Key Points
Induction led to response in 41% and 32%, survival of 10.8 and 6 months, and transplant in 40% and 42% of responders in MDS and AML.
Treatment with high-dose cytarabine improved response rates in MDS and an anthracycline-containing regimen increased survival in AML.
Abstract
Hypomethylating agent (HMA) failure in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) carries a poor prognosis with limited treatment options. Although intensive, remission induction chemotherapy is often used subsequently, in particular to bridge to allogeneic transplantation, it is not clear whether an advantage exists for any particular regimen. Based on an international collaboration, we retrospectively analyzed induction response rate and survival in 366 patients after HMA failure. Patients received 7+3, intermediate- to high-dose cytarabine (IDAC), or purine nucleoside analog–based regimens. For the MDS cohort (n = 307), the overall response rate (ORR) was 41%; median overall survival (OS) was 10.8 months, and 40% of responding patients bridged to allogeneic stem cell transplant (allo-SCT). For the AML cohort (n = 59), the ORR was 32%, OS 6 months, and 42% of responding patients bridged to allo-SCT. Prognostic factors for response in MDS included adverse cytogenetics (odds ratio [OR], 0.46, P = .01), age ≥65 years (OR, 0.47; P < .01), and use of IDAC (OR, 2.91, P = .01). Shorter survival was associated with adverse cytogenetics (hazard ratio [HR], 1.43; P = .06). In the AML cohort, OS was decreased by disease progression at time of HMA failure (HR, 2.66; P = .02) and prolonged with use of an anthracycline-containing regimen (HR, 0.37; P = .01). In conclusion, intensive chemotherapy after HMA failure may be a reasonable treatment option for selected patients as a bridge to allogeneic transplantation and should be considered a potential platform for future investigations.
Introduction
The hypomethylating agents (HMAs) decitabine and azacitidine are commonly used in the treatment of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). In higher-risk MDS, both HMAs delay the progression to AML, and azacitidine prolongs overall survival (OS).1-3 Both agents are also active in AML, with a modest improvement of OS of treatment-naive older patients.4,5 Several studies have also explored the use of HMAs as salvage therapy after failure of frontline AML treatment,6-10 and in an effort to improve overall response rates (ORRs) and duration, HMAs are being investigated in combination with other agents, including lenalidomide and histone deacetylase inhibitors.11,12
Despite these efforts, all patients will eventually experience treatment failure, as HMAs do not represent a curative option, unless they are used as a bridge to allogeneic transplantation. The outcome after HMA failure in both high-risk MDS and AML after MDS is poor, with a median survival of 4 to 6 months and 3 to 4 months, respectively.13-16 Consequently, it is strongly recommended to enroll patients with HMA failure in clinical trials.17
Allogeneic stem cell transplantation, although potentially curative, is limited to a selected population based on criteria such as age and availability of donors. The availability of transplantation is also dependent on disease control, with a majority of patients requiring cytoreductive treatments prior to transplantation.18 Besides clinical trials, the most common modality used to bridge to transplantation is intensive chemotherapy.
Data specifically addressing the question of intensive chemotherapy after HMA failure remain limited to a small series of patients.18-22 The described induction regimens of these smaller studies are heterogeneous, ranging from classical 7+3, use of intermediate- to high-dose cytarabine (IDAC), or purine nucleoside analog (PNA) based (fludarabine, cladribine, clofarabine). In higher-risk MDS, a retrospective study of 35 patients treated with AML-like induction chemotherapy and a phase 2 study of 70 patients treated with low-dose clofarabine plus cytarabine showed a median OS of 8.9 and 10 months, respectively.18,22 The outcomes for secondary AML and HMA failure treated with induction chemotherapy are more dismal with median survival of 3.7 months and ORR of 35%.23 To date, there is no single study evaluating the safety and efficacy of multiple chemotherapy regimens.
Because a prospective evaluation of these “standard of care” regimens will be a practical challenge, we designed a retrospective international study aimed specifically at the evaluation of intensive chemotherapy outcomes in AML and MDS patients experiencing HMA failure.
