Key Points
Reduced membrane deformability is not a key factor in sequestration of red blood cells by the human spleen.
Abstract
The current paradigm in the pathogenesis of several hemolytic red blood cell disorders is that reduced cellular deformability is a key determinant of splenic sequestration of affected red cells. Three distinct features regulate cellular deformability: membrane deformability, surface area-to-volume ratio (cell sphericity), and cytoplasmic viscosity. By perfusing normal human spleens ex vivo, we had previously showed that red cells with increased sphericity are rapidly sequestered by the spleen. Here, we assessed the retention kinetics of red cells with decreased membrane deformability but without marked shape changes. A controlled decrease in membrane deformability (increased membrane rigidity) was induced by treating normal red cells with increasing concentrations of diamide. Following perfusion, diamide-treated red blood cells (RBCs) were rapidly retained in the spleen with a mean clearance half-time of 5.9 minutes (range, 4.0-13.0). Splenic clearance correlated positively with increased membrane rigidity (r = 0.93; P < .0001). To determine to what extent this increased retention was related to mechanical blockade in the spleen, diamide-treated red cells were filtered through microsphere layers that mimic the mechanical sensing of red cells by the spleen. Diamide-treated red cells were retained in the microsphilters (median, 7.5%; range, 0%-38.6%), although to a lesser extent compared with the spleen (median, 44.1%; range, 7.3%-64.0%; P < .0001). Taken together, these results have implications for understanding the sensitivity of the human spleen to sequester red cells with altered cellular deformability due to various cellular alterations and for explaining clinical heterogeneity of RBC membrane disorders.
Introduction
Although red blood cells (RBCs) undergo repeated, extensive, and reversible deformations in the vascular bed of tissues, squeezing through interendothelial slits (IESs) of the venous sinus of the spleen red pulp is the ultimate test for sensing their altered cellular deformability.1,2 As a consequence, RBCs with reduced cellular deformability are retained in the cords and eventually destroyed by red pulp macrophages.
Three principal parameters determine the ability of intact RBCs to deform: the cell surface area to volume (S/V) ratio, intracellular viscosity, and membrane viscoelasticity.3-5 Several hemolytic RBC disorders are accompanied by the alteration of ≥1 of these parameters, thus contributing to the splenic entrapment of affected RBCs and anemia.6,7 Although decreased S/V ratio has previously been shown to be a major determinant of splenic sequestration,5 the contribution of reduced membrane deformability to splenic entrapment of altered RBCs remains unclear.
Southeast Asian ovalocytosis (SAO) is an inherited disorder that is characterized by a marked decrease in membrane deformability (increased membrane rigidity) of RBCs8,9 that circulate freely in the vasculature bed without significant sequestration by the spleen. This suggests that the SAO RBCs with increased membrane rigidity, in contrast to spherical red cells, are able to cross venous sinus IESs of the splenic red pulp without splenic sequestration. Splenic sequestration of α,β-thalassemic RBCs has been associated with their reduced deformability.10,11 Because this reduced deformability of thalassemic red cells has been linked to altered intracellular viscosity and increased membrane rigidity,3,4 the individual contribution of each parameter on splenic sequestration of these abnormal cells cannot be distinguished.
In this study, we wanted to document the ability of red cells with decreased membrane deformability to traverse the spleen. To this end, we used the ex vivo perfusion of human spleen system.2,12,13 Using this experimental approach, we recently clarified the relationship between the increased cell sphericity and RBC clearance by the spleen.5 Here, we explore the retention of RBCs exposed to increasing concentrations of diamide by the spleen. Diamide is a sulfhydryl oxidant that induces the formation of inter- and intramolecular disulfide bonds, thereby increasing protein–protein associations14 and leading to increased membrane rigidity.14 We confirmed that diamide reduces membrane deformability without affecting cell shape or intracellular viscosity. We studied the clearance of diamide-exposed RBCs (dRBCs) by the perfused human spleen and analyzed the correlation between increased membrane rigidity and splenic clearance. Furthermore, we studied the mechanical retention of dRBCs using microsphiltration, in which layers of metal microspheres generate narrow spaces resembling the geometry of splenic IESs.2
Materials and methods
Human spleen retrieval
Spleens were retrieved and processed as previously described.2,12,13 Medical and surgical care was not modified, and patient written consent was obtained. All patients underwent left splenopancreatectomy for pancreas disease (neuroendocrine tumor, proven or suspected adenocarcinoma, cyst, unspecified tumor, or chronic pancreatitis). The spleen was macroscopically and microscopically normal in all cases. After a 30 to 60–minute period of warm ischemia linked to the surgical procedure, the main splenic artery was cannulated once the macroscopic aspect of the spleen had been examined by the pathologist, and a pre-experiment biopsy was performed whenever required. The spleens were flushed with cold Roswell Park Memorial Institute (RPMI) 1640–albumin solution for transport to the laboratory. This study was approved by the Ile-de-France II Investigational Review Board (Paris, France).
RBC labeling with PKH67 and/or PKH26
Concentrated RBCs from the French blood bank (Établissement Français du Sang, Rungis, France) were washed 3 times in RPMI 1640 to remove saline-adenine-glucose-mannitol and white blood cells. RBCs were labeled using lipophilic fluorescent probes PKH67 and/or PKH26 (Sigma Aldrich) (5% hematocrit).5 RBCs were single labeled with PKH (PKH67, dilution 1/1000 or PKH26, dilution 1/250) or doubled labeled using PKH at different concentrations (PKH67, dilution 1/2000 + PKH26, dilution 1/500 or PKH67, dilution 1/1000 + PKH26, dilution 1/3000).
Treatment of RBCs with diamide
PKH-labeled RBCs were resuspended in diamide (Sigma-Aldrich; 10-500 µmol/L) in isotonic phosphate buffer (90 mmol/L KCl, 45 mmol/L NaCl, 44 mmol/L sucrose, 10 mmol/L NaPO4, pH 8.0) at 1% hematocrit level. Following incubation for 1 hour at 37°C, samples were washed 3 times with phosphate-buffered saline (PBS) and resuspended in Krebs-albumin for further analysis.
Measurement of RBC deformability
RBC deformability was measured by ektacytometry using a laser-assisted optical rotational cell analyzer (Lorrca; Mechatronics, Hoorn, The Netherlands), as previously described.5,13,15 The unit of RBC deformability (ie., elongation index [EI]), was defined as the ratio between the difference between the 2 axes of the ellipsoid diffraction pattern and the sum of these 2 axes. RBC deformability was assessed over a range of shear stresses (0.3-30 Pa).
Ex vivo spleen perfusion
Isolated-perfused spleen experiments were performed, as previously described,2,5,12,13 in a Plexiglas chamber maintained at 37°C by a regulated warm airflow. Briefly, once in the laboratory (cold ischemia time, 60-90 minutes), the spleen was connected to the perfusion device and a progressive warming from 8°C to 37°C was performed by increasing the Krebs-albumin medium flow from 1 mL/min to 50 to 150 mL/min over 40 to 60 minutes. During this adaptation period, the donor RBCs were flushed from the spleen (hematocrit at the end of the warming period was <0.1%). When the spleen temperature reached 35°C, untreated O+ RBCs were added (final hematocrit: 5%-10%) and allowed to circulate for 30 to 60 minutes. Glucose, Na, and K concentrations, O2 and CO2 partial pressures, and pH were monitored in the vein, artery, and reservoir every 10 to 30 minutes using an i-STAT device (Abbott Laboratories, Abbott Park, IL). In the steady-state, key physiologic markers were maintained in the following ranges: perfusate flow, 0.8 to 1.2 mL/g of perfused spleen parenchyma per minute; temperature of the spleen capsule, 36.7°C to 37.2°C; Na, 135 to 148 mEq/L; K, 4 to 5 mEq/L; glucose, 4 to 12 mM; pH, 7.2 to 7.35; hematocrit, 4% to 10%; and arterial-venous oxygen partial pressure decay 60 to 120 mm Hg. Finally, a mixture of PKH-labeled RBCs, untreated or treated with diamide, was introduced in the circulating system for 2 hours after normal RBCs had been rinsed out for 5 to 10 minutes (hematocrit before the introduction of RBC was <0.2%). The percentage of circulating dRBCs was quantified by flow cytometric analysis (FACSCalibur; BD Biosciences, San Jose, CA). Data were analyzed using CellQuest software (BD Biosciences).
