Abstract
Patients treated with allogeneic hematopoietic cell transplantation (HCT) are at risk of cytomegalovirus (CMV) reactivation and disease, which results in increased morbidity and mortality. Although universal antiviral prophylaxis against CMV improves outcomes in solid organ transplant recipients, data have been conflicting regarding such prophylaxis in patients undergoing allogeneic HCT. We conducted a systematic review of randomized trials of prophylactic antivirals against CMV after allogeneic HCT to summarize the evolution of the field over the last 35 years and evaluate the prophylactic potential of antiviral agents against CMV after allogeneic HCT. Electronic databases were queried from database inception through 31 December 2017. For included studies, incidence of CMV infection and all-cause mortality were collected as primary outcomes; CMV disease incidence, use of preemptive therapy, and drug toxicities were collected as secondary outcomes. Nineteen clinical trials conducted between 1981 and 2017 involving a total of 4173 patients were included for review. Prophylactic strategies included use of acyclovir, valacyclovir, ganciclovir, maribavir, brincidofovir, and letermovir compared with placebo or a comparator antiviral. Fourteen trials that compared antiviral prophylaxis with placebo demonstrated overall effectiveness in reducing incidence of CMV infection (odds ratio [OR], 0.49; 95% confidence interval [CI], 0.42-0.58), CMV disease (OR, 0.56; 95% CI, 0.40-0.80), and use of preemptive therapy (OR, 0.51; 95% CI, 0.42-0.62; 6 trials); however, none demonstrated reduction in all-cause mortality (OR, 0.96; 95% CI, 0.78-1.18) except the phase 3 trial of letermovir (week-24 OR, 0.59; 95% CI, 0.38-0.98). Additional research is warranted to determine patient groups most likely to benefit from antiviral prophylaxis and its optimal deployment after allogeneic HCT.
Introduction
Human cytomegalovirus (CMV) is an enveloped double-stranded DNA virus that belongs to the herpesviridae family. Infection with CMV is common, with seroprevalences ranging from 50% to over 90% depending on age, geographical location, and socioeconomic factors.1 CMV establishes latency in human epithelial tissue, polymorphonuclear cells, myeloid progenitors, and T lymphocytes and is normally controlled by the host’s immune system.2,3 Immunosuppression after allogeneic hematopoietic cell transplantation (HCT) frequently leads to CMV reactivation, which is associated with increased morbidity and mortality in this patient population.4-6 Primary CMV infection and reactivation increase the risk of CMV disease after allogeneic HCT, which can manifest clinically in diverse ways, including colitis, pneumonitis, retinitis, and hepatitis.7,8 Recent studies have also shown that despite use of preemptive therapy, CMV reactivation may be associated with an increased risk of invasive fungal disease8 and is also an independent risk factor for nonrelapse mortality.9
The evolution of effective antiviral agents against CMV has resulted in the emergence of 2 distinctive strategies to prevent CMV-related outcomes among patients undergoing HCT: universal prophylaxis and preemptive therapy. The latter is defined as antiviral treatment triggered by early detection of active CMV infection, before clinical disease occurs. Specifically, patients undergo blood CMV surveillance with viral DNA or antigen detection, and antiviral therapy is initiated above a certain detection threshold.10,11 However, any level of CMV viremia has been associated with increased nonrelapse mortality after allogeneic HCT, despite use of highly sensitive diagnostic assays to detect low-level CMV viremia.12 This disadvantage highlights the need for safe and effective antiviral agents to be used in prophylactic strategies.
The quest for successful prophylactic strategies against CMV for allogeneic HCT patients started in the 1980s. Although universal prophylaxis was effective in preventing CMV primary infection and reactivation after transplantation in some trials, the overall benefit of prophylactic agents has been difficult to assess. Universal prophylaxis has been associated with toxicities particularly detrimental after HCT, including clinically significant myelosuppression associated with ganciclovir and valganciclovir use, which may increase nonrelapse mortality.13
We conducted a systematic review of all antivirals that have been studied for universal prophylaxis to reduce risk of CMV infection among patients undergoing allogeneic HCT. This review assesses the overall efficacy of antiviral prophylaxis in view of novel antiviral therapies and increasingly sensitive diagnostic tests and puts these into perspective with letermovir, which was recently approved for CMV prophylaxis in this patient population.
Methods
Data sources and searches
This manuscript was prepared and reported using PRISMA guidelines and registered in PROSPERO in 2016 as #CRD42016052180.14 PubMed electronic databases were queried from database inception to 31 December 2017. Search terms combined MeSH terms, text words, and exploding terms, including cytomegalovirus, CMV, allogeneic, stem-cell transplant, hematopoietic cell transplant, bone marrow transplant, and prophylaxis. The complete strategy and search terms are listed in supplemental Table 1 of the supplemental Material. The search was limited to articles published in English. Additional studies were identified from references from relevant articles.
Study selection and quality assessment
We included randomized clinical trials involving IV or oral antiviral prophylaxis where CMV infection or CMV disease was a measured outcome. Nonrandomized trials, nonprophylactic trials involving preemptive therapy, and nonantiviral therapies were excluded. Studies of patients who had undergone allogeneic HCT irrespective of age, CMV serostatus, transplantation conditioning regimen, or HLA matching were included. Any intervention that compared an antiviral agent with either placebo or a different antiviral early after HCT (before day +100) with the intent of preventing initial episodes of CMV infection or disease posttransplantation was considered. Authors of selected papers were contacted for additional data if key outcomes were not reported. Study quality was assessed using a standardized tool in Review Manager 5.3 (Cochrane Collaboration, Copenhagen, Denmark) based on the Cochrane handbook, where selection, performance, detection, attrition, and reporting biases were assessed.15
Outcome measures
To evaluate the efficacy of CMV prophylaxis, 2 primary outcomes were assessed. The first was to determine the effect of antiviral prophylaxis on incident CMV primary infection or reactivation. The second was to determine all-cause mortality through follow-up. Secondary end points included rate of preemptive therapy for CMV reactivation while receiving prophylaxis, incidence of CMV disease, and antiviral drug–related toxicity. The primary and secondary outcomes were obtained by referring to end points prespecified in the individual studies, but outcomes up to a year were captured if data were available.
Data extraction and collection
Two independent reviewers (K.C. and M.P.C.) first assessed the titles and abstracts of the search results for eligibility. The full text of the eligible studies was then reviewed for inclusion. The reviewers then designed a data collection form to document authors, year of publication, definition of CMV infection, CMV infection rate, all-cause mortality rate, preemptive therapy rate, antiviral drug used, dosing information and timeline, days of treatment, days of follow-up, type and frequency of CMV measurement, and predefined end points for each study. A formal metaanalysis was not performed because of the heterogeneity in study design and diagnostic methods over time.
Data synthesis and analysis
Data summary and figures were generated in Review Manager 5.3 (Cochrane Collaboration).15 Dichotomous data were analyzed and presented using forest plots, which summarized treatment effect on all-cause mortality, CMV disease, CMV incidence (as defined by study parameters), and preemptive therapy rate if available. Secondary outcomes, such as drug toxicity and study characteristics, were summarized in tables for qualitative analysis.
Results
Included studies
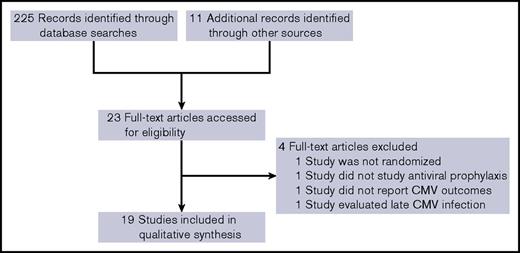
The electronic searches retrieved 225 unique results. After reading the titles and abstracts, 11 trials were selected for full review. Of these, 1 was not a prophylaxis trial, and 1 was not randomized. Ten additional studies were selected through identification of relevant references. A trial of late CMV prophylaxis comparing valganciclovir with placebo beginning after day +100 that included patients with prior episodes of CMV infection was not considered further.16 Nineteen trials were included in this review (Figure 1). Fourteen studies compared an antiviral prophylaxis with placebo, whereas 5 studies compared one antiviral with another.
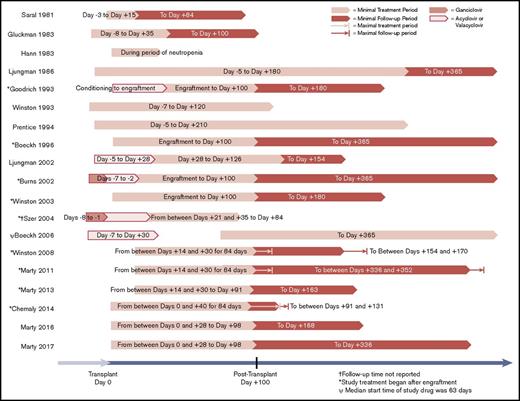
A total of 4173 patients were randomly assigned in prospective trials and analyzed for the primary outcomes analyses. Of these patients, 58% were male, with an estimated median age of 41 years (range, 1-78 years). The most common underlying malignancy was acute leukemia (46%), followed by myelodysplastic syndrome (12%), lymphoma (12%), and chronic myeloid leukemia (11%). Description of key study characteristics, trial intervention, and standard-of-care CMV management of each study can be found in Table 1. Antiviral drugs used included acyclovir, valacyclovir, ganciclovir, maribavir, brincidofovir, and letermovir. Follow-up times after antiviral prophylaxis ranged from 0 to 265 days, with 14 of 19 studies having at least 28 days of follow-up. Study design outlines, including length of prophylactic treatment and follow-up period, are shown in Figure 2.