Patients and methods
Patient selection
Patients included in this study received at least 1 cycle of azacitidine or decitabine for either MDS (ie, International Prognostic Scoring System [IPSS] intermediate-1 or higher) or AML as defined by World Health Organization 2008 Classification.24,25 After failure of the HMA, all patients received at least 1 cycle of AML-type induction chemotherapy (as defined in “Treatment strategies”). Additional criteria for inclusion were age >18 years, primary or secondary HMA failure, and treatment with an intensive chemotherapy regimen. This study was approved by the institutional review board at Yale University. Data were obtained from the following patient registries: Yale New Haven Hospital, Lee Moffitt Cancer Center, Johns Hopkins University, Taussig Cancer Institute at the Cleveland Clinic, Centre Hospitalier Universitaire de Nice, French Azacitidine Compassionate Program (Groupe Francophone des Myelodysplasies), Dusseldorf registry, Portuguese Institute of Oncology, Austrian Azacitidine Registry (Center of Salzburg only), and the MDS Zentrum Dresden. Cytogenetic classification was assessed on the basis of the IPSS for MDS and the UK Medical Research Council for AML.24,26,27
Definition of HMA failure
Patients were treated with azacitidine or decitabine until progression occurred or a lack of response persisted after at least 4 cycles. Disease status at the end of hypomethylating treatment was categorized as stable in the absence of treatment response and signs of progression, and as progressive disease, if patients had lost their responses to hypomethylating treatment or had experienced progression during treatment. Patients who discontinued hypomethylating therapy for intolerance or who were allotransplanted while responding to HMA were excluded from the analyses.
Treatment strategies
After HMA failure, patients were treated with various induction regimens. We classified the regimens into 3 groups, defined by the first cycle of induction therapy: 7+3 for combinations of cytarabine and anthracycline; IDAC for intermediate- and high-dose cytarabine regimens (>1000 mg/m2 per day for at least 3 days, ± anthracycline, ± topoisomerase inhibitor); and PNA for purine nucleoside analog based inductions (ie, fludarabine, cladribine, clofarabine) (supplemental Table 3). After the first induction cycle, the use of salvage chemotherapy, consolidation, and allogeneic transplantation was at the discretion of the host institution.
Response criteria and outcome measurements
Response to HMA was evaluated by the 2006 MDS International Working Group response criteria.28 Response to salvage induction chemotherapy was assessed according to the response criteria for AML 2003.29 OS was calculated from the time of induction to death or last follow-up. For the subgroup of patients with documented response to intensive chemotherapy, cumulative incidence of relapse (CIR) was calculated from time of response to induction chemotherapy to relapse or last follow-up. Additional secondary outcomes included the 8-week all-cause mortality after induction and the incidence of allo-SCT.
Statistical analysis
Data were summarized by frequency and percentage for categorical variables and by median and range for continuous variables. All statistical tests were 2 sided at 5% level of significance. Baseline characteristics were evaluated by χ2 analysis and Student t tests. Kaplan-Meier estimates were used to summarize OS and CIR. A logistic regression model was used for response evaluation and a Cox regression model for survival. All variables with an impact on response or survival in univariate analyses (P < .15) were included in the multivariate analysis. We used a 0.05 threshold to determine statistical significance for all analyses. The evaluation of the impact of allogeneic transplantation was performed using a landmark analysis at 3 months after transplantation. All statistical analyses were adjusted on region of origin of the patient (Europe vs United States). The statistics were performed using IBM SPSS 21 software, and graphs were designed and edited using GraphPad Prism 7 software.
Results
Patient characteristics
A total of 366 patients, including 307 patients with MDS and 59 patients with AML, treated between January 2005 and September 2015 met criteria for inclusion in this study. Baseline characteristics for the MDS and AML cohorts are depicted in Table 1 and supplemental Table 1, respectively.
Demographic and clinical characteristics of patients with MDS after HMA failure receiving induction chemotherapy
| Variable . | Global . | 7+3 . | IDAC . | PNA . | P . |
|---|---|---|---|---|---|
| N | 307 | 173 | 44 | 90 | |
| Median age (range), y | 64 (22-85) | 65 (22-81) | 58 (24-85) | 66 (43-80) | <.01 |
| Sex, male/female (%) | 196/111 (64) | 116/57 (67) | 26/18 (59) | 54/36 (60) | .41 |
| t-MN, n (%) | 43 (14) | 23 (13) | 6 (14) | 14 (16) | .879 |
| Median WBC at induction (range), ×109/L | 2.6 (0.1-341) | 2.6 (0.1-341) | 3.3 (0.77-70) | 2.4 (1.1-14.7) | .829 |
| Cytogenetic risk, n (%) | |||||
| Favorable | 128 (42) | 69 (40) | 14 (32) | 45 (50) | .54 |
| Intermediate | 65 (21) | 36 (21) | 11 (25) | 18 (20) | |
| Unfavorable | 109 (36) | 65 (38) | 18 (41) | 26 (29) | |
| IPSS, n (%) | |||||
| Low | 8 (3.4) | 5 (4) | 0 (0) | 3 (4) | .191 |
| Int-1 | 67 (28) | 27 (22) | 11 (37) | 29 (35) | |
| Int-2 | 81 (34) | 42 (34) | 13 (43) | 26 (31) | |
| High | 80 (34) | 48 (40) | 6 (20) | 26 (31) | |
| Blast, % | 9.6 | 9.6 | 8.5 | 10.1 | .34 |
| Prior induction, n (%) | 7 (2) | 2 (1) | 4 (9) | 1 (1) | .42 |
| Prior allo-SCT, n (%) | 10 (3) | 2 (15) | 7 (16) | 1 (1) | <.01 |
| AZA/DEC (%) | 279/28 (91) | 156/17 (90) | 38/6 (86) | 85/5 (94) | .27 |
| Cycles of HMA, median (range) | 6 (1-72) | 6 (1-72) | 6 (1-38) | 6 (2-47) | .573 |
| Initiation of HMA to start of induction, median time (range), mo | 7.9 (0-72) | 8.4 (0-72) | 6.2 (0.6- 52.7) | 8.5 (1.8-51) | .23 |
| Variable . | Global . | 7+3 . | IDAC . | PNA . | P . |
|---|---|---|---|---|---|
| N | 307 | 173 | 44 | 90 | |
| Median age (range), y | 64 (22-85) | 65 (22-81) | 58 (24-85) | 66 (43-80) | <.01 |
| Sex, male/female (%) | 196/111 (64) | 116/57 (67) | 26/18 (59) | 54/36 (60) | .41 |
| t-MN, n (%) | 43 (14) | 23 (13) | 6 (14) | 14 (16) | .879 |
| Median WBC at induction (range), ×109/L | 2.6 (0.1-341) | 2.6 (0.1-341) | 3.3 (0.77-70) | 2.4 (1.1-14.7) | .829 |
| Cytogenetic risk, n (%) | |||||
| Favorable | 128 (42) | 69 (40) | 14 (32) | 45 (50) | .54 |
| Intermediate | 65 (21) | 36 (21) | 11 (25) | 18 (20) | |
| Unfavorable | 109 (36) | 65 (38) | 18 (41) | 26 (29) | |
| IPSS, n (%) | |||||
| Low | 8 (3.4) | 5 (4) | 0 (0) | 3 (4) | .191 |
| Int-1 | 67 (28) | 27 (22) | 11 (37) | 29 (35) | |
| Int-2 | 81 (34) | 42 (34) | 13 (43) | 26 (31) | |
| High | 80 (34) | 48 (40) | 6 (20) | 26 (31) | |
| Blast, % | 9.6 | 9.6 | 8.5 | 10.1 | .34 |
| Prior induction, n (%) | 7 (2) | 2 (1) | 4 (9) | 1 (1) | .42 |
| Prior allo-SCT, n (%) | 10 (3) | 2 (15) | 7 (16) | 1 (1) | <.01 |
| AZA/DEC (%) | 279/28 (91) | 156/17 (90) | 38/6 (86) | 85/5 (94) | .27 |
| Cycles of HMA, median (range) | 6 (1-72) | 6 (1-72) | 6 (1-38) | 6 (2-47) | .573 |
| Initiation of HMA to start of induction, median time (range), mo | 7.9 (0-72) | 8.4 (0-72) | 6.2 (0.6- 52.7) | 8.5 (1.8-51) | .23 |
AZA, azacitidine; DEC, decitabine; prior allo, prior allogeneic stem cell transplant; t-MN, therapy-related myeloid neoplasm.
In the MDS population, median age was 64 years (range, 22-85 years). For the cohort, 42% (n = 128) had favorable; 21% (n = 65) had intermediate, and 36% (n = 109) had unfavorable cytogenetics based on IPSS criteria. At initial diagnosis, 68% (n = 161) of the patients with available IPSS scores had higher-risk MDS (intermediate-2 or high risk). Patients were treated with azacitidine (91%, n = 279) or decitabine (9%, n = 28) for a median of 6 cycles (range, 1-72). Thirty-four patients had a documented progression to AML after failure of HMA. After HMA failure, 56% (n = 173) received 7+3; 14% (n = 44) received IDAC, and 29% (n = 90) received PNA. The median time between documentation of HMA failure and induction was 2.4 months. Baseline demographics, including sex, IPSS, cytogenetic risk stratification, HMA type, and prior number of HMA cycles, were similar among the treatment groups (Table 1). The IDAC cohort was significantly younger (median age: 7+3, 65 years; IDAC, 58 years; PNA, 66 years; P < .01) and more heavily treated prior to induction therapy with allogeneic transplantation (prior transplantation: 7+3, 15%; IDAC, 16%; PNA, 1%; P < .01), or intensive chemotherapy (prior chemotherapy: 7+3, 1%; IDAC, 9%; PNA, 1%; P = .02) (Table 1).
Among patients with AML prior to initiation of HMA, 24 had a documentation of prior MDS. Median age was 64 years (range, 22-85). Most AML patients had intermediate-risk cytogenetics, 67% (n = 39), as compared with unfavorable, 32% (n = 19), and only 1 patient had favorable cytogenetics. Patients were treated with azacitidine, 88% (n = 52), and decitabine, 12% (n = 7), for a median 5 cycles (range, 1-39). The median time between HMA failure and induction was 1 month. Baseline demographics, including sex, cytogenetic risk stratification, HMA type, and prior number of HMA cycles, were similar among the treatment groups (supplemental Table 1). Among the treatment regimens, the IDAC and PNA groups had significantly fewer cycles of HMA (median HMA cycles: 7+3, 7 cycles; IDAC, 4 cycles; PNA, 4 cycles; P = .04) and higher rates of induction chemotherapy (prior induction: 7+3, 13%; IDAC, 42%; PNA, 59%; P < .01) and allogeneic transplant (prior transplantation: 7+3, 0%; IDAC, 25%; PNA, 19%; P = .02) prior to HMA therapy than the 7+3 cohort. The IDAC group was also significantly younger (age: 7+3, 67 years; IDAC, 57 years; PNA, 61 years; P < .01) (supplemental Table 1).
Response and survival after induction chemotherapy in MDS
Of 307 patients, 167 (54%) died and 140 (46%) were alive at last follow-up. The median duration of follow-up was 10 months (range, 2.7-84 months). For MDS, the ORR to induction chemotherapy was 41% (n = 125, 95% confidence interval [CI; 35.5-46.5]); 8-week mortality was 6.5% (n = 20, 95% CI [3.7-9.3]), and median OS was 10.8 months (95% CI [8.6-13.0]) (Table 2). The CIR was 59% at 1 year and 81% at 2 years (Table 2).
Differential outcome of patients with MDS and AML after HMA failure based on induction strategy
| . | MDS . | AML . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Global (n = 307) . | 7+3 (n = 173) . | IDAC (n = 44) . | PNA (n = 90) . | P . | Global (n = 59) . | 7+3 (n = 30) . | IDAC (n = 12) . | PNA (n = 17) . | P . |
| Response, CR+CRi% | 41 | 39 | 64 | 34 | .04 | 32 | 63 | 25 | 21 | .43 |
| 8-wk mortality, % | 6.5 | 8.7 | 2.3 | 4.4 | .19 | 15.3 | 16.7 | 0 | 23.5 | .21 |
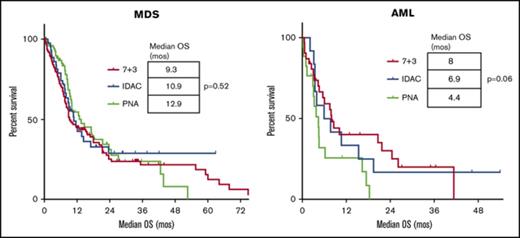
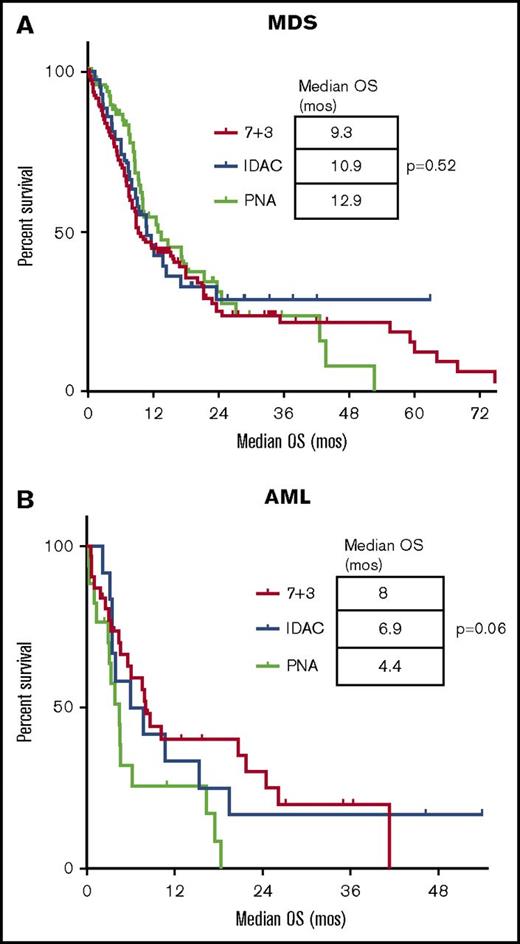
| Median OS, mo | 10.8 | 9.3 | 10.9 | 12.9 | .52 | 6.0 | 8.0 | 6.9 | 4.4 | .06 |
| Median CIR, mo | 11.6 | 11.3 | 12.5 | 14.1 | .60 | 14.4 | 16.6 | 13.0 | 13.9 | .15 |
| Responders undergoing Allo, % | 39.7 | 37.3 | 64.3 | 22.6 | <.01 | 42 | 42 | 67 | 25 | .54 |
| . | MDS . | AML . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Global (n = 307) . | 7+3 (n = 173) . | IDAC (n = 44) . | PNA (n = 90) . | P . | Global (n = 59) . | 7+3 (n = 30) . | IDAC (n = 12) . | PNA (n = 17) . | P . |
| Response, CR+CRi% | 41 | 39 | 64 | 34 | .04 | 32 | 63 | 25 | 21 | .43 |
| 8-wk mortality, % | 6.5 | 8.7 | 2.3 | 4.4 | .19 | 15.3 | 16.7 | 0 | 23.5 | .21 |
| Median OS, mo | 10.8 | 9.3 | 10.9 | 12.9 | .52 | 6.0 | 8.0 | 6.9 | 4.4 | .06 |
| Median CIR, mo | 11.6 | 11.3 | 12.5 | 14.1 | .60 | 14.4 | 16.6 | 13.0 | 13.9 | .15 |
| Responders undergoing Allo, % | 39.7 | 37.3 | 64.3 | 22.6 | <.01 | 42 | 42 | 67 | 25 | .54 |
CR, complete remission; CRi, complete remission with incomplete blood count recovery; Responders undergoing Allo, percentage of responders to induction chemotherapy undergoing allogeneic transplant.
Among the different chemotherapy regimens, patients receiving IDAC had a significantly higher ORR (IDAC, 64%; 7+3, 39%; PNA, 34%; P = .04) and percentage of responders bridging to allogeneic stem cell transplant (IDAC, 64%; 7+3, 37%; PNA, 23%; P < .01) (Table 2). There was, however, no difference in the median OS, 8-week mortality, or the median CIR between the 3 defined groups of induction (Table 2; Figure 1A; supplemental Figure 1). We also looked at the impact of the addition of anthracycline in the subgroup of MDS patients not treated with 7+3 (ie, IDAC and PNA groups). In univariate analysis, the ORR was 34% without vs 49.2% with anthracyclines (P = .10), and the OS was 9.3 months vs 12.6 months (P = .63) for patients treated without or with anthracyclines, respectively. In multivariate analysis, the ORR was negatively impacted by age ≥65 (odds ratio [OR] 0.47; 95% CI [0.28-0.78]; P < .01) and unfavorable cytogenetic risk (OR 0.46; 95% CI [0.25-0.83]; P = .01). Treatment with IDAC was associated with an improved response rate (OR 2.91; 95% CI [1.24-6.90]; P = .01) (Table 3). There was no impact of IPSS score or therapy-related myeloid neoplasm in the logistic regression model.
Kaplan-Meier estimates of the OS for different induction chemotherapy regimens. In MDS (A) and AML (B). Survival is expressed in months after induction. Each tick mark represents a censored patient. (A-B) No significant difference in survival among the induction regimens for patients with MDS or AML.
Kaplan-Meier estimates of the OS for different induction chemotherapy regimens. In MDS (A) and AML (B). Survival is expressed in months after induction. Each tick mark represents a censored patient. (A-B) No significant difference in survival among the induction regimens for patients with MDS or AML.
Multivariate analyses models for response and survival after induction chemotherapy in MDS patients experiencing HMA failure
| Variable . | ORR, % . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| Age, y | 0.28-0.78 | <.01 | ||
| <65 | 48 | 1 | ||
| ≥65 | 34 | 0.47 | ||
| Cytogenetic risk | