Key Points
Platelet activation in vitro results in a more rapid and greater upregulation of TLT-1 surface expression compared with P-selectin.
TLT-1 is more rapidly translocated to the surface of activated platelets than P-selectin during thrombus formation in vivo.
Introduction
Formation of hemostatic thrombi in vivo is a dynamic process involving platelets with heterogeneous levels of activation. Pioneering work by Stalker et al1 and Nesbitt et al2 has shown developing thrombi are characterized by a core of fully activated platelets, which have undergone α-granule exocytosis, surrounded by a less activated platelet shell. Platelets in the activated core, directly adjacent to the site of injury, are tightly packed and can be identified by surface exposure of P-selectin. In contrast, the surrounding shell contains loosely adhered platelets not expressing P-selectin.1
P-selectin is a type 1 transmembrane protein present in platelet and megakaryocyte α-granules. Upon platelet activation, fusion of α-granules with the plasma membrane results in P-selectin exposure on the platelet surface.3 Once there, the primary role of P-selectin is to mediate interactions with leukocytes (monocytes and neutrophils) via its ligand, P-selectin glycoprotein ligand 1, facilitating efficient recruitment of these cells to sites of vascular injury.3 P-selectin is therefore commonly used as a marker of α-granule secretion and irreversible platelet activation.1,4-6
TREM (triggering receptor expressed on myeloid cells)–like transcript 1 (TLT-1) is another membrane receptor found in platelet and megakaryocyte α-granules and is rapidly translocated to the surface upon activation.7,8 TLT-1 is abundantly present in platelets, with expression levels greater than P-selectin in both human (TLT-1, 14 200 copies; P-selectin, 8900 copies) and mice (TLT-1, 154 769 copies; P-selectin, 35 970 copies) as determined by quantitative proteomics-based approaches.9,10 Early findings revealed TLT-1 and P-selectin are both present in platelet α-granules, but a portion did not colocalize, suggesting distinct storage compartments.11 Elevated levels of soluble TLT-1 can also be detected in plasma of patients with thrombotic diseases.12 TLT-1 belongs to the immunoreceptor tyrosine-based inhibition motif (ITIM)–containing receptor family; however, unlike other conventional ITIM-containing receptors, deletion of TLT-1 in mice was shown to reduce platelet aggregation in response to binding its physiological ligand, fibrinogen.8,11,13
In this study, we show that TLT-1 is more rapidly and abundantly upregulated on the surface of activated platelets than the current gold standard P-selectin. Furthermore, we show that in vivo TLT-1 is present in both the highly activated core and less activated platelet shell of thrombi, whereas P-selectin is only detectable in the highly activated core, providing for the first time direct evidence of activated platelets in the thrombus shell. Collectively, these results suggest that surface expression of TLT-1 is a more sensitive marker of platelet activation than P-selectin.
Methods
Animals
Wild-type mice were all on a C57BL/6 background. All procedures were in accordance with the Animal (Scientific Procedures) Act 1986 and undertaken with United Kingdom Home Office approval.
Antibodies and reagents
Antibodies used are listed supplemental Table 1. All other reagents were sourced from Sigma-Aldrich (Poole, United Kingdom) or as previously described.14
Platelet preparation
Blood was collected from the inferior vena cava of CO2-asphyxiated mice into 1:10 (v:v) acid-citrate-dextrose anticoagulant. Washed platelets were prepared as previously described.14 Briefly, platelets were resuspended in modified Tyrodes N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 1 mM MgCl2, and 5 mM glucose, pH 7.3) and used at 2 × 107/mL for the spreading experiment.
Megakaryocyte preparation
Megakaryocytes were prepared as previously described.14 In brief, bone marrow (BM) cells were obtained from mouse femurs and tibias by flushing, and cells expressing lineage-specific surface markers (CD16/CD32+, Gr1+, B220+, or CD11b+) were depleted. The remaining population was cultured in serum-supplemented StemPro medium for 2 days with murine stem cell factor (20 ng/mL) and a further 4 days in the presence of stem cell factor and 50 ng/mL thrombopoietin (37°C, 5% CO2). Mature megakaryocytes were then enriched using a 1.5%/3% bovine serum albumin gradient.
Western blotting
Whole-cell lysates were prepared from megakaryocyte culture each day following thrombopoietin addition as previously described.14 Equal amounts of proteins were resolved on 4% to 12% NuPAGE Bis-Tris gradient gels and immunoblotted with primary antibodies (anti-mouse TLT-1, 1/1000 [R&D Systems]; anti-mouse P-selectin, 1/500 [Santa Cruz]; and anti-Erk1/2, 1/1000 [Cell Signaling]) and horseradish peroxidase–conjugated secondary antibody. Proteins were detected by enhanced chemiluminescence and autoradiography.