Randomized trials of CMV prophylaxis in allogeneic HCT patients analyzed
| Study . | Year . | Methods . | Participant characteristics . | Study intervention . | SOC CMV management . | Outcomes measured . |
|---|---|---|---|---|---|---|
| 19 | 1981 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: allogeneic (16), autologous (3), syngeneic (1) | Patients randomized 1:1 to IV acyclovir 250 mg/m2 every 8 h or placebo from d −3 for total of 18 d | No additional management specified | HSV Infection, CMV infection |
| Age range: 6-51 y | ||||||
| Inclusion: HSV antibody titers ≥1:8 | ||||||
| 17 | 1983 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: matched RD or syngeneic | Patients randomized 1:1 to oral acyclovir 200 mg every 6 h or placebo from d −8 to d +35 | No additional management specified | HSV infection, CMV infection, effect of acyclovir on GVHD and survival |
| 18 | 1983 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: allogeneic matched or mismatched RD | Patients randomized 1:1 to IV acyclovir 5 mg/kg every 12 h or placebo during neutropenia (ANC <1 × 109/L) | No additional management specified | Incidence of HSV, incidence of CMV infection or disease, days of neutropenia |
| Serostatus: HSV R+ | ||||||
| 20 | 1986 | Randomized, double blind, placebo controlled, parallel groups, stratified by HSV serostatus | Transplantation type: allogeneic marrow transplantation | Patients randomized 1:1 to IV acyclovir 250 mg/m2 twice a day from d −5 to d +35, transitioned to oral acyclovir 400 mg every 8 h until d +180 | No additional management specified | Incidences of HSV infection, VZV infection, CMV infection |
| Age: ≥2 y | ||||||
| 25 | 1993 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: allogeneic marrow transplantation using total-body irradiation or busulfan-cyclophosphamide | Patients randomized 1:1 to IV ganciclovir 5 mg/kg twice daily or placebo beginning at engraftment for 5 d, then 5 mg/kg per day to d +100 | Upon positive viral cultures, patients removed from blinded study drug and treated with IV ganciclovir | Incidences of CMV infection, CMV disease, neutropenia |
| Age: ≥2 y | ||||||
| Serostatus: CMV R+ | ||||||
| 26 | 1993 | Randomized, double blind, parallel groups, placebo controlled | Transplantation type: allogeneic | Patients randomized 1:1 to IV ganciclovir 2.5mg/kg every 8 h from d −7 to d −1, then from engraftment (ANC ≥1 × 109/L) to d +120 6 mg/kg per day Mondays through Fridays, or placebo | Upon diagnosis of clinical CMV disease, patients removed from blinded study and treated with IV ganciclovir | CMV infection, CMV disease, study drug toxicity |
| Age: ≥12 y | Drug held for ANC <1 × 109/L, resumed at 6 mg/kg per d 3 times per wk | |||||
| Serostatus: CMV R+ | ||||||
| 21 | 1994 | Randomized, double blind, double dummy, 3 parallel groups | Transplantation type: HLA matched related or unrelated allogeneic | Patients randomized 1:1:1 to 1 of 3 arms | CMV disease or CMV infection treated at discretion of site investigator | Time to CMV infection, survival, time to CMV viremia |
| Age: ≥2 y | Arm A: IV acyclovir 500 mg/m2 every 8 h from d −5 to d +30, followed by oral acyclovir 800 mg every 6 h until 6 mo posttransplantation, adjusted to renal function | |||||
| Serostatus: CMV R+ or D+ | Arm B: IV acyclovir like arm A, placebo until 6 mo posttransplantation | |||||
| Arm C: 400 mg oral acyclovir every 6 h from d −5 to d +30, then placebo | ||||||
| 30 | 1996 | Randomized, double blind, parallel groups | Transplantation type: all allogeneic | Patients randomized 1:1 to IV ganciclovir 5 mg/kg twice daily or placebo beginning at engraftment for 5 d, then 5 mg/kg per day 6 d per wk to d +100 | If antigenemia or CMV viremia detected, study drug stopped, and IV ganciclovir administered | CMV disease, neutropenia |
| Age: all ages | Patients underwent CMV antigenemia testing and treated with IV ganciclovir with antigenemia of ≥3 cells per 2 slides | |||||
| Serostatus: CMV R+ | ||||||
| 23 | 2002 | Randomized, double blind, parallel groups | Transplantation type: allogeneic matched or mismatched, related or unrelated | All patients treated with IV acyclovir 500 mg/m2 every 8 h from d −5 to d +28, then randomized 1:1 into 2 arms | Preemptive therapy with IV ganciclovir or foscarnet based on laboratory evidence of infection, according to study site usual practice | Time to detection of CMV, time to death, time to CMV viremia, time to onset of CMV disease, time to other antiviral use, time to onset of CMV infection |
| Age: ≥13 y | Arm A: oral valacyclovir 2 g every 6 h until end of wk 22 posttransplantation | |||||
| Serostatus: CMV R+ or D+ | Arm B: oral acyclovir 800 mg every 6 h until end of wk 22 posttransplantation | |||||
| All doses adjusted to creatinine clearance | ||||||
| 24 | 2002 | Randomized trial, parallel groups | Transplantation type: allogeneic related or unrelated | All patients received IV ganciclovir 5 mg/kg twice daily from d −7 to −2, followed by IV acyclovir 10 mg/kg every 8 h from d −1 to engraftment; patients then randomized 1:1 into 2 arms | Upon detection of antigenemia, prophylaxis discontinued, and patients treated preemptively with administration of IV ganciclovir or foscarnet | Incidences of CMV antigenemia and CMV disease, adverse events |
| Age range: 1-55 y | Arm A: oral acyclovir 800 mg in adults, 18 mg/kg children, 5 times per day | |||||
| Serostatus: CMV R+ | Arm B: IV ganciclovir 5 mg/kg every weekday until d +100 | |||||
| All patients received IV immunoglobulin 500 mg/kg on days −6, 0, 7, 21, 35, 55, 76, 98 | ||||||
| 28 | 2003 | Randomized trial, parallel groups | Transplantation type: allogeneic related or unrelated | All patients received IV acyclovir 500 mg/m2 every 8 h from d 0 to engraftment (ANC ≥750 cells/µL for 2 d); patients then randomized 1:1 into 2 arms | Treatment of CMV infection or disease at discretion of site investigators | Incidences of CMV infection and CMV disease, incidence of neutropenia, survival |
| Age: ≥13 y | Arm A: oral valacyclovir 2 g every 6 h from engraftment to d +100 | |||||
| Serostatus: CMV R+ | ||||||
| Arm B: IV ganciclovir 5 mg/kg twice daily for 1 wk, then 6 mg/kg once daily 5 d per week | ||||||
| Doses reduced for patients with renal dysfunction | ||||||
| 27 | 2004 | Randomized trial, parallel groups | Transplantation type: allogeneic | IV ganciclovir 5 mg/kg twice daily from d −8 to d −1 for CMV R+ | No additional management specified; patients who stopped oral ganciclovir early treated with IV ganciclovir | Incidences of CMV infection, CMV disease |
| Serostatus: CMV R+ or D+ | Acyclovir 400 mg orally or 250 mg IV every 8 h for HSV R+ | |||||
| Randomized at engraftment 1:1 (ANC 500/µL and unsupported platelet recovery >20 × 109/L) | ||||||
| Arm A: IV ganciclovir 5 mg/kg 3 times weekly to d +84 | ||||||
| Arm B: oral ganciclovir 1 g every 8 h to d +84 | ||||||
| 22 | 2006 | Randomized, double blind, placebo controlled, stratified by presence of GVHD | Transplantation type: allogeneic | All patients seropositive to HSV given prophylactic acyclovir 250 mg/m2 every 12 h from d −7 to d +30 | CMV-specific management not mentioned | VZV disease at 1 y after transplantation, VZV infection after 1 y, HSV infection, CMV disease, toxicity, survival, VZV- and HSV-specific immune reconstitutions |
| Age: ≥10 y | Patients randomized 1:1 oral acyclovir 800 mg twice daily and started study drug between d 30 and d 100 and continued until 1 y after transplantation | |||||
| Inclusion: history of chicken pox | ||||||
| 29 | 2008 | Randomized, double blind, placebo controlled, stratified by transplantation type, sequential dose-escalation groups | Transplantation type: allogeneic | Randomized 3:1 to maribavir or placebo, dosing began at engraftment, d 14-30 after transplantation | Upon detection of CMV infection or diagnosis of CMV disease, study drug discontinued, and patients treated with anti-CMV antiviral at discretion of site investigators | Incidence and time of onset of CMV infection or CMV disease, incidence of CMV disease alone, use of preemptive antiviral therapy |
| Age: ≥18 y | Arm A: maribavir 100 mg twice daily | |||||
| Serostatus: CMV R+ | Arm B: maribavir 400 mg daily | |||||
| Arm C: maribavir 400 mg twice daily | ||||||
| Arm D: matching placebo twice daily, pooled from each dose level | ||||||
| 34 | 2011 | Randomized, double blind, placebo controlled, stratified by CMV serostatus and conditioning regimen, parallel groups | Transplantation type: allogeneic | Randomized 2:1 to maribavir or placebo 100 mg twice daily | Upon detection of CMV infection or diagnosis of CMV disease, study drug discontinued, and patients treated with anti-CMV antiviral at discretion of site investigators | Incidence of CMV disease through wk 24 after transplantation, time to onset of CMV disease, use of preemptive therapy, incidence of CMV infection, time to onset of CMV infection by antigenemia and PCR |
