bound BCMA and TACI. To this end, TriPRIL CARTs were cocultured with MM.1S target cells at different effector-to-target (E:T) ratios over a range of concentrations of sBCMA, sTACI, and sAPRIL. At the highest tested concentrations (1000 ng/mL) of sBCMA and sAPRIL, reduced cytotoxicity of TriPRIL CARTs was observed only at the lowest E:T ratios (1:3 and 1:10; Figure 6A). Interestingly, high concentrations of TACI did not reduce cytotoxicity of TriPRIL CARTs. Importantly, the concentrations of sTACI and sAPRIL that we tested far exceeded the levels reported in MM patients. However, sBCMA concentrations of up to 2000 ng/mL have been reported in the blood of patients with advanced MM.59
Translating TriPRIL CARTs into clinical application. (A) Luciferase-based cytotoxicity assays of TriPRIL CARTs against MM.1S myeloma in the presence of sBCMA, sTACI, and sAPRIL. Cells were cocultured at various E:T ratios, as indicated on the x-axis. The concentrations of sBCMA, sTACI, and sAPRIL added to the assay are denoted in the graph legend. Luciferase activity of targets was measured after 8 hours. Bars indicate means ± SEM of triplicates from 1 normal donor; †closest to median concentration reported in BM of MM patients; ‡closest to median concentration reported in PB of MM patients. (B-C) Flow cytometry-based cytotoxicity assay of TriPRIL CARTs or UTDs from the same donors against primary myeloma cells from r/r MM patients. T cells and MM bone marrow mononuclear cells (BMMCs) were cocultured at a 1:1 ratio for 24 hours. (B) Bar graphs depict mean percentage of MM cell lysis ± SD of 3 representative MM patient cells cocultured each with T cells from 3 allogeneic normal donors. (C) Scatter plot indicating mean percentage of MM cell lysis ± SD of combined data set of 7 MM patients and 4 normal T-cell donors. *P < .05; ***P < .001, by 2-tailed Student t test.
Translating TriPRIL CARTs into clinical application. (A) Luciferase-based cytotoxicity assays of TriPRIL CARTs against MM.1S myeloma in the presence of sBCMA, sTACI, and sAPRIL. Cells were cocultured at various E:T ratios, as indicated on the x-axis. The concentrations of sBCMA, sTACI, and sAPRIL added to the assay are denoted in the graph legend. Luciferase activity of targets was measured after 8 hours. Bars indicate means ± SEM of triplicates from 1 normal donor; †closest to median concentration reported in BM of MM patients; ‡closest to median concentration reported in PB of MM patients. (B-C) Flow cytometry-based cytotoxicity assay of TriPRIL CARTs or UTDs from the same donors against primary myeloma cells from r/r MM patients. T cells and MM bone marrow mononuclear cells (BMMCs) were cocultured at a 1:1 ratio for 24 hours. (B) Bar graphs depict mean percentage of MM cell lysis ± SD of 3 representative MM patient cells cocultured each with T cells from 3 allogeneic normal donors. (C) Scatter plot indicating mean percentage of MM cell lysis ± SD of combined data set of 7 MM patients and 4 normal T-cell donors. *P < .05; ***P < .001, by 2-tailed Student t test.
To confirm the function of TriPRIL CARTs in the context of primary MM tumor cells, we performed flow cytometry–based cytotoxicity assays of TriPRIL CARTs against fresh myeloma cells recovered from bone marrow aspirates of 7 patients with r/r MM. Cocultures of bone marrow mononuclear cells with allogeneic TriPRIL CARTs or UTDs were set up at a 1:1 ratio. The number of viable MM cells was determined initially and after 24 hours of coculture by gating on CD138+ cells, and the percentage of MM cell lysis was calculated. TriPRIL CARTs induced significantly higher lysis of MM than did the UTD control cells (P < .05), as shown in cells from 3 representative r/r MM patients that were cocultured with 3 T-cell donors each (Figure 6B). Pooled analysis of the data from 7 r/r MM patients and 4 T-cell donors demonstrated that TriPRIL CARTs lysed primary myeloma cells at a significantly higher rate than the UTDs (P < .001; Figure 6C). Thus, TriPRIL CARTs are functional against MM cell lines in vitro and in vivo and against primary MM patient tumor cells.