Flow cytometry
Surface protein expression of TLT-1 and P-selectin was analyzed in resting and stimulated whole blood (BD Accuri C6 flow cytometer) or BM-derived megakaryocytes (BD FACSCalibur) following fixation and staining with indicated conjugated antibodies as previously described.14
In vivo thrombosis assay
Laser-induced injury of cremaster arterioles and ferric chloride (FeCl3)–induced injury of carotid arteries were performed in mice (20-25 g) as previously described.14 Briefly, mice were anesthetized, and the cremaster muscle or carotid artery was exposed. Thrombi were then generated by laser or FeCl3-soaked filter paper application (10%, 3 minutes). Mice were injected with indicated conjugated antibodies prior to injury. Fluorescence and bright-field images were captured simultaneously using an Olympus BX61WI, upright spinning disk confocal microscope (40× 0.8 numerical aperture [NA] water-immersion lens/4× 0.13 NA air lens) with a Photometrics Evolve camera. Images were analyzed using Slidebook6 software (Intelligent Imaging Innovations).
Immunofluorescence microscopy
Resting platelets were fixed in suspension and plated on poly-l-lysine–coated coverslips and centrifuged at 1000 rpm for 10 minutes. Megakaryocytes were plated on fibrinogen-coated coverslips (100 µg/mL) for 15 minutes at 37°C then fixed. Platelets and megakaryocytes were then permeabilized and stained as previous.14 Platelets were imaged by Zeiss LSM880 confocal microscope (40×, 1.2 NA water-immersion lens) with Airyscan processing. Megakaryocytes were imaged using a Zeiss Observer 7 epifluorescent microscope (63×, 1.4 NA oil-immersion lens) and Hamamatsu ORCA Flash 4 LT sCMOS camera. Images were acquired using Zen Pro V2.3. Deconvolution was performed on representative Z-stacks before maximum intensity projection for quantification.
Image analysis
Maximum intensity projections were used for image analysis. Analysis performed using Icy software.15 Colocalization of TLT-1 (488 channel) and P-selectin (647 channel) in platelets was determined by Manders overlap coefficients M1 (TLT-1:P-selectin) and M2 (P-selectin:TLT-1) as previously described.16 Megakaryocyte images were processed using Icy’s Spot Detector plug-in (http://icy.bioimageanalysis.org/plugin/Spot_Detector) to effectively threshold granules positive for TLT-1 (488 channel) and P-selectin (647 channel) and determine an object based colocalization measure. In large, complex cells like megakaryocytes, this approach can effectively threshold and measure colocalization between granules of interest.
Statistical analysis
Data presented as mean ± standard deviation (SD) unless stated otherwise. Statistical significance was analyzed using analysis of variance. P < .05 was considered statistically significant.
Results and discussion
TLT-1 is expressed in mature megakaryocytes and upregulated to the surface upon activation
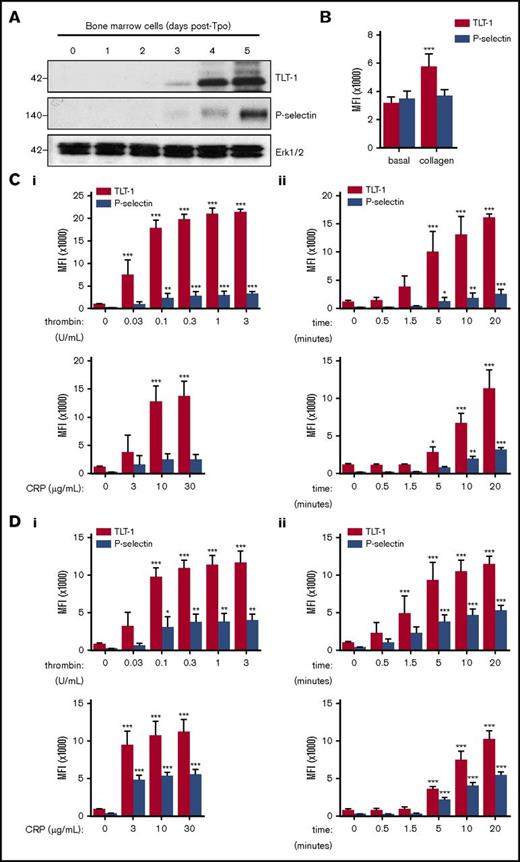
TLT-1 was only detectable in mature BM-derived mouse megakaryocytes 4 days after thrombopoietin addition (Figure 1A). Mature megakaryocytes show increased TLT-1 surface expression in response to collagen stimulation, which was not mirrored by P-selectin (Figure 1B).