| Age: ≥18 y | Treatment had to start after engraftment and continue for up to 12 wk of treatment | |||||
| Serostatus: CMV D+ or R+ | ||||||
| 33 | 2013 | Randomized, double blind, placebo controlled, sequential dose-escalation groups | Transplantation type: allogeneic | Patients randomized 3:1 to brincidofovir (CMX001) or matching placebo; randomization and dosing initiated after engraftment, 14-30 d after transplantation | Preemptive therapy per study center standards | Failure to prevent progressive CMV infection: occurrence of CMV infection or increase in plasma CMV DNA level; rates of and reasons for discontinuation of study drug, use of preemptive therapy, trough levels of brincidofovir and cidofovir |
| Age: ≥18 y | Arm A: brincidofovir 40 mg weekly | |||||
| Serostatus: CMV R+ | Arm B: brincidofovir 100 mg weekly | |||||
| Arm C: brincidofovir 200 mg weekly | ||||||
| Arm D: brincidofovir 200 mg twice weekly | ||||||
| Arm E: brincidofovir 100 mg twice weekly | ||||||
| Arm F: matching placebo, pooled from each dose level | ||||||
| Treated for 9-11 wk depending on day of randomization after transplantation until wk 13 posttransplantation | ||||||
| 35 | 2014 | Randomized, double blind, placebo controlled, sequential dose-escalation groups | Transplantation type: allogeneic | Randomized 3:1 to letermovir or matching placebo; randomization and dosing began at engraftment between d 0 and d +40 after transplantation | Upon detection of active viral replication, study drug was discontinued and an alternative drug was initiated per study center standards | Incidence and time to onset of all-cause failure of prophylaxis against CMV infection; incidence and time of onset of CMV end-organ disease; detection of CMV replication; and discontinuation of study drug during the 12-wk period |
| Age: ≥18 y | Arm A: letermovir 60 mg daily | |||||
| Serostatus: CMV R+ | Arm B: letermovir 120 mg daily | |||||
| Arm C: letermovir 240 mg daily | ||||||
| Arm D: matching placebo daily | ||||||
| 32 | 2016 | Randomized, double blind, placebo controlled, stratified by CMV infection risk and center, parallel groups | Transplantation type: allogeneic | Randomized 2:1 to brincidofovir 100 mg twice weekly or matching placebo; treatment started between d 0 and d +28 independent of engraftment, continued until d +100 (wk 14 after transplantation) | Preemptive treatment per study center standards | Incidence of clinically significant CMV infection (defined as CMV disease or initiation of preemptive therapy) through wk 24 after transplantation; discontinuation for any reason or missing data on wk 24 considered event |
| Age: ≥18 y | Study requested CMV VL threshold of 1000 copies/mL to start preemptive therapy | |||||
| Serostatus: CMV R+ | ||||||
| 31 | 2017 | Randomized, double blind, placebo controlled, stratified by CMV risk and center, parallel groups | Type of transplantation: allogeneic | Randomized 2:1 to letermovir 480 mg per d (240 mg per d with concomitant cyclosporine use) or matching placebo | Preemptive treatment per study center standards | Incidence of clinically significant CMV infection (defined as CMV disease or initiation of preemptive therapy through wk 24 after transplantation); discontinuation for any reason or missing data on wk 24 considered event |
| Age: ≥18 y | Treatment started between d 0 and d +28 independent of engraftment, continued until d +100 (wk 14 after transplantation) | Study suggested CMV VL thresholds to initiate preemptive therapy: >150 copies/mL for high-risk patients, >300 copies/mL for low-risk patients | Proportion and time to clinically significant CMV infection through wk 14 posttransplantation; all-cause mortality through wk 24 | |||
| Serostatus: CMV R+ |
| Study . | Year . | Methods . | Participant characteristics . | Study intervention . | SOC CMV management . | Outcomes measured . |
|---|---|---|---|---|---|---|
| 19 | 1981 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: allogeneic (16), autologous (3), syngeneic (1) | Patients randomized 1:1 to IV acyclovir 250 mg/m2 every 8 h or placebo from d −3 for total of 18 d | No additional management specified | HSV Infection, CMV infection |
| Age range: 6-51 y | ||||||
| Inclusion: HSV antibody titers ≥1:8 | ||||||
| 17 | 1983 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: matched RD or syngeneic | Patients randomized 1:1 to oral acyclovir 200 mg every 6 h or placebo from d −8 to d +35 | No additional management specified | HSV infection, CMV infection, effect of acyclovir on GVHD and survival |
| 18 | 1983 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: allogeneic matched or mismatched RD | Patients randomized 1:1 to IV acyclovir 5 mg/kg every 12 h or placebo during neutropenia (ANC <1 × 109/L) | No additional management specified | Incidence of HSV, incidence of CMV infection or disease, days of neutropenia |
| Serostatus: HSV R+ | ||||||
| 20 | 1986 | Randomized, double blind, placebo controlled, parallel groups, stratified by HSV serostatus | Transplantation type: allogeneic marrow transplantation | Patients randomized 1:1 to IV acyclovir 250 mg/m2 twice a day from d −5 to d +35, transitioned to oral acyclovir 400 mg every 8 h until d +180 | No additional management specified | Incidences of HSV infection, VZV infection, CMV infection |
| Age: ≥2 y | ||||||
| 25 | 1993 | Randomized, double blind, placebo controlled, parallel groups | Transplantation type: allogeneic marrow transplantation using total-body irradiation or busulfan-cyclophosphamide | Patients randomized 1:1 to IV ganciclovir 5 mg/kg twice daily or placebo beginning at engraftment for 5 d, then 5 mg/kg per day to d +100 | Upon positive viral cultures, patients removed from blinded study drug and treated with IV ganciclovir | Incidences of CMV infection, CMV disease, neutropenia |
| Age: ≥2 y | ||||||
| Serostatus: CMV R+ | ||||||
| 26 | 1993 | Randomized, double blind, parallel groups, placebo controlled | Transplantation type: allogeneic | Patients randomized 1:1 to IV ganciclovir 2.5mg/kg every 8 h from d −7 to d −1, then from engraftment (ANC ≥1 × 109/L) to d +120 6 mg/kg per day Mondays through Fridays, or placebo | Upon diagnosis of clinical CMV disease, patients removed from blinded study and treated with IV ganciclovir | CMV infection, CMV disease, study drug toxicity |
| Age: ≥12 y | Drug held for ANC <1 × 109/L, resumed at 6 mg/kg per d 3 times per wk | |||||
| Serostatus: CMV R+ | ||||||
| 21 | 1994 | Randomized, double blind, double dummy, 3 parallel groups | Transplantation type: HLA matched related or unrelated allogeneic | Patients randomized 1:1:1 to 1 of 3 arms | CMV disease or CMV infection treated at discretion of site investigator | Time to CMV infection, survival, time to CMV viremia |
| Age: ≥2 y | Arm A: IV acyclovir 500 mg/m2 every 8 h from d −5 to d +30, followed by oral acyclovir 800 mg every 6 h until 6 mo posttransplantation, adjusted to renal function | |||||
| Serostatus: CMV R+ or D+ | Arm B: IV acyclovir like arm A, placebo until 6 mo posttransplantation | |||||
| Arm C: 400 mg oral acyclovir every 6 h from d −5 to d +30, then placebo | ||||||
| 30 | 1996 | Randomized, double blind, parallel groups | Transplantation type: all allogeneic | Patients randomized 1:1 to IV ganciclovir 5 mg/kg twice daily or placebo beginning at engraftment for 5 d, then 5 mg/kg per day 6 d per wk to d +100 | If antigenemia or CMV viremia detected, study drug stopped, and IV ganciclovir administered | CMV disease, neutropenia |
| Age: all ages | Patients underwent CMV antigenemia testing and treated with IV ganciclovir with antigenemia of ≥3 cells per 2 slides | |||||
| Serostatus: CMV R+ | ||||||
| 23 | 2002 | Randomized, double blind, parallel groups | Transplantation type: allogeneic matched or mismatched, related or unrelated | All patients treated with IV acyclovir 500 mg/m2 every 8 h from d −5 to d +28, then randomized 1:1 into 2 arms | Preemptive therapy with IV ganciclovir or foscarnet based on laboratory evidence of infection, according to study site usual practice | Time to detection of CMV, time to death, time to CMV viremia, time to onset of CMV disease, time to other antiviral use, time to onset of CMV infection |
| Age: ≥13 y | Arm A: oral valacyclovir 2 g every 6 h until end of wk 22 posttransplantation | |||||
| Serostatus: CMV R+ or D+ | Arm B: oral acyclovir 800 mg every 6 h until end of wk 22 posttransplantation | |||||
| All doses adjusted to creatinine clearance | ||||||
| 24 | 2002 | Randomized trial, parallel groups | Transplantation type: allogeneic related or unrelated | All patients received IV ganciclovir 5 mg/kg twice daily from d −7 to −2, followed by IV acyclovir 10 mg/kg every 8 h from d −1 to engraftment; patients then randomized 1:1 into 2 arms | Upon detection of antigenemia, prophylaxis discontinued, and patients treated preemptively with administration of IV ganciclovir or foscarnet | Incidences of CMV antigenemia and CMV disease, adverse events |