Microsphiltration
Filtration of RBCs on microsphilters was performed as previously described.2 In brief, calibrated metal microspheres (Industrie des Poudres Spheriques, Annemasse, France) with 2 different size distributions (5-15 µm in diameter and 15-25 µm in diameter), each from a single batch, were used. A total of 2 g of dry microspheres of each size range was mixed and suspended in 8 mL of Krebs with 1% AlbuMAX II (Invitrogen). A total of 600 µL of this bead suspension was poured into an inverted 1000-µL antiaerosol pipette tip (Neptune barrier tips) and allowed to settle, leading to the formation of a 5-mm-thick bead layer above the antiaerosol filter. A total of 600 µL of a 2% hematocrit RBC suspension containing <10% of dRBCs or control RBCs was introduced upstream from the microsphere layer. Cells were perfused through the bead layer at a flow rate of 60 mL/h using an electric pump (Syramed µSP6000; Arcomed Ag), and the bead layer was washed with 8 mL of Krebs/1% AlbuMAX II. Lysophosphatidylcholine (LPC)–exposed or heated RBCs were used as a control for the perfusion. PKH-labeled RBCs were resuspended in different concentrations of LPC in PBS at 1% hematocrit level and incubated for 5 minutes at room temperature to induce a controlled loss of membrane surface area. Following incubation, samples were washed 3 times with PBS and resuspended in Krebs-albumin for further analysis. For the heat treatment, RBCs were suspended in RPMI 1640 (1% hematocrit) and heated at 50°C for 15 minutes.
Analysis of RBC morphology and dimensions
Untreated RBCs or dRBCs were fixed with PBS-paraformaldehyde (1%) for analysis. Image acquisition and data analysis were done as previously described.2 Briefly, images were acquired on the ImageStream imaging cytometer (Amnis, Seattle, WA). At least 10 000 images were collected for each sample. Debris was eliminated from the data set by setting a minimum object area in channel 2 and/or 3, and ∼50 mW of laser power was used to excite the probes. Cells were collected with the laser scatter image in channel 1, the PKH26 and PKH67 images in channel 2 (red) and channel 3 (green), respectively, and the bright-field image in channel 5 (white). Postacquisition data analysis was performed using the IDEAS image analysis software package (Amnis). Morphological (compactness, circularity, and aspect and shape ratios) and dimensional (surface area and diameter) features of RBC were calculated, using images of RBCs, by IDEAS software. LPC-exposed RBCs were used as a positive control, because LPC can induce release of microvesicules from normal RBCs by accumulating in the external leaflet of the lipid bilayer of the membrane,16,17 and RBCs became spherical.4,5,16-18
Osmotic fragility test
Osmotic fragility of RBCs was determined as previously described.19 Untreated RBCs or dRBCs were incubated for 30 minutes in a hypotonic solution with NaCl ranging from 0.1% to 0.9% (hematocrit: 1%). After centrifugation, absorbance of the supernatant was measured at 540 nm using a spectrophotometer, and the percentage hemolysis was calculated for each supernatant and plotted against NaCl concentrations. The resulting osmotic fragility curves of dRBCs were then compared with those obtained with normal control RBCs. Because RBCs with reduced membrane surface area and S/V ratio are osmotically fragile,4 LPC-exposed RBCs were used as a positive control.
Statistical analysis
We used the Wilcoxon signed-rank test for statistical analysis. The correlation between different continuous measures was determined using the Spearman correlation coefficient. P < .05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software (version 5.0).
Results
Diamide treatment of RBCs induces a dose-dependent increase in membrane rigidity
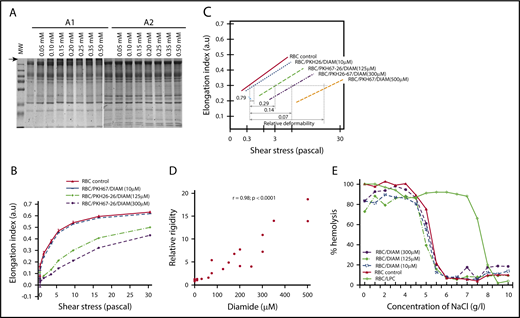
RBCs were exposed to increasing concentrations of diamide (10-500 µM) in isotonic phosphate buffer. Analysis of RBC morphology and shape using imaging flow cytometry (ImageStream apparatus) showed that diamide treatment had little impact, because the distributions of surface area and diameter were similar in diamide-treated and untreated RBCs (Figure 1A-B). However, circularity and sphericity (aspect and shape ratios) were slightly increased, with the presence of 2 distinct subpopulations at high concentration of diamide (500 µM) characterized by the bimodal form of the circularity distribution curves (Figure 1C). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis showed that, as expected,20 the membrane of dRBCs contained high-molecular-weight complexes (Figure 2A1, arrow), which disappeared upon reduction with dithiothreitol prior to electrophoresis (Figure 2A2). Diamide treatment did not alter internal viscosity, because mean corpuscular hemoglobin concentration (MCHC) was similar in exposed and unexposed RBCs. The characteristics of dRBCs are summarized in Table 1.
Dimensional and morphological characteristics of dRBCs. Projected surface area (A), diameter (B), circularity (C), aspect ratio (D), and shape ratio (E) (sphericity) of untreated RBCs and RBCs treated with increasing concentrations of diamide (10-500 µM) and analyzed with an ImageStream imaging cytometer. RBCs treated with LPC to induce a control loss of cell surface area5 were used as a positive control for imaging. Unlike LPC treatment, exposure to diamide had no effect on RBC dimensions (surface area and diameter [A-B]) and only a slight effect on cell morphology (circularity [C] and sphericity [D-E]).
Dimensional and morphological characteristics of dRBCs. Projected surface area (A), diameter (B), circularity (C), aspect ratio (D), and shape ratio (E) (sphericity) of untreated RBCs and RBCs treated with increasing concentrations of diamide (10-500 µM) and analyzed with an ImageStream imaging cytometer. RBCs treated with LPC to induce a control loss of cell surface area5 were used as a positive control for imaging. Unlike LPC treatment, exposure to diamide had no effect on RBC dimensions (surface area and diameter [A-B]) and only a slight effect on cell morphology (circularity [C] and sphericity [D-E]).
Effect of diamide exposure on RBC membrane protein–protein associations and deformability. (A) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of dRBCs, run in nonreducing (A1) or reducing (A2) conditions. (B) Deformability profile of dRBCs or untreated control RBCs before the spleen perfusion (shown is a representative experiment, n = 8), as assessed by Lorrca. (C) EI (RBC deformability parameter) is plotted as a function of the logarithm of the shear stress. dRBCs required significantly greater applied shear stress to reach equivalent deformation as RBCs with normal membrane. Because the lines are parallel, one can see that RBC membranes treated with 10 µM diamide required 1.3-fold greater shear stress than normal membranes to reach equivalent deformation at all points along the curve, indicating that the diamide-treated membranes had 0.79 times the normal deformability. Treatment with 125, 300, and 500 mM diamide resulted in membranes that had 0.29-, 0.14-, and 0.07-fold the normal deformability, respectively. (D) Linear regression fit of the correlation between diamide concentrations and the relative rigidity of dRBCs. (E) Osmotic fragility of dRBCs (a representative experiment).
Effect of diamide exposure on RBC membrane protein–protein associations and deformability. (A) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of dRBCs, run in nonreducing (A1) or reducing (A2) conditions. (B) Deformability profile of dRBCs or untreated control RBCs before the spleen perfusion (shown is a representative experiment, n = 8), as assessed by Lorrca. (C) EI (RBC deformability parameter) is plotted as a function of the logarithm of the shear stress. dRBCs required significantly greater applied shear stress to reach equivalent deformation as RBCs with normal membrane. Because the lines are parallel, one can see that RBC membranes treated with 10 µM diamide required 1.3-fold greater shear stress than normal membranes to reach equivalent deformation at all points along the curve, indicating that the diamide-treated membranes had 0.79 times the normal deformability. Treatment with 125, 300, and 500 mM diamide resulted in membranes that had 0.29-, 0.14-, and 0.07-fold the normal deformability, respectively. (D) Linear regression fit of the correlation between diamide concentrations and the relative rigidity of dRBCs. (E) Osmotic fragility of dRBCs (a representative experiment).
Characteristics of unexposed RBCs and dRBCs
| Sample . | Hb, g/dL . | MCHC, g/dL . | Diameter, µm . | MCV, µm3 . | Projected area, µm2 . | S/V, µm−1 . | Sphericity . | Relative rigidity . | Retention rate, % . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Spleen . | Microsphilters . | |||||||||
| Untreated RBCs | 11.3 (1.3) | 34 (2) | 9.2 (0.003) | 92 (2) | 67 (0.01) | 0.73 (0.02) | 0.82 (0.01) | 1.0 (0.2) | 9 (5) | 0 (0) |
| Diamide-treated RBCs | ||||||||||
| 10-60 µM | 11.8 (1.9) | 34 (2) | 9.4 (0.04) | 91 (2) | 70 (0.4) | 0.77 (0.02) | 0.82 (0.001) | 1.3 (0.4) | 24 (13) | 2 (3) |
| 75-250 µM | 11.5 (1.6) | 34 (1) | 9.4 (0.1) | 91 (4) | 70 (2) | 0.77 (0.04) | 0.83 (0.01) | 4.1 (1.7) | 41 (5) | 19 (16) |
| 300-500 µM | 11.4 (1.3) | 33 (1) | 9.5 (0.1) | 95 (4) | 72 (2) | 0.76 (0.04) | 0.83 (0.02) | 17.4 (4.3) | 58 (5) | 13 (13) |
| Sample . | Hb, g/dL . | MCHC, g/dL . | Diameter, µm . | MCV, µm3 . | Projected area, µm2 . | S/V, µm−1 . | Sphericity . | Relative rigidity . | Retention rate, % . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Spleen . | Microsphilters . | |||||||||
| Untreated RBCs | 11.3 (1.3) | 34 (2) | 9.2 (0.003) | 92 (2) | 67 (0.01) | 0.73 (0.02) | 0.82 (0.01) | 1.0 (0.2) | 9 (5) | 0 (0) |
| Diamide-treated RBCs | ||||||||||
| 10-60 µM | 11.8 (1.9) | 34 (2) | 9.4 (0.04) | 91 (2) | 70 (0.4) | 0.77 (0.02) | 0.82 (0.001) | 1.3 (0.4) | 24 (13) | 2 (3) |
| 75-250 µM | 11.5 (1.6) | 34 (1) | 9.4 (0.1) | 91 (4) | 70 (2) | 0.77 (0.04) | 0.83 (0.01) | 4.1 (1.7) | 41 (5) | 19 (16) |
| 300-500 µM | 11.4 (1.3) | 33 (1) | 9.5 (0.1) | 95 (4) | 72 (2) | 0.76 (0.04) | 0.83 (0.02) | 17.4 (4.3) | 58 (5) | 13 (13) |
Hemoglobin (Hb), MCHC, and mean cell volume (MCV) were measured with an ADVIA hematology system. Sphericity = aspect ratio. All data are mean (standard deviation).
Diamide treatment resulted in a gradual loss of RBC elongation (a representative experiment is illustrated in Figure 2B-C). There was a strong positive correlation (r = 0.98; P < .0001) between the concentrations of diamide and relative rigidity of the dRBCs (Figure 2D). In Figure 2B, EI max of dRBCs was not reached at a shear stress of 30 Pa, and the deformability profiles were not parallel with those of untreated cells at high shear stress values. Such a pattern is characteristic of RBCs with decreased cellular elongation as a consequence of increased membrane rigidity.4 This conclusion was supported by the similar osmotic-resistance profiles of dRBCs and control RBCs, implying no changes in membrane surface area (Figure 2E).
Retention of dRBCs by isolated-perfused human spleen increases with cell membrane rigidity
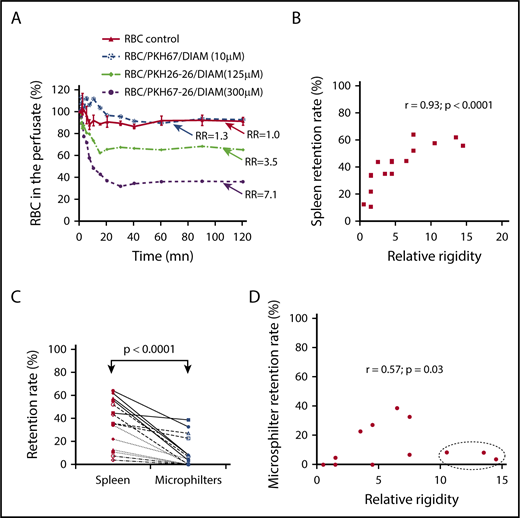
Ex vivo normal human spleens were perfused with dRBCs, and normal untreated RBCs were used as control. Each spleen was perfused with a mixture of differentially PKH-labeled diamide-exposed cells, such that 3 diamide concentrations could be tested alongside untreated red cells in a single experiment. The kinetics of RBC retention during perfusion was assessed by flow cytometry analysis of the perfusate (allowing quantification of the circulating differentially PKH-labeled cells at different time points during the perfusion). dRBCs were rapidly cleared, with maximum clearance observed between 10 and 40 minutes, depending on the dose of diamide (a representative study is illustrated in Figure 3A). The mean clearance half-time and range were 5.9 and 4.0 to 13.0 minutes, respectively. Retention increased (range, 7.3%-64.0%) with increasing red cell membrane rigidity (Figure 3B). There was a strong positive correlation (r = 0.93; P < .0001) between the percentage of RBCs retained in the spleen and relative rigidity of the dRBC preparation perfused (Figure 3B). This splenic retention of dRBCs was not due to saturation of the splenic filtration function, because diamide-treated RBCs represented only a small percentage of the cells perfused (∼4-5% of the total RBCs). Likewise, this retention could not be linked to the dysfunction of the spleens perfused with dRBCs, because the organs were morphologically and histological normal (data not shown).
Retention of dRBCs by the isolated-perfused human spleen and the microsphiltration system. (A) Kinetics of dRBC clearance in the perfused spleen, monitored by measuring PKH-labeled counts in the perfusate (a representative experiment, n = 8). (B) Linear regression fit of the correlation between levels of dRBC retention within the spleen and the relative rigidity. (C) Retention rates of dRBCs in the isolated-perfused human spleen 2 hours after the onset of perfusion or in the microspheres filter. (D) Linear regression fit of the correlation between levels of dRBC retention within the microsphilters and the relative rigidity.
Retention of dRBCs by the isolated-perfused human spleen and the microsphiltration system. (A) Kinetics of dRBC clearance in the perfused spleen, monitored by measuring PKH-labeled counts in the perfusate (a representative experiment, n = 8). (B) Linear regression fit of the correlation between levels of dRBC retention within the spleen and the relative rigidity. (C) Retention rates of dRBCs in the isolated-perfused human spleen 2 hours after the onset of perfusion or in the microspheres filter. (D) Linear regression fit of the correlation between levels of dRBC retention within the microsphilters and the relative rigidity.
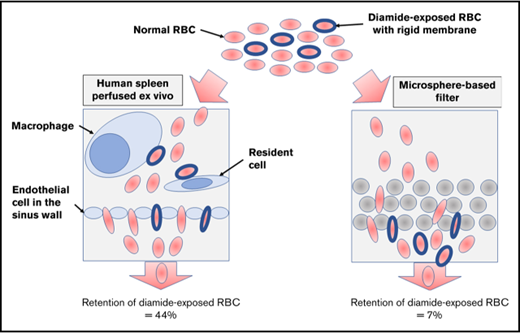
To evaluate the mechanical process of the splenic entrapment of dRBCs, we perfused, in parallel, dRBCs through ex vivo human spleen and through the microsphiltering device, a microbead-based technology that mimics the mechanical filtering function of the spleen.2 There was retention of dRBCs in the microsphilters (median, 7.5%; range, 0-38.6%), but to a much lower extent compared with the isolated spleen (median, 44.1%; range, 7.3%-64.0%; P < .0001) (Figure 3C). There was a mild correlation between the percentage of dRBCs retained in the microsphilters and membrane rigidity (r = 0.57; P = .03) (Figure 3D). Three dRBC populations with relative membrane rigidity > 10 were not retained in the microsphilters (Figure 3D; dashed oval). Taken together, these results suggest that cellular rigidity per se is not the main contributor to the splenic entrapment of dRBCs.
Discussion
This study confirms that exposure of RBCs to diamide resulted in reduced cellular deformability, mainly due to increased membrane rigidity.3,4 We did not find any evidence of alteration of RBC intracellular viscosity following diamide treatment. dRBCs were rapidly retained in the human spleen. This retention was dose dependent and correlated strongly with the increased RBC membrane rigidity. However, there was only a modest relationship between the retention rate and increased membrane rigidity of dRBCs with the microsphere-filtering device that challenges mechanical elasticity of the cells and filterability through narrow slits that mimic the mechanical filtering function of the spleen. Moreover, dRBC populations with the highest reduced membrane deformability were not retained in the microsphilters, suggesting that the mechanical process is not the main contributor to the splenic entrapment of dRBCs. This result is in contrast with our earlier findings,5,21 where we found that RBCs with reduced S/V ratio after exposition to LPC were rapidly entrapped to the same extent in the spleen5 and in the microsphilters.21 Taken together, these results suggest that increased membrane rigidity per se might not markedly reduce the ability of RBCs to traverse the spleen. This concept is supported by the finding in SAO RBCs, which display similar cellular characteristic (reduced membrane deformability) as dRBCs but do not sequester in the spleen of patients.
The discordance in the retention rates of dRBCs between the spleen and the microsphilters suggests that others factors may drive the splenic retention of dRBCs. It is well established that treatment of RBCs with diamide induces aggregation of several membrane proteins, including band 3, as well as membrane skeletal proteins (eg, spectrin)3,4,14 (Figure 2A). It might induce externalization of ligands22,23 that possibly trigger splenic entrapment of the dRBCs through adhesion to the structures of the reticular meshwork of the spleen red pulp and their phagocytosis by the macrophages.24-26 This hypothesis is supported by the fact that dRBCs with a relatively low increase in membrane rigidity (range of relative rigidity, 1.1-1.6) were efficiently retained in the spleen (range, 22%-34%).
The comparison of the human spleen with the microsphiltration system clearly demonstrates that increased red cell membrane rigidity is not the principal reason for the splenic entrapment of dRBCs. Thus, a model involving decreased deformability, acting in conjunction with cellular adhesion in the splenic red pulp, fits with clinical observations and epidemiological studies,10,11 as well as animal studies.27,28 This could explain the heterogeneity of inherited RBC disorders, such as ovalocytosis, α- or β-thalassemia, and sickle cell disease. Further investigations are needed to validate this model and to identify the putative ligand–receptor interactions involved. dRBCs might not be a good model for SAO RBC handling by the spleen. The microspheres do not capture other important parameters influencing splenic retention, in particular, display of ligands, which can trigger the splenic entrapment of these cells through ligand–receptor interactions, with 1 possible end point being their phagocytosis by the red pulp macrophages.24-26
Acknowledgments
This work was supported by the “Fonds dédié Combattre les maladies parasitaires,” Sanofi Aventis-Ministère de l’Enseignement Supérieur et de la Recherche-Institut Pasteur, the Centre National de la Recherche Scientifique, and the Institut Pasteur. I.S. received a fellowship from the Région Ile de France. G.D. was supported by the Délégation Générale à l’Armement (fellowship 056000032) and the Région Ile de France. The research leading to these results received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement 242095 (Evimalar), the project Mechanisms of Erythrocytic Infection and Anemia in Malaria (principal investigator, Kasturi Haldar), and National Institutes of Health, National Heart, Lung, and Blood Institute (award 5P01HL078826-06). N.M. acknowledges the “Fonds dédié Combattre les maladies parasitaires,” Sanofi Aventis-Ministère de l’Enseignement Supérieur et de la Recherche-Institut Pasteur, which made possible his sabbatical stay at Institut Pasteur, and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant DK26263.
Authorship
Contribution: I.S. and P.A.B. designed and performed the research, contributed vital analytical tools, analyzed the data, and wrote the manuscript; G.D. and S.P. designed and performed the research, contributed vital analytical tools, and analyzed the data; V.B. designed and performed the research and analyzed the data; A.S., B.A., S.D., A.C., and D.C.-H. contributed reagents/materials/analysis tools and wrote the manuscript; and O.M.-P., G.M., P.H.D., and N.M. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Innocent Safeukui, Department of Biological Sciences, University of Notre Dame, 103 Galvin Life Sciences, Notre Dame, IN 46556; e-mail: innocent.safeukui@nd.edu.

![Figure 1. Dimensional and morphological characteristics of dRBCs. Projected surface area (A), diameter (B), circularity (C), aspect ratio (D), and shape ratio (E) (sphericity) of untreated RBCs and RBCs treated with increasing concentrations of diamide (10-500 µM) and analyzed with an ImageStream imaging cytometer. RBCs treated with LPC to induce a control loss of cell surface area5 were used as a positive control for imaging. Unlike LPC treatment, exposure to diamide had no effect on RBC dimensions (surface area and diameter [A-B]) and only a slight effect on cell morphology (circularity [C] and sphericity [D-E]).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/20/10.1182_bloodadvances.2018024562/4/m_advances024562f1.png?Expires=1769092321&Signature=IiAKly5pDQz0iKxyAFRVET2yf4CIM804XGqyXzyuc6lJDswZ0AORMFQxh40pLbRFd2J5E3Yqjd6JTyyIndHC1ddZRmR3kCKvU-g~y1xB4lr9LwuMei22CyvB7Q0GXi1NvTjeOMWomJzc26hDHK~pQCsM5aiqVNclM2ucvT2gCw4e0E1PyrsYd2Jyvo9rp-tGRbEdpw3rljFt0bMCcKKP4qvif60fRwC4P4BJcbsc86YaK9DVfxDLPJlr6JqlFYkLLdGlH7~ZZfHJKj0gzQdzgZU6eKHMVVpJWfiB99IOWqfXNMUN770Wodext7I6cO1jXBycm8oWlpXb7iOumAXeaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)