TLT-1 expression is greater than P-selectin expression on the surface of activated megakaryocytes and platelets in vitro. (A) Whole-cell lysates of primary BM-derived mouse megakaryocytes 0 to 5 days after thrombopoietin (Tpo) administration were western blotted for TLT-1, P-selectin, and ERK1/2. Representative blots of 2 independent experiments. (B) BM-derived megakaryocyte surface expression of TLT-1 and P-selectin following collagen stimulation (30 µg/mL) for 20 minutes at 37°C. Data are presented as mean fluorescence intensity (MFI) ± SD; n = 7-9. (C-D) Platelet surface expression of TLT-1 and P-selectin was measured by flow cytometry in response to the protease activated receptor agonist thrombin and the glycoprotein VI–specific agonist CRP in murine (C) and human (D) whole blood. (i) Dose-response (thrombin, 0.03-3 U/mL; CRP, 3-30 µg/mL) and (ii) time course (thrombin, 0.1 U/mL; CRP, 10 µg/mL). Data are presented as median fluorescence intensity ± SD; n = 3-6 independent experiments per condition. *P < .05, **P < .01, ***P < .001 vs basal.
TLT-1 expression is greater than P-selectin expression on the surface of activated megakaryocytes and platelets in vitro. (A) Whole-cell lysates of primary BM-derived mouse megakaryocytes 0 to 5 days after thrombopoietin (Tpo) administration were western blotted for TLT-1, P-selectin, and ERK1/2. Representative blots of 2 independent experiments. (B) BM-derived megakaryocyte surface expression of TLT-1 and P-selectin following collagen stimulation (30 µg/mL) for 20 minutes at 37°C. Data are presented as mean fluorescence intensity (MFI) ± SD; n = 7-9. (C-D) Platelet surface expression of TLT-1 and P-selectin was measured by flow cytometry in response to the protease activated receptor agonist thrombin and the glycoprotein VI–specific agonist CRP in murine (C) and human (D) whole blood. (i) Dose-response (thrombin, 0.03-3 U/mL; CRP, 3-30 µg/mL) and (ii) time course (thrombin, 0.1 U/mL; CRP, 10 µg/mL). Data are presented as median fluorescence intensity ± SD; n = 3-6 independent experiments per condition. *P < .05, **P < .01, ***P < .001 vs basal.
TLT-1 is rapidly upregulated to the platelet surface upon activation
Increased surface expression of TLT-1 and P-selectin were detected in mouse platelets following stimulation with thrombin and glycoprotein VI–specific agonist collagen-related peptide (CRP), with TLT-1 upregulated to significantly greater extent than P-selectin (Figure 1Ci). TLT-1 was also more rapidly upregulated to the surface following thrombin and CRP stimulation compared with P-selectin (Figure 1Cii).
Differences in upregulation may possibly be explained by the fact that TLT-1 abundance is >4 times greater than P-selectin abundance in murine platelets.9 Therefore experiments were repeated with human platelets, where abundance is more comparable.10 Consistent with findings in murine platelets, upregulation of TLT-1 was greater (Figure 1Di) and more rapid than upregulation of P-selectin (Figure 1Dii).
TLT-1 is expressed throughout the core and shell of thrombi in vivo
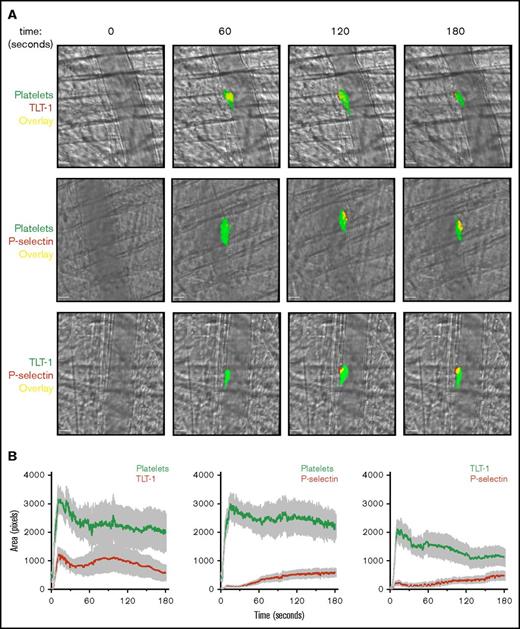
In agreement with our in vitro findings, TLT-1 was detected more quickly than P-selectin in vivo after laser-induced injury in mouse cremaster arterioles (Figure 2A; supplemental Videos 1-3). Interestingly, TLT-1 expression (unlike P-selectin) was not limited to the core and was present throughout the thrombus (Figure 2B). This differs with the current model of thrombus formation, in which only platelets in the core undergo α-granule secretion.1 While TLT-1 expression in the shell does not necessarily suggest the thrombus core (which has other defining characteristics, such as packing density) is larger than previously thought, it does suggest a greater level of platelet activation in the shell.1,17 Interestingly, in FeCl3-induced thrombi, the platelet core and shell are not discernible. However, consistent with previous results, TLT-1 was detected more rapidly than P-selectin and was present throughout thrombi (supplemental Figure 1; supplemental Videos 4-6).
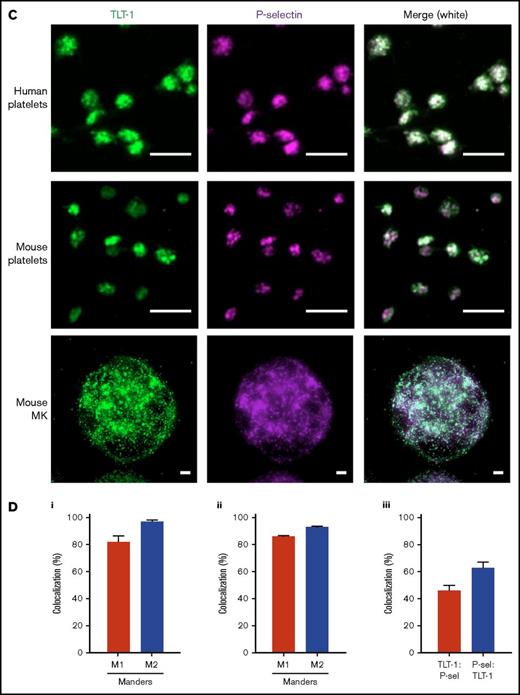
TLT-1 appears more rapidly and is detected throughout laser-induced thrombi in vivo. Mice were injected with anti-GPIbβ, anti-TLT-1, or anti-CD62P antibody. Arterioles of cremaster muscles were subsequently injured by laser. (A) Representative composite bright-field and fluorescence images of platelets (GPIbβ), TLT-1, and P-selectin in thrombi. Scale bars, 10 µm. (B) Quantification of fluorescence area in pixels (data represent mean ± standard error of the mean; n = 25-32 injuries from 4 to 6 mice). (C) Distinct and overlapping staining of TLT-1 and P-selectin in platelets and megakaryocytes. Resting human (top row) and mouse (middle row) platelets seeded on poly-l-lysine and primary mouse BM-derived megakaryocytes (MK) (bottom row) spread on fibrinogen matrix for 15 minutes at 37°C were fixed, permeabilized, and stained with Alexa Fluor 488 anti-TLT-1 and Alexa Fluor 647 anti-P-selectin antibodies. The right column represented an overlay of both images. Scale bars, 5 µm. Images are representative of 3 to 4 independent experiments. (D) Degree of TLT-1 and P-selectin colocalization as determined by the Manders overlap coefficients (M1: TLT-1:P-selectin and M2: P-selectin:TLT-1) in resting human (i) and mouse platelets (ii), and object-based colocalization in mouse BM-derived megakaryocytes (iii) (n = 3-4 independent experiments per condition, 400-600 platelets, and 25-35 megakaryocytes per experiment).
TLT-1 appears more rapidly and is detected throughout laser-induced thrombi in vivo. Mice were injected with anti-GPIbβ, anti-TLT-1, or anti-CD62P antibody. Arterioles of cremaster muscles were subsequently injured by laser. (A) Representative composite bright-field and fluorescence images of platelets (GPIbβ), TLT-1, and P-selectin in thrombi. Scale bars, 10 µm. (B) Quantification of fluorescence area in pixels (data represent mean ± standard error of the mean; n = 25-32 injuries from 4 to 6 mice). (C) Distinct and overlapping staining of TLT-1 and P-selectin in platelets and megakaryocytes. Resting human (top row) and mouse (middle row) platelets seeded on poly-l-lysine and primary mouse BM-derived megakaryocytes (MK) (bottom row) spread on fibrinogen matrix for 15 minutes at 37°C were fixed, permeabilized, and stained with Alexa Fluor 488 anti-TLT-1 and Alexa Fluor 647 anti-P-selectin antibodies. The right column represented an overlay of both images. Scale bars, 5 µm. Images are representative of 3 to 4 independent experiments. (D) Degree of TLT-1 and P-selectin colocalization as determined by the Manders overlap coefficients (M1: TLT-1:P-selectin and M2: P-selectin:TLT-1) in resting human (i) and mouse platelets (ii), and object-based colocalization in mouse BM-derived megakaryocytes (iii) (n = 3-4 independent experiments per condition, 400-600 platelets, and 25-35 megakaryocytes per experiment).
Distinct and overlapping TLT-1 and P-selectin staining
To explain the different localization of TLT-1 and P-selectin, immunofluorescence microscopy was performed on human and mouse platelets and primary BM-derived megakaryocytes. We found that TLT-1 colocalized with P-selectin within α-granules. However, a proportion was present in distinct granules, as determined by Manders overlap coefficients and object-based colocalization, suggesting differential distribution or localization to another as-yet-unidentified compartment (Figure 2C-D).
Collectively, findings from this study demonstrate that TLT-1 is a more sensitive marker of megakaryocyte and platelet activation than P-selectin. The stronger signal and rapid upregulation of TLT-1 compared with P-selectin following platelet activation is most likely due to the greater abundance of TLT-1; however, differential compartmentalization of the 2 receptors and differences in antibody-binding affinities and fluorescence may also contribute to the stronger TLT-1 signal. The latter is unlikely to be the case, as all antibodies tested yielded the same results, irrespective of labeling (data not shown). Differences in surface translocation rates of the 2 receptors and distribution within thrombi suggest distinct mechanisms of upregulation, even though both are reportedly localized in platelet α-granules.3,7 Another possibility is that TLT-1 is expressed in an early-release granule that is devoid of P-selectin. This may be either an α-granule subpopulation or a distinct type of granule.
Previous studies have shown stimulation of platelets with different agonists causes differential release of α-granule cargo and led to the proposal of distinct subpopulations of α-granules that undergo release to specific agonists.18-22 Alternatively, it has been suggested that cargo is randomly distributed among α-granules but segregated into subregions within each granule, with differential release resulting from partial activation.23,24 Identification of tubular α-granules has also given rise to the possibility of polarized granule secretion.25 Either of these mechanisms could explain the differences in TLT-1 and P-selectin localization throughout thrombi, as agonists and extent of platelet activation at sites of vascular damage are stratified, with stronger agonists and full platelet activation in the core and partial platelet activation by weaker agonists in the surrounding shell.1,17,18 Conversely, the rapid upregulation of TLT-1 to the surface suggests a proportion of TLT-1 may localize distinctly from P-selectin to other as-yet-unidentified compartments of α-granules and puts forward the possibility of an early-release granule.11 Consistent with this hypothesis, a distinct staining pattern of TLT-1 and P-selectin within platelets and megakaryocytes was observed, suggesting differential compartmentalization of a proportion of these 2 receptors. However, additional work is required to characterize these distinct P-selectin–negative granules and mechanism of translocation to the surface.
In conclusion, TLT-1 is a more sensitive marker of platelet activation than P-selectin that can be detected in both the core and shell of thrombi in vivo. This opens the possibility of TLT-1 being used as a biomarker for early detection of platelet activation in various pathologies, including coronary artery disease and deep vein thrombosis, as well as megakaryocyte activation in BM in myeloproliferative disorders and myelofibrosis.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the members of the Biomedical Service Unit for their technical support and Jeremy A. Pike for his help analyzing and quantifying the degree of colocalization.
This work was supported by the British Heart Foundation. A.M. is a BHF Intermediate Basic Science Research Fellow (FS/15/58/31784). Y.A.S. is a BHF Senior Basic Science Research Fellow (FS/13/1/29894). C.W.S. and A.O.K. are college-funded PhD students. Z.R. is a postdoctoral research fellow. P.P. is a BHF-funded research technician.
Authorship
Contribution: C.W.S. performed experiments, collected, analyzed and interpreted data and wrote and revised the manuscript; L.P. performed experiments and collected, analyzed, and interpreted data; Z.R. designed and performed experiments and collected, analyzed, and interpreted data; A.O.K. performed microscopy imaging; P.P. maintained mouse colonies and assisted with experiments; Y.A.S. contributed intellectually and revised the manuscript; and A.M. conceived the project, contributed intellectually, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandra Mazharian, Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Wolfson Dr, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: a.mazharian@bham.ac.uk.