| Age range: 1-55 y | Arm A: oral acyclovir 800 mg in adults, 18 mg/kg children, 5 times per day | |||||
| Serostatus: CMV R+ | Arm B: IV ganciclovir 5 mg/kg every weekday until d +100 | |||||
| All patients received IV immunoglobulin 500 mg/kg on days −6, 0, 7, 21, 35, 55, 76, 98 | ||||||
| 28 | 2003 | Randomized trial, parallel groups | Transplantation type: allogeneic related or unrelated | All patients received IV acyclovir 500 mg/m2 every 8 h from d 0 to engraftment (ANC ≥750 cells/µL for 2 d); patients then randomized 1:1 into 2 arms | Treatment of CMV infection or disease at discretion of site investigators | Incidences of CMV infection and CMV disease, incidence of neutropenia, survival |
| Age: ≥13 y | Arm A: oral valacyclovir 2 g every 6 h from engraftment to d +100 | |||||
| Serostatus: CMV R+ | ||||||
| Arm B: IV ganciclovir 5 mg/kg twice daily for 1 wk, then 6 mg/kg once daily 5 d per week | ||||||
| Doses reduced for patients with renal dysfunction | ||||||
| 27 | 2004 | Randomized trial, parallel groups | Transplantation type: allogeneic | IV ganciclovir 5 mg/kg twice daily from d −8 to d −1 for CMV R+ | No additional management specified; patients who stopped oral ganciclovir early treated with IV ganciclovir | Incidences of CMV infection, CMV disease |
| Serostatus: CMV R+ or D+ | Acyclovir 400 mg orally or 250 mg IV every 8 h for HSV R+ | |||||
| Randomized at engraftment 1:1 (ANC 500/µL and unsupported platelet recovery >20 × 109/L) | ||||||
| Arm A: IV ganciclovir 5 mg/kg 3 times weekly to d +84 | ||||||
| Arm B: oral ganciclovir 1 g every 8 h to d +84 | ||||||
| 22 | 2006 | Randomized, double blind, placebo controlled, stratified by presence of GVHD | Transplantation type: allogeneic | All patients seropositive to HSV given prophylactic acyclovir 250 mg/m2 every 12 h from d −7 to d +30 | CMV-specific management not mentioned | VZV disease at 1 y after transplantation, VZV infection after 1 y, HSV infection, CMV disease, toxicity, survival, VZV- and HSV-specific immune reconstitutions |
| Age: ≥10 y | Patients randomized 1:1 oral acyclovir 800 mg twice daily and started study drug between d 30 and d 100 and continued until 1 y after transplantation | |||||
| Inclusion: history of chicken pox | ||||||
| 29 | 2008 | Randomized, double blind, placebo controlled, stratified by transplantation type, sequential dose-escalation groups | Transplantation type: allogeneic | Randomized 3:1 to maribavir or placebo, dosing began at engraftment, d 14-30 after transplantation | Upon detection of CMV infection or diagnosis of CMV disease, study drug discontinued, and patients treated with anti-CMV antiviral at discretion of site investigators | Incidence and time of onset of CMV infection or CMV disease, incidence of CMV disease alone, use of preemptive antiviral therapy |
| Age: ≥18 y | Arm A: maribavir 100 mg twice daily | |||||
| Serostatus: CMV R+ | Arm B: maribavir 400 mg daily | |||||
| Arm C: maribavir 400 mg twice daily | ||||||
| Arm D: matching placebo twice daily, pooled from each dose level | ||||||
| 34 | 2011 | Randomized, double blind, placebo controlled, stratified by CMV serostatus and conditioning regimen, parallel groups | Transplantation type: allogeneic | Randomized 2:1 to maribavir or placebo 100 mg twice daily | Upon detection of CMV infection or diagnosis of CMV disease, study drug discontinued, and patients treated with anti-CMV antiviral at discretion of site investigators | Incidence of CMV disease through wk 24 after transplantation, time to onset of CMV disease, use of preemptive therapy, incidence of CMV infection, time to onset of CMV infection by antigenemia and PCR |
| Age: ≥18 y | Treatment had to start after engraftment and continue for up to 12 wk of treatment | |||||
| Serostatus: CMV D+ or R+ | ||||||
| 33 | 2013 | Randomized, double blind, placebo controlled, sequential dose-escalation groups | Transplantation type: allogeneic | Patients randomized 3:1 to brincidofovir (CMX001) or matching placebo; randomization and dosing initiated after engraftment, 14-30 d after transplantation | Preemptive therapy per study center standards | Failure to prevent progressive CMV infection: occurrence of CMV infection or increase in plasma CMV DNA level; rates of and reasons for discontinuation of study drug, use of preemptive therapy, trough levels of brincidofovir and cidofovir |
| Age: ≥18 y | Arm A: brincidofovir 40 mg weekly | |||||
| Serostatus: CMV R+ | Arm B: brincidofovir 100 mg weekly | |||||
| Arm C: brincidofovir 200 mg weekly | ||||||
| Arm D: brincidofovir 200 mg twice weekly | ||||||
| Arm E: brincidofovir 100 mg twice weekly | ||||||
| Arm F: matching placebo, pooled from each dose level | ||||||
| Treated for 9-11 wk depending on day of randomization after transplantation until wk 13 posttransplantation | ||||||
| 35 | 2014 | Randomized, double blind, placebo controlled, sequential dose-escalation groups | Transplantation type: allogeneic | Randomized 3:1 to letermovir or matching placebo; randomization and dosing began at engraftment between d 0 and d +40 after transplantation | Upon detection of active viral replication, study drug was discontinued and an alternative drug was initiated per study center standards | Incidence and time to onset of all-cause failure of prophylaxis against CMV infection; incidence and time of onset of CMV end-organ disease; detection of CMV replication; and discontinuation of study drug during the 12-wk period |
| Age: ≥18 y | Arm A: letermovir 60 mg daily | |||||
| Serostatus: CMV R+ | Arm B: letermovir 120 mg daily | |||||
| Arm C: letermovir 240 mg daily | ||||||
| Arm D: matching placebo daily | ||||||
| 32 | 2016 | Randomized, double blind, placebo controlled, stratified by CMV infection risk and center, parallel groups | Transplantation type: allogeneic | Randomized 2:1 to brincidofovir 100 mg twice weekly or matching placebo; treatment started between d 0 and d +28 independent of engraftment, continued until d +100 (wk 14 after transplantation) | Preemptive treatment per study center standards | Incidence of clinically significant CMV infection (defined as CMV disease or initiation of preemptive therapy) through wk 24 after transplantation; discontinuation for any reason or missing data on wk 24 considered event |
| Age: ≥18 y | Study requested CMV VL threshold of 1000 copies/mL to start preemptive therapy | |||||
| Serostatus: CMV R+ | ||||||
| 31 | 2017 | Randomized, double blind, placebo controlled, stratified by CMV risk and center, parallel groups | Type of transplantation: allogeneic | Randomized 2:1 to letermovir 480 mg per d (240 mg per d with concomitant cyclosporine use) or matching placebo | Preemptive treatment per study center standards | Incidence of clinically significant CMV infection (defined as CMV disease or initiation of preemptive therapy through wk 24 after transplantation); discontinuation for any reason or missing data on wk 24 considered event |
| Age: ≥18 y | Treatment started between d 0 and d +28 independent of engraftment, continued until d +100 (wk 14 after transplantation) | Study suggested CMV VL thresholds to initiate preemptive therapy: >150 copies/mL for high-risk patients, >300 copies/mL for low-risk patients | Proportion and time to clinically significant CMV infection through wk 14 posttransplantation; all-cause mortality through wk 24 | |||
| Serostatus: CMV R+ |
ANC, absolute neutrophil count; D, donor; GVHD, graft-versus-host disease; HSV, herpes simplex virus; PCR, polymerase chain reaction; R, recipient; RD, related donor; SOC, standard of care; VZV, varicella zoster virus.
Risk of bias in included studies
Four studies17-20 were judged to be at high risk of reporting bias because of incomplete reporting of outcome measures. The full summary and graphs for risk of bias can be found in supplemental Figures 1 and 2. The overall quality of the studies was good. Allocation bias was the most common risk identified, because most studies did not report their allocation or randomization methods.20-30 Four studies randomly assigned patients using a form of interactive voice or Web response system.31-35 One study randomly assigned patients using a computer-generated table of random numbers.19 Fourteen of 19 studies were double blinded,18-20,22,23,25,26,29-35 whereas 5 studies did not mention blinding and were assumed to be open label.17,21,24,27,28 Because adverse event reporting can be biased in open-label studies, these studies should be judged carefully in their reporting of drug-related toxicity. A minority of studies had incomplete outcome data.17,18,27 One study did not have results on CMV infection rate.22 Three studies lacked results on CMV disease in treatment or control arm as well as adverse event or toxicity data17,18,20 (Tables 1 and 2).