Discussion
CARTs are emerging as a novel and potentially curative therapeutic approach for hematological malignancies. However, relapse rates as high as 45% have been observed for CD19-targeted CART therapy in patients with r/r B-cell acute lymphoblastic leukemia.8,60-63 Because of limited follow-up, the incidence of disease relapse in MM patients receiving anti-BCMA CARTs is currently unknown, but in 1 trial, median progression-free survival was 11.8 months.25 Furthermore, reports of disease resistance related to BCMA loss under the selective pressure of anti-BCMA CART treatment are emerging.24,26 Anticipating the development of antigen escape variants, in this study, we engineered a multimeric APRIL-based CAR to target MM and plasma cells. We demonstrate that preserving APRIL’s natural trimeric conformation may be superior to previously described approaches that employed APRIL in a nonnative conformation. Furthermore, TriPRIL CAR compared favorably to a monospecific anti-BCMA CAR with retained function against BCMA− TACI+ MM cells, making it a promising strategy to treat MM.
An APRIL-based antigen-binding domain allows for dual targeting of 2 related myeloma-associated antigens: BCMA and TACI. Although the concept of using APRIL as an extracellular moiety to target BCMA and TACI was initially published by Lee et al,36 we note that their third-generation monomeric APRIL-based CARs were not directly compared with anti-BCMA CARs, required high T-cell doses to achieve disease responses, had a short follow-up duration (11 days), and reported no models of BCMA− myeloma. In contrast, we developed a lead candidate that constitutes a second-generation CAR with a rationally designed trimeric APRIL format as the antigen-binding domain. Furthermore, we demonstrate for the first time that myeloma cells retain TACI expression in the absence of BCMA expression. Given that BCMA and TACI are closely related members of the same TNFR superfamily, these findings are not obvious and provide further rationale for pursuing combinatorial antigen recognition approaches that target 2 molecules with redundant functionality. We found that TriPRIL CARTs were sufficient to eliminate myeloma cells in vivo, despite the absence of BCMA expression.
As reported by us and others,18,36 most MM patient samples express TACI. In a small patient cohort, Novak and colleagues18 reported BCMA and TACI expression on 3 of 3 freshly isolated MM samples. In a larger patient cohort, Lee and colleagues36 reported BCMA and TACI expression on 50 of 50 and 39 of 50 primary bone marrow–derived MM cells, respectively. Our study found that 27 of 29 and 28 of 29 of MM patients had BCMA and TACI expression levels of ≥20% of total plasma cells, respectively. Taken together, these data sets suggest that both BCMA and TACI are expressed on most MM samples. In addition, TACI expression has been reported on presumable myeloma stem cell populations, which makes it a promising target.64,65 Thus, TriPRIL CARTs may be suited for both preventing and treating BCMA escape variants. In addition, combinatorial antigen targeting increases the density of targetable molecules on tumor cells, which has been shown to enhance CART functionality.48,66 Furthermore, efficient dual targeting of BCMA and TACI may improve efficiency against myeloma that has low expression of BCMA. Importantly, some clinical trials investigating BCMA-directed CART treatment of MM excluded patients whose plasma cells had a BCMA expression level <50%, as measured by immunohistochemistry in early cohorts.67,68 Because TriPRIL CAR does not rely solely on BCMA expression, it may broaden eligibility for adoptive cell therapy of MM.
To date, most CARs, especially the ones in clinical application, employ antibody-derived scFvs for antigen recognition. However, there is growing interest in the use of natural ligands as a CAR-binding domain with several successful reports.36,69-73 Because they originate from fully human sequences, natural ligand binders may be less likely to prompt immune responses and trigger rejection of CARTs than murine scFv sequences, which may be one of the other reasons for the limited duration of response in reported trials.74,75 In turn, the use of natural ligands as CAR-binding domains may also give rise to potential issues. scFv-based CARs can easily be affinity tuned,76 but alternative approaches to binding enhancement may be required. We developed solutions to increase the stability of binder–target interactions between APRIL and BCMA/TACI to trigger CAR-mediated signaling. Specifically, we engineered a trimeric binding configuration of APRIL as the extracellular domain and also took care to eliminate domains that mediate off-target binding and cleavage domains that could result in shedding of APRIL. We observed that employing a trimeric form of the APRIL binding moiety rather than a monomeric form increased recognition of MM antigens in vitro and in vivo. Importantly, compared with the APRIL CAR with one binding domain per CAR, the TriPRIL CAR with 3 binding domains per CAR, resulted in improved long-term proliferative capacity and polyfunctionality of T cells, which have been shown to correlate with favorable patient outcomes.8,10,52 We also observed that better results were obtained by modifying the extracellular binding moiety as opposed to modifying canonical CAR structures, such as the transmembrane domain.
In addition to antigen escape and immune rejection, a third potential mechanism of resistance to CART therapies is blocking of the CAR by soluble versions of the target antigen. Our data, in conjunction with published work by others,36,59 suggests that, of the 3 soluble TNF/TNFR family members examined, sBCMA is the most likely to reduce the efficiency of TriPRIL CARTs. Nevertheless, lymphodepleting conditioning regimens included in most CART protocols could be used to reduce the levels of these soluble proteins to below clinical relevance. Alternatively, elegant work by Pont and colleagues suggests that coadministration with γ-secretase inhibitors may prevent shedding of soluble BCMA.77 Since sBCMA, sTACI, and sAPRIL can affect responsiveness to all BCMA-targeted therapies, standard clinical measurement of these proteins and correlative studies in MM patients would be desirable in future clinical investigations to confirm mechanisms of resistance. Along the same lines, incorporation of BCMA and TACI staining into standard clinical flow cytometry diagnostic and measurable residual disease assessment would help to identify potential eligible patients and mechanisms of therapy resistance, rather than post hoc measurements of BCMA or TACI expression by immunohistochemistry, which has much lower sensitivity.
In summary, TriPRIL, a rationally designed, high-affinity, trimeric APRIL-based CAR using only human sequences, holds promise for the treatment of MM and may prevent or treat antigen escape from targeting a single antigen, while also avoiding antimurine immune rejection responses.
Acknowledgments
The authors thank the Flow Cytometry Laboratory Department of Pathology, Massachusetts General Hospital, for help with the analysis of BCMA and TACI expression on primary MM patient plasma cells.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) under Germany’s Excellence Strategy through EXC294 (BIOSS) and EXC2189 (CIBSS; Project ID 390939984) (W.S.); and by the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie grant agreement GA721358 (W.S.). This work and M.V.M. were supported by the Gabrielle’s Angel Foundation and the V Foundation. A.S. receives support from a German Cancer Aid Post-Doctoral Research Fellowship (57406718; 91709023).
Authorship
Contribution: A.S. and M.V.M. designed the study; A.S., M.O., B.D.C., A.O.T., A.A.B., I.S., R.C.L., M.J.F., K.G., A.P.C., L.S.R., M.L.C., A.C.B., R.M.-H.V.C., W.S., Y.-T.T., K.C.A., and M.V.M. designed and executed individual experiments; J.Z. and S.M. performed single-cell cytokine analysis; A.S. and M.V.M. interpreted data and wrote the manuscript; and all authors read, edited, and approved the manuscript.
Conflict-of-interest disclosure: M.V.M., A.S., and B.D.C. are contributors to patents related to TriPRIL CARTs. M.V.M. has received consulting income or honoraria from Adaptimmune, Agentus, Arcellx, BluebirdBio, CRISPR Therapeutics, Becton Dickinson, KitePharma, Novartis, Takeda, TCR2, Torque, and WindMIL, none of which are related to the constructs described in this manuscript. W.S. received consulting income from TCR2 Therapeutics. J.Z. is employed by and has equity ownership in IsoPlexis. S.M. is cofounder of, has equity ownership in, and holds patents with IsoPlexis. M.J.F. has received consulting income or honoraria from Novartis, Xenetic, Arcellx, Nkarta, Incyte, Kite/Gilead and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Marcela V. Maus, Massachusetts General Hospital, 149 13th St, Room 3.216, Charlestown, MA 02129; e-mail: mvmaus@mgh.harvard.edu.
References
Author notes
The full-text version of this article contains a data supplement.
For original data, please contact the corresponding author.