Summary of adverse events
| Study . | Study characteristics . | Serious adverse event incidence . | Most common drug-related adverse events . | |||
|---|---|---|---|---|---|---|
| Experimental intervention . | Control intervention . | Experimental arm . | Control arm . | Experimental arm . | Control arm . | |
| 19 | IV acyclovir | Placebo | Not provided | Not provided | Serum AST > twice normal (60%) | Serum AST > twice normal (50%) |
| 17 | Oral acyclovir | Placebo | Not provided | Not provided | Not provided | Not provided |
| 18 | IV acyclovir | Placebo | Not provided | Not provided | Nausea and vomiting (50%) | Nausea and vomiting (80%) |
| 20 | IV acyclovir | Placebo | Not provided | Not provided | Not provided | Not provided |
| 25 | IV ganciclovir | Placebo | Not provided | Not provided | Neutropenia (30%) | Neutropenia (0%) |
| 26 | IV ganciclovir | Placebo | Not provided | Not provided | Neutropenia requiring treatment interruption (58%) | Neutropenia requiring treatment interruption (28%) |
| 21 | IV acyclovir extended | Oral acyclovir | Not provided | Not provided | Nausea (4%-8%)* | Nausea (4%) |
| IV acyclovir short course | Vomiting (3%) | Vomiting (3%) | ||||
| Renal failure (7%-13%)* | Renal failure (9%) | |||||
| 30 | IV ganciclovir | Placebo | Not provided | Not provided | Neutropenia (25%) | Neutropenia (32%) |
| Any bacterial infection (45%) | Any bacterial infection (40%) | |||||
| Invasive fungal disease (16%) | Invasive fungal disease (6%) | |||||
| 23 | Valacyclovir | Oral acyclovir | Not provided | Not provided | Nausea (24%) | Nausea (21%) |
| Vomiting (22%) | Vomiting (22%) | |||||
| Abdominal pain (19%) | Abdominal pain (16%) | |||||
| Diarrhea (19%) | Diarrhea (21%) | |||||
| 24 | IV ganciclovir | Oral acyclovir | Not provided | Not provided | Secondary neutropenia (62%) | Secondary neutropenia (39%) |
| Renal insufficiency (38%) | Renal insufficiency (33%) | |||||
| 28 | Valacyclovir | IV ganciclovir | Not provided | Not provided | Neutropenia (32%) | Neutropenia (13%) |
| Renal insufficiency (6%) | Renal insufficiency (10%) | |||||
| Nausea, vomiting (5%) | Nausea, vomiting (8%) | |||||
| 27 | IV ganciclovir | Oral ganciclovir | Not provided | Not provided | Not provided | Not provided |
| 22 | Oral acyclovir | Placebo | Not provided | Not provided | Gastrointestinal effects (8%) | Neutropenia (3%) |
| Pancytopenia (3%) | ||||||
| Increased liver function tests (3%) | ||||||
| 29 | Maribavir 100 mg twice daily | Placebo | Not provided | Not provided | Taste disturbance (18%-31%)* | Taste disturbance (0%) |
| Maribavir 400 mg daily | Placebo | Nausea (7%-15%)* | Nausea (0%) | |||
| Maribavir 400 mg twice daily | Placebo | Vomiting (4%-11%)* | Vomiting (4%) | |||
| 34 | Maribavir 100 mg twice daily | Placebo | 197/451 (44%) | 98/223 (44%) | Acute GVHD (36%) | Acute GVHD (33%) |
| Diarrhea (21%) | Diarrhea (19%) | |||||
| Fatigue (16%) | Fatigue (10%) | |||||
| 33 | Brincidofovir 40 mg weekly | Placebo | 12/25 (48%) | 27/59 (46%) | Diarrhea (12%-70%)* | Diarrhea (27%) |
| Brincidofovir 100 mg weekly | Placebo | 10/27 (37%) | Vomiting (8%-44%)* | Abdominal pain (7%) | ||
| Brincidofovir 200 mg weekly | Placebo | 19/39 (49%) | Acute GVHD (32%-80%)* | Acute GVHD (29%) | ||
| Brincidofovir 200 mg biweekly | Placebo | 21/30 (70%) | ||||
| Brincidofovir 100 mg biweekly | Placebo | 30/50 (60%) | ||||
| 35 | Letermovir 60 mg daily | Placebo | 9/33 (27%) | 12/33 (36%) | Gastrointestinal disorders (52%-76%)* | Gastrointestinal disorders (61%) |
| Letermovir 120 mg daily | Placebo | 12/31 (39%) | Infections (52%-59%)* | Infections (76%) | ||
| Letermovir 240 mg daily | Placebo | 9/34 (26%) | Skin and cutaneous tissue disorders (39%-55%)* | Skin and cutaneous tissue disorders (33%) | ||
| 32 | Brincidofovir 100 mg biweekly | Placebo | 173/303 (57%) | 56/149 (38%) | Diarrhea (61%) | Diarrhea (36%) |
| Acute GVHD (57%) | Acute GVHD (32%) | |||||
| Abdominal pain (34%) | Abdominal pain (17%) | |||||
| 31 | Letermovir 480 mg daily | Placebo | 193/373 (52%) | 109/192 (57%) | GVHD (39%) | GVHD (39%) |
| Diarrhea (26%) | Diarrhea (25%) | |||||
| Nausea (27%) | Nausea (23%) | |||||
| Study . | Study characteristics . | Serious adverse event incidence . | Most common drug-related adverse events . | |||
|---|---|---|---|---|---|---|
| Experimental intervention . | Control intervention . | Experimental arm . | Control arm . | Experimental arm . | Control arm . | |
| 19 | IV acyclovir | Placebo | Not provided | Not provided | Serum AST > twice normal (60%) | Serum AST > twice normal (50%) |
| 17 | Oral acyclovir | Placebo | Not provided | Not provided | Not provided | Not provided |
| 18 | IV acyclovir | Placebo | Not provided | Not provided | Nausea and vomiting (50%) | Nausea and vomiting (80%) |
| 20 | IV acyclovir | Placebo | Not provided | Not provided | Not provided | Not provided |
| 25 | IV ganciclovir | Placebo | Not provided | Not provided | Neutropenia (30%) | Neutropenia (0%) |
| 26 | IV ganciclovir | Placebo | Not provided | Not provided | Neutropenia requiring treatment interruption (58%) | Neutropenia requiring treatment interruption (28%) |
| 21 | IV acyclovir extended | Oral acyclovir | Not provided | Not provided | Nausea (4%-8%)* | Nausea (4%) |
| IV acyclovir short course | Vomiting (3%) | Vomiting (3%) | ||||
| Renal failure (7%-13%)* | Renal failure (9%) | |||||
| 30 | IV ganciclovir | Placebo | Not provided | Not provided | Neutropenia (25%) | Neutropenia (32%) |
| Any bacterial infection (45%) | Any bacterial infection (40%) | |||||
| Invasive fungal disease (16%) | Invasive fungal disease (6%) | |||||
| 23 | Valacyclovir | Oral acyclovir | Not provided | Not provided | Nausea (24%) | Nausea (21%) |
| Vomiting (22%) | Vomiting (22%) | |||||
| Abdominal pain (19%) | Abdominal pain (16%) | |||||
| Diarrhea (19%) | Diarrhea (21%) | |||||
| 24 | IV ganciclovir | Oral acyclovir | Not provided | Not provided | Secondary neutropenia (62%) | Secondary neutropenia (39%) |
| Renal insufficiency (38%) | Renal insufficiency (33%) | |||||
| 28 | Valacyclovir | IV ganciclovir | Not provided | Not provided | Neutropenia (32%) | Neutropenia (13%) |
| Renal insufficiency (6%) | Renal insufficiency (10%) | |||||
| Nausea, vomiting (5%) | Nausea, vomiting (8%) | |||||
| 27 | IV ganciclovir | Oral ganciclovir | Not provided | Not provided | Not provided | Not provided |
| 22 | Oral acyclovir | Placebo | Not provided | Not provided | Gastrointestinal effects (8%) | Neutropenia (3%) |
| Pancytopenia (3%) | ||||||
| Increased liver function tests (3%) | ||||||
| 29 | Maribavir 100 mg twice daily | Placebo | Not provided | Not provided | Taste disturbance (18%-31%)* | Taste disturbance (0%) |
| Maribavir 400 mg daily | Placebo | Nausea (7%-15%)* | Nausea (0%) | |||
| Maribavir 400 mg twice daily | Placebo | Vomiting (4%-11%)* | Vomiting (4%) | |||
| 34 | Maribavir 100 mg twice daily | Placebo | 197/451 (44%) | 98/223 (44%) | Acute GVHD (36%) | Acute GVHD (33%) |
| Diarrhea (21%) | Diarrhea (19%) | |||||
| Fatigue (16%) | Fatigue (10%) | |||||
| 33 | Brincidofovir 40 mg weekly | Placebo | 12/25 (48%) | 27/59 (46%) | Diarrhea (12%-70%)* | Diarrhea (27%) |
| Brincidofovir 100 mg weekly | Placebo | 10/27 (37%) | Vomiting (8%-44%)* | Abdominal pain (7%) | ||
| Brincidofovir 200 mg weekly | Placebo | 19/39 (49%) | Acute GVHD (32%-80%)* | Acute GVHD (29%) | ||
| Brincidofovir 200 mg biweekly | Placebo | 21/30 (70%) | ||||
| Brincidofovir 100 mg biweekly | Placebo | 30/50 (60%) | ||||
| 35 | Letermovir 60 mg daily | Placebo | 9/33 (27%) | 12/33 (36%) | Gastrointestinal disorders (52%-76%)* | Gastrointestinal disorders (61%) |
| Letermovir 120 mg daily | Placebo | 12/31 (39%) | Infections (52%-59%)* | Infections (76%) | ||
| Letermovir 240 mg daily | Placebo | 9/34 (26%) | Skin and cutaneous tissue disorders (39%-55%)* | Skin and cutaneous tissue disorders (33%) | ||
| 32 | Brincidofovir 100 mg biweekly | Placebo | 173/303 (57%) | 56/149 (38%) | Diarrhea (61%) | Diarrhea (36%) |
| Acute GVHD (57%) | Acute GVHD (32%) | |||||
| Abdominal pain (34%) | Abdominal pain (17%) | |||||
| 31 | Letermovir 480 mg daily | Placebo | 193/373 (52%) | 109/192 (57%) | GVHD (39%) | GVHD (39%) |
| Diarrhea (26%) | Diarrhea (25%) | |||||
| Nausea (27%) | Nausea (23%) | |||||
AST, aspartate aminotransferase.
Range provided for different treatment arms and dose levels.
Effects of interventions
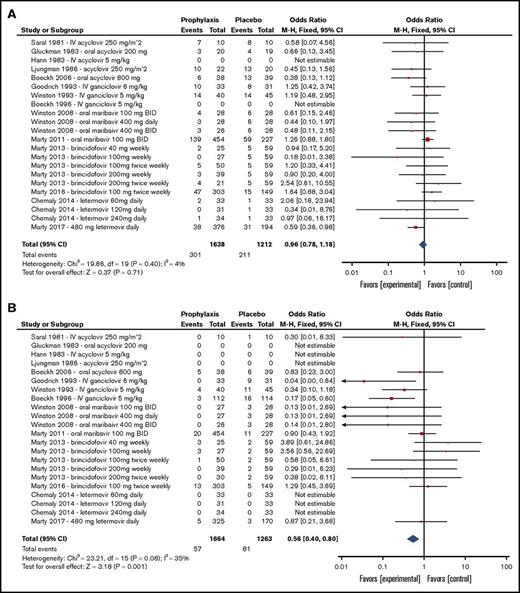
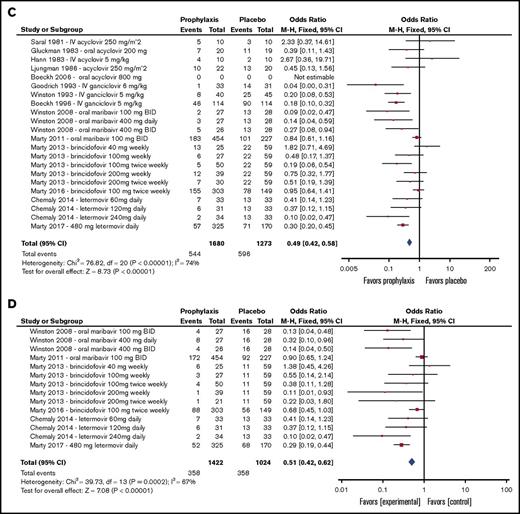
Because of changing CMV detection standards, the approach to CMV management has evolved over time. Before 2002, a majority of CMV primary infection, reactivation, and disease diagnoses were based on CMV culture. Around 2004, trial diagnostic methods shifted toward culture-independent techniques, including antigenemia and polymerase chain reaction (supplemental Table 3). Supplemental Table 2 summarizes each CMV detection technique used by each study. For phase 2 dose-ranging studies, each dose level is presented separately. Forest plots summarizing all-cause mortality, CMV disease, CMV infection, and preemptive therapy incidences to the predefined end point periods of the individual trials that compared antivirals with placebo are presented in Figure 3. Overall, the 14 trials that compared antiviral prophylaxis with placebo demonstrated effectiveness in reducing incident CMV infection (odds ratio [OR], 0.49; 95% confidence interval [CI], 0.42-0.58), CMV disease (OR, 0.56; 95% CI, 0.40-0.80), and use of preemptive therapy (OR, 0.51; 95% CI, 0.42-0.62; 6 trials) but not all-cause mortality (OR, 0.96; 95% CI, 0.78-1.18). The most relevant toxicity and adverse event data are shown in Table 2.
Forest plots summarizing outcomes of trials of antiviral prophylaxis vs placebo in HCT patients. (A) All-cause mortality. (B) CMV disease. (C) CMV infection (reactivation). (D) Preemptive therapy. BID, twice per day; df, degree of freedom.
Forest plots summarizing outcomes of trials of antiviral prophylaxis vs placebo in HCT patients. (A) All-cause mortality. (B) CMV disease. (C) CMV infection (reactivation). (D) Preemptive therapy. BID, twice per day; df, degree of freedom.
Acyclovir
The first antiviral studied for universal prophylaxis against CMV was acyclovir, with a total of 8 studies published from 1981 to 2006; some studies did not report enrollment periods.17-24 Five studies were placebo controlled, and 1 study each compared acyclovir with IV ganciclovir, IV acyclovir, and oral valacyclovir. These studies had a total of 1347 participants and initiated prophylaxis between days −8 to −3 before transplantation. Treatment continued for a median time of 102 days (range, 18-216 days), and follow-up continued for a median time of 69 days (range, 0-335 days). The overall results were mixed (Figure 3) and suggested that acyclovir was associated with low toxicity after allogeneic HCT (Table 2). However, although a delay in the onset of CMV reactivation was demonstrated, acyclovir showed nonsignificant efficacy in preventing CMV disease (Figure 3; supplemental Figure 3).
Ganciclovir
The next antiviral agent studied was ganciclovir, with 5 reported studies spanning from 1990 to 1998.25-28,30 Three studies compared IV ganciclovir against placebo, 1 compared IV ganciclovir against valacyclovir, and 1 compared oral ganciclovir against IV ganciclovir (supplemental Figure 4). A total of 647 participants were included in these studies. Median treatment time was 101 days (range, 84-128 days), and median follow-up time was 80 days after treatment (range, 0-265 days). These studies suggested that ganciclovir was effective in reducing incidence of CMV infection and CMV disease after allogeneic HCT but was not associated with a reduction in all-cause mortality, likely secondary to drug discontinuation related to clinically significant myelosuppression (Figure 3; supplemental Figure 4; Table 2).
Maribavir
In the last 10 years, 3 novel antivirals have emerged as potential CMV prophylactic candidates and have been studied in an era of highly sensitive molecular testing. Two studies involved maribavir, which is a UL97 viral protein kinase inhibitor that prevents nuclear egress of CMV virions.36,37 The studies included 792 HCT participants recruited from 2004 to 2008.29,34 Median treatment time was 84 days (range, 1-92 days), and median follow-up time after treatment was 147 days (range, 56-238 days). Although a dose-escalation phase 2 trial had demonstrated antiviral activity at doses ranging from 100 to 400 mg twice daily,29 the results of the phase 3 trial demonstrated that maribavir at 100 mg twice daily started after engraftment had no significant effect on incidence of CMV disease, CMV reactivation, or preemptive therapy for CMV after allogeneic HCT compared with placebo by HCT week 24 and had no statistically significant effect on mortality (Figure 3).34,38 Maribavir was largely well tolerated; the proportion of patients with adverse events leading to study drug discontinuation and serious adverse events was largely the same between maribavir and placebo arms. However, patients receiving 400 mg of maribavir twice daily in the phase 2 trial experienced increased rates of nausea and taste disturbance (Table 2).29
Brincidofovir
Brincidofovir (CMX001) is an oral lipid conjugate formulation of cidofovir and was recently evaluated in 2 randomized placebo-controlled studies for prevention of CMV infection in HCT recipients from 2009 to 2015.32,33 In total, 682 participants were treated for a median duration of 66.5 days (range, 1-99 days) and followed for a median time of 71 days (range, 70-72 days) after treatment. Although a phase 2 dose-ranging trial demonstrated significantly lower CMV events with brincidofovir at a dose of 100 mg twice weekly and a treatment completion rate of 60% when started after engraftment through week 13 post-HCT,33 brincidofovir did not improve CMV-related outcomes in the phase 3 trial that evaluated treatment at 100 mg twice weekly against placebo beginning a median of 15 days post-HCT (Figure 3), with a low completion rate (38%).32 Furthermore, brincidofovir was associated with increased rates of diarrhea, acute GVHD with gastrointestinal involvement, other gastrointestinal adverse events, and a nonsignificant increased risk of death when compared with placebo (Table 2).32,33
Letermovir
Letermovir is an antiviral agent with a novel mechanism of action involving inhibition of the human CMV terminase complex.39-43 It was studied in 2 randomized placebo-controlled studies for CMV prophylaxis from 2010 to 2016 with a total of 686 HCT participants.31,35 In these studies, median treatment time was 77.5 days (range, 1-113 days), and median follow-up time was 122.5 days (range, 7-238 days). Among patients undergoing allogeneic HCT, letermovir at a dose of 480 mg per day (or 240 mg per day when administered concomitantly with cyclosporine) was found to significantly reduce CMV reactivation, use of preemptive anti-CMV therapy, and all-cause mortality by week 24 posttransplantation (Figure 3). Letermovir had a favorable adverse event profile and high treatment completion rate (71%) despite being started preengraftment in a majority of patients (Table 2). Reduction in CMV reactivation and mortality was prominent in patients at higher risk of CMV reactivation and CMV disease, including those undergoing haploidentical HCT or mismatched-donor HCT and those receiving antithymocyte globulin. All-cause mortality was nonsignificantly lower in patients who received letermovir compared with placebo by week 48. The results of this trial led to regulatory approvals by the US Food and Drug Administration, European Medicines Agency, and Health Canada in late 2017.44-46
Letermovir is not myelosuppressive and is available in oral and IV formulations, allowing treatment to start a median of 9 days after HCT in the phase 3 trial. Letermovir is excreted by the liver and does not require dose adjustments based on renal or hepatic function except in patients with advanced cirrhosis (Child-Pugh class C).44,47 Although pharmacokinetic studies have found that letermovir increased exposure to certain drugs,44 including atorvastatin, tacrolimus, sirolimus, midazolam, and other medications that may require dosing adjustments, letermovir itself only required dose adjustment (50% reduction) when administered with cyclosporine.48 Detailed letermovir characteristics are presented in Table 3.
Letermovir characteristics and clinical guide
| Characteristic . | Key information . | Practical recommendations by authors . |
|---|---|---|
| Chemical name | (4S)-2-{8-fluoro-2-[4-(3-methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-3,4-dihydroquinazolin-4-yl}acetic acid | — |
| Chemical structure |  | — |
| Other names | BAY-73-6327, AIC-090027, AIC-246, MK-8228, Prevymis | — |
| Mechanism of action | Inhibition of human CMV terminase complex (UL51, UL56, UL89) by binding to UL56, UL51, or both39-41,67 | — |
| Antiviral activity | Active against human CMV; median EC50 2.1 nM (range, 0.7-6.1 nM) against all CMV gB genotypes44,68 | Patients should receive antiviral prophylaxis against HSV and VZV with acyclovir, valacyclovir, or famciclovir as clinically indicated31 |
| No activity against other herpesviruses | ||
| No antagonism when combined with CMV DNA polymerase inhibitor ganciclovir, foscarnet, or cidofovir69 | ||
| Letermovir resistance | UL56 V236M clinically resistant mutant has been identified in patients in phase 235,70 and phase 3 trials31,49 ; UL56 C325W breakthrough mutant was also identified in phase 3 trial in patient who began treatment with letermovir with detectable plasma CMV DNA (not part of primary efficacy population)43,44 | Letermovir resistance testing is available in research71,72 and reference73 laboratories. |
| UL56 V236M and other letermovir-associated UL5671,72,74,75 and UL5167 mutations have been identified in vitro | ||
| Approved indication | Prophylaxis of CMV infection and disease in adult CMV-seropositive recipients of allogeneic HCT44-46 | Phase 3 trial comparing valganciclovir vs letermovir for CMV prophylaxis in CMV donor–seropositive, CMV-seronegative kidney transplant recipients is ongoing76 |
| Letermovir has been used for secondary CMV prophylaxis in solid organ transplant recipients with ganciclovir-resistant infections77 | ||
| Formulations | Tablets: 240 and 480 mg | In HCT clinical trial, 26% of patients received IV letermovir for median of 12 d,31 usually in setting of mucositis or other gastrointestinal issues that precluded oral administration; given higher bioavailability of IV letermovir, consider starting treatment with IV formulation in similar situations |
| IV: 240 mg per 12 mL and 480 mg per 24 mL in single-dose vials (20 mg/mL) dissolved in hydroxypropyl-β-cyclodextrin, at ratio of 1800 mg per 240 mg of letermovir31,78 | Although IV administration of letermovir is advisable for patients who cannot take oral tablets, and letermovir package insert does not recommend it because of lack of data,44 we have been successful in administering crushed letermovir tablets via gastrostomy tube in the outpatient setting | |
| Dosage | 480 mg per d | Letermovir exposure in patients receiving 240 mg per d of letermovir and concomitant cyclosporine was ∼50% higher than in patients who received 480 mg per d of letermovir without concomitant cyclosporine use31 |
| 240 mg per d when coadministered with cyclosporine31,48 | ||
| Bioavailability | 35% of 480 mg per d dose without cyclosporine use31 | Letermovir can be taken with or without food |
| 85% of 240 mg per d dose with cyclosporine use31 | No plasma letermovir concentration measurements available in reference laboratories to date | |
| No appreciable food effect; t1/2 11 to 18 h79 | ||
| Metabolism | Hepatic uptake via OATP1B1/3, 93% excreted in feces, 70% unchanged; highly protein bound in plasma (99%)44 | These metabolism pathways should be taken into consideration when administering concomitant medications not mentioned below |
| Substrate of metabolizing enzymes CYP3A, CYP2D6, UGT1A1, UGT1A3; transporters OATP1B1/3 and P-gp.44 | ||
| Drug interactions | Drug-drug interactions are likely more intense if using concomitant cyclosporine | Empirical dose adjustment of tacrolimus or sirolimus used for GVHD prophylaxis usually depends on drug levels when letermovir is started, as well as desired target trough levels; in our experience, in patients with low levels at beginning of coadministration (≤4 ng/mL), tacrolimus and sirolimus levels can be monitored without empirical adjustments; for patients with levels >8 ng/mL, empirical reductions of 50% and monitoring of drug levels are advisable |
| Via CYP3A inhibition (weak to moderate) | ||
| Increased levels of amiodarone, antidiabetic agents (glyburide, repaglinide, rosiglitazone), fentanyl, midazolam (2.0× AUC), quinidine, sirolimus (3.0× AUC), tacrolimus (1.8× AUC)48,80 | Consider reducing dose of statins if coadministered; we favor use of pravastatin or rosuvastatin given their lack of significant CYP metabolism81 | |
| Via induction of CYP2C9/19 (weak to moderate) | ||
| Decreased levels of voriconazole (0.56× AUC),79 warfarin, phenytoin, proton pump inhibitors (omeprazole, pantoprazole) | ||
| Via OATP1B1/3 | ||
| Increased levels of statins: atorvastatin (3.0× AUC), fluvastatin, lovastatin, pravastatin, rosuvastatin82,83 | ||
| Increased levels of cyclosporine (1.7× AUC)48,80 | ||
| No significant interactions in studies with acyclovir, digoxin, mycophenolate, posaconazole,79 ethinyl estradiol, levonorgestel44 | ||
| Contraindications | Pimozide and ergotamine, because of letermovir inhibition of CYP3A and increased levels of pimozide (increased QTc) and ergot alkaloids (ergotism)44 | These are uncommonly used drugs in HCT patients |
| Pitavastatin and simvastatin if given concurrently with cyclosporine, because of increased statin levels via OATP1B1/344 | ||
| Common adverse events | Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, and abdominal pain (>10% and >2% over placebo events)31,35,44 | Consider letermovir as potential cause of persistent nausea or vomiting |
| Adverse events of note | Atrial fibrillation or flutter occurred in 4.6% of letermovir-treated patients vs 1% in those who received placebo31 ; letermovir does not prolong QTc interval31,44 | We would not start letermovir soon after conditioning regimens that involve use of CYP-metabolized drugs (eg, busulfan, cyclophosphamide); we would also withhold it during or right after posttransplantation cyclophosphamide administration to minimize risk of hepatotoxicity resulting from drug-drug and drug-metabolite interactions |
| Alanine aminotransferase levels >5× ULN were 3.5% in letermovir-treated patients vs 1.6% in those receiving placebo31 | ||
| Renal dysfunction | Increased letermovir exposure in patients with GFR <60 mL/min (< twofold)84 ; increased exposure does not require dose adjustments44 | Some caution is advised if GFR <50 mL/min and using IV formulation because of potential hydroxypropyl-β-cyclodextrin accumulation and consequent osmotic toxicity44,78 |
| No dosing recommendations for GFR <10 mL/min31,44 | The phase 3 trial enrolled patients with GFR >10 mL/min, yet no increased nephrotoxicity was observed31 | |
| No recommendation for severe renal impairment based on lack of data in this subpopulation; consider risks and benefits of letermovir prophylaxis for patients in this situation | ||
| Liver dysfunction | Patients with cirrhosis and moderate liver dysfunction (Child-Pugh class B) have < twofold higher letermovir exposures; patients with severe liver dysfunction (Child-Pugh class C) have ∼fourfold increased letermovir exposures47 | No letermovir dose adjustments are necessary for patients with cirrhosis and mild to moderate liver dysfunction (Child-Pugh class A or B), but letermovir is not recommended for cirrhotic patients with severe impairment (Child-Pugh class C)44 |
| Characteristic . | Key information . | Practical recommendations by authors . |
|---|---|---|
| Chemical name | (4S)-2-{8-fluoro-2-[4-(3-methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-3,4-dihydroquinazolin-4-yl}acetic acid | — |
| Chemical structure |  | — |
| Other names | BAY-73-6327, AIC-090027, AIC-246, MK-8228, Prevymis | — |
| Mechanism of action | Inhibition of human CMV terminase complex (UL51, UL56, UL89) by binding to UL56, UL51, or both39-41,67 | — |
| Antiviral activity | Active against human CMV; median EC50 2.1 nM (range, 0.7-6.1 nM) against all CMV gB genotypes44,68 | Patients should receive antiviral prophylaxis against HSV and VZV with acyclovir, valacyclovir, or famciclovir as clinically indicated31 |
| No activity against other herpesviruses | ||
| No antagonism when combined with CMV DNA polymerase inhibitor ganciclovir, foscarnet, or cidofovir69 | ||
| Letermovir resistance | UL56 V236M clinically resistant mutant has been identified in patients in phase 235,70 and phase 3 trials31,49 ; UL56 C325W breakthrough mutant was also identified in phase 3 trial in patient who began treatment with letermovir with detectable plasma CMV DNA (not part of primary efficacy population)43,44 | Letermovir resistance testing is available in research71,72 and reference73 laboratories. |
| UL56 V236M and other letermovir-associated UL5671,72,74,75 and UL5167 mutations have been identified in vitro | ||
| Approved indication | Prophylaxis of CMV infection and disease in adult CMV-seropositive recipients of allogeneic HCT44-46 | Phase 3 trial comparing valganciclovir vs letermovir for CMV prophylaxis in CMV donor–seropositive, CMV-seronegative kidney transplant recipients is ongoing76 |
| Letermovir has been used for secondary CMV prophylaxis in solid organ transplant recipients with ganciclovir-resistant infections77 | ||
| Formulations | Tablets: 240 and 480 mg | In HCT clinical trial, 26% of patients received IV letermovir for median of 12 d,31 usually in setting of mucositis or other gastrointestinal issues that precluded oral administration; given higher bioavailability of IV letermovir, consider starting treatment with IV formulation in similar situations |
| IV: 240 mg per 12 mL and 480 mg per 24 mL in single-dose vials (20 mg/mL) dissolved in hydroxypropyl-β-cyclodextrin, at ratio of 1800 mg per 240 mg of letermovir31,78 | Although IV administration of letermovir is advisable for patients who cannot take oral tablets, and letermovir package insert does not recommend it because of lack of data,44 we have been successful in administering crushed letermovir tablets via gastrostomy tube in the outpatient setting | |
| Dosage | 480 mg per d | Letermovir exposure in patients receiving 240 mg per d of letermovir and concomitant cyclosporine was ∼50% higher than in patients who received 480 mg per d of letermovir without concomitant cyclosporine use31 |
| 240 mg per d when coadministered with cyclosporine31,48 | ||
| Bioavailability | 35% of 480 mg per d dose without cyclosporine use31 | Letermovir can be taken with or without food |
| 85% of 240 mg per d dose with cyclosporine use31 | No plasma letermovir concentration measurements available in reference laboratories to date | |
| No appreciable food effect; t1/2 11 to 18 h79 | ||
| Metabolism | Hepatic uptake via OATP1B1/3, 93% excreted in feces, 70% unchanged; highly protein bound in plasma (99%)44 | These metabolism pathways should be taken into consideration when administering concomitant medications not mentioned below |
| Substrate of metabolizing enzymes CYP3A, CYP2D6, UGT1A1, UGT1A3; transporters OATP1B1/3 and P-gp.44 | ||
| Drug interactions | Drug-drug interactions are likely more intense if using concomitant cyclosporine | Empirical dose adjustment of tacrolimus or sirolimus used for GVHD prophylaxis usually depends on drug levels when letermovir is started, as well as desired target trough levels; in our experience, in patients with low levels at beginning of coadministration (≤4 ng/mL), tacrolimus and sirolimus levels can be monitored without empirical adjustments; for patients with levels >8 ng/mL, empirical reductions of 50% and monitoring of drug levels are advisable |
| Via CYP3A inhibition (weak to moderate) | ||
| Increased levels of amiodarone, antidiabetic agents (glyburide, repaglinide, rosiglitazone), fentanyl, midazolam (2.0× AUC), quinidine, sirolimus (3.0× AUC), tacrolimus (1.8× AUC)48,80 | Consider reducing dose of statins if coadministered; we favor use of pravastatin or rosuvastatin given their lack of significant CYP metabolism81 | |
| Via induction of CYP2C9/19 (weak to moderate) | ||
| Decreased levels of voriconazole (0.56× AUC),79 warfarin, phenytoin, proton pump inhibitors (omeprazole, pantoprazole) | ||
| Via OATP1B1/3 | ||
| Increased levels of statins: atorvastatin (3.0× AUC), fluvastatin, lovastatin, pravastatin, rosuvastatin82,83 | ||
| Increased levels of cyclosporine (1.7× AUC)48,80 | ||
| No significant interactions in studies with acyclovir, digoxin, mycophenolate, posaconazole,79 ethinyl estradiol, levonorgestel44 | ||
| Contraindications | Pimozide and ergotamine, because of letermovir inhibition of CYP3A and increased levels of pimozide (increased QTc) and ergot alkaloids (ergotism)44 | These are uncommonly used drugs in HCT patients |
| Pitavastatin and simvastatin if given concurrently with cyclosporine, because of increased statin levels via OATP1B1/344 | ||
| Common adverse events | Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, and abdominal pain (>10% and >2% over placebo events)31,35,44 | Consider letermovir as potential cause of persistent nausea or vomiting |
| Adverse events of note | Atrial fibrillation or flutter occurred in 4.6% of letermovir-treated patients vs 1% in those who received placebo31 ; letermovir does not prolong QTc interval31,44 | We would not start letermovir soon after conditioning regimens that involve use of CYP-metabolized drugs (eg, busulfan, cyclophosphamide); we would also withhold it during or right after posttransplantation cyclophosphamide administration to minimize risk of hepatotoxicity resulting from drug-drug and drug-metabolite interactions |
| Alanine aminotransferase levels >5× ULN were 3.5% in letermovir-treated patients vs 1.6% in those receiving placebo31 | ||
| Renal dysfunction | Increased letermovir exposure in patients with GFR <60 mL/min (< twofold)84 ; increased exposure does not require dose adjustments44 | Some caution is advised if GFR <50 mL/min and using IV formulation because of potential hydroxypropyl-β-cyclodextrin accumulation and consequent osmotic toxicity44,78 |
| No dosing recommendations for GFR <10 mL/min31,44 | The phase 3 trial enrolled patients with GFR >10 mL/min, yet no increased nephrotoxicity was observed31 | |
| No recommendation for severe renal impairment based on lack of data in this subpopulation; consider risks and benefits of letermovir prophylaxis for patients in this situation | ||
| Liver dysfunction | Patients with cirrhosis and moderate liver dysfunction (Child-Pugh class B) have < twofold higher letermovir exposures; patients with severe liver dysfunction (Child-Pugh class C) have ∼fourfold increased letermovir exposures47 | No letermovir dose adjustments are necessary for patients with cirrhosis and mild to moderate liver dysfunction (Child-Pugh class A or B), but letermovir is not recommended for cirrhotic patients with severe impairment (Child-Pugh class C)44 |
AUC, area under the curve; GFR, glomerular filtration rate.
It is important to note that the last 2 phase 3 trials of CMV prophylaxis conducted in patients undergoing HCT31,32 imputed premature trial discontinuations for any reason (eg, withdrawal of consent, death, or loss to follow-up) as primary end point events, a conservative approach requested by regulatory agencies, which helps inform the clinical benefit and drug tolerability of the overall strategy. However, for the letermovir phase 3 trial, the proportion of patients with CMV-specific end points was 25 (7.7%) of 325 at the end of the treatment period (week 14, day +100). Of these events, 12 (3.7%) occurred after patients had ended letermovir treatment for a median of 43 days (range, 14-75 days), and 1 patient began preemptive therapy for CMV within the week-14 study window after stopping letermovir. Another 12 events (3.7%) of preemptive therapy occurred while patients were receiving letermovir, but 10 of these events had nonquantifiable (<137 IU/mL) CMV DNA in the central laboratory; only 2 patients (0.6%) had quantifiable CMV viral loads at the time of preemptive therapy, and in 1 (0.3%) of these 2 patients, a mutation (UL56 V236M) that confers letermovir resistance was documented.31 Furthermore, no mutations associated resistance were found in patients who experienced CMV reactivation after discontinuation of letermovir.49 An additional mutation (UL56 C325W) was identified in 1 of 48 patients who began letermovir treatment with detectable CMV DNA (not part of the primary efficacy population); the patient developed breakthrough CMV viremia a few weeks into treatment.43,44 Therefore, the on-treatment efficacy of letermovir when used in patients without CMV viremia at the start of prophylaxis was high.
Discussion
CMV infection has been an obstacle to improved outcomes for patients who undergo allogeneic HCT and are CMV seropositive. Several studies in the past 3 decades have evaluated different antiviral agents in an attempt to find a safe and effective agent to be used as universal prophylaxis. The scope of the treatments covered in this review highlights the longstanding search for suitable CMV prophylaxis stretching from 1981 to present day.
The randomized trials reviewed demonstrate that among the 6 antiviral therapies studied, ganciclovir and letermovir were the most effective in reducing incidence of CMV reactivation when used as universal prophylaxis agents. Furthermore, CMV disease rates have decreased over the study period (Figure 3), in part because of the introduction of more sensitive molecular methods for CMV surveillance and use of preemptive therapy during this time.50,51 Given the known disadvantages of preemptive therapy, such as treatment with drugs that have frequent toxic effects and an increased overall risk of mortality associated with CMV reactivation, the results presented suggest that patients undergoing allogeneic HCT would significantly benefit from universal prophylaxis with an agent that is tolerable after HCT. The data suggest that although effective at reducing CMV reactivation and disease, ganciclovir use cannot be recommended as a universal prophylaxis agent because of an increased risk of myelosuppression and subsequent drug discontinuation.
In contrast, the data suggest that letermovir has an excellent safety profile, and its use should be considered for this indication in patients at risk. Letermovir was associated with a decrease in CMV-related outcomes and all-cause mortality through 24 weeks after HCT. These benefits are likely due in part to its tolerability, which allowed patients to continue treatment through week 14 posttransplantation, and the possibility of administering IV treatment in patients who were acutely ill or could not take oral medications. Although there were several cases of CMV reactivation in the letermovir arm in the phase 3 trial, a majority of these occurred after the period of drug administration or in patients who discontinued letermovir therapy prematurely.31 Given these data, weekly surveillance for CMV reactivation during administration of letermovir may not be necessary for a majority of patients; targeted testing when CMV reactivation is clinically suspected may be a reasonable approach, including the evaluation of fever, cytopenias, or clinical syndromes that could be due to CMV disease. CMV monitoring after discontinuation of letermovir prophylaxis is advisable in patients who remain at higher risk of CMV infection, especially those with GVHD.
Risk of CMV reactivation remains a concern among high-risk patients undergoing HCT, including those undergoing haploidentical HCT, cord-blood recipients, ex vivo T cell–depleted graft recipients, antithymocyte globulin recipients, and patients with grade ≥2 GVHD requiring systemic glucocorticoids for treatment. As such, letermovir use may be preferentially considered in this patient population to prevent CMV reactivation. However, because letermovir does not have any activity against other human herpesviruses, concomitant acyclovir, valacyclovir, or famciclovir should be prescribed to reduce the risk of herpes simplex and varicella zoster clinical events.
On the basis of the criteria for study inclusion at the onset of this review, the trials presented are compelling in their quality. This review prioritized the selection of prospective, randomized studies, most of them double blinded and placebo controlled, which allow more direct comparisons. Through randomization, these studies minimized selection bias and addressed the most important outcomes in thoroughly understanding the feasibility of an antiviral CMV prophylaxis: the impact of the intervention on CMV infection incidence and all-cause mortality. The large number of overall participants also strengthens the qualitative conclusions reached by this review.
A key limitation of the evidence is that many of the studies identified had a small sample size. Only 3 studies had established dosing regimens and large sample sizes (N > 300 patients). Another 3 studies evaluated varying drug doses, affecting the confidence of the overall outcome. Most of the trials included in this review had wide CIs, making it difficult to measure the true efficacy of these interventions. Heterogeneity among studies resulting from vastly differing interventional methods, outcome measures, and study designs over >30 years further limited their comparability, so a formal metaanalysis was not pursued. Three earlier studies also lacked details about primary study end points and experimental design.17,18,20 Changes in diagnostic sensitivity is another confounding factor when analyzing the results. Because culture-based methods are less sensitive than molecular methods for CMV detection,51 antiviral interventions during the era of culture-based testing may have seemed more favorable than they were in actuality.
The results from this review reflect the current clinical consensus that most antiviral prophylaxis options to date have been inadequate in overall efficacy, and those that are efficacious against CMV reactivation introduce undesirable toxicities that limit their use. Although the decision to pursue CMV prophylaxis in post-HCT patients has historically been nuanced to balance drug-related toxicity with CMV-related outcomes, the results of recent studies have changed this landscape.
Patients at increased risk for primary CMV reactivation and CMV disease are most likely to benefit from anti-CMV prophylaxis. Additional research is warranted to further refine which particular HCT populations would benefit most from anti-CMV prophylaxis in a rapidly evolving landscape. Further research is also warranted to study the impact of CMV surveillance after the prophylactic period, the optimal threshold at which to initiate preemptive therapy after prophylaxis, and the role of CMV-specific immune monitoring for guiding prophylactic and preemptive CMV strategies.49,52-55 These parameters will remain fluid and are likely to change in the future with the incorporation of CMV immunotherapies56-61 and CMV vaccines.62-66
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank professor Robert H. Rubin for his enduring mentorship in the study of CMV in transplantation and for his discussion of ideas presented here.
M.P.C. receives salary support from the Detweiler Travelling Fellowship, provided by the Royal College of Physicians and Surgeons of Canada.
Authorship
Contribution: F.M.M. and K.C. conceptualized this systematic review; K.C., M.P.C., and F.M.M. performed data collection and analyses; K.C. wrote the first draft of the manuscript; M.P.C., S.P.H., H.E., and F.M.M. revised the manuscript and provided intellectual content; and all authors reviewed the manuscript and agreed to its submission in its current form.
Conflict-of-interest disclosure: S.P.H. has received institutional research support from Merck. H.E. has participated in advisory boards for Chimerix, Clinicgene, and Merck. F.M.M. has received institutional research support from Astellas, Chimerix, Merck, and Shire and consulting honoraria from Alexion, Chimerix, Fate Therapeutics, GlaxoSmithKline, LFB, Merck, Roche Molecular Diagnostics, and Shire. The remaining authors declare no competing financial interests.
Correspondence: Francisco M. Marty, Division of Infectious Diseases, Dana-Farber Cancer Institute and Brigham & Women’s Hospital, 75 Francis St, Boston, MA 02115; e-mail: fmarty@bwh.harvard.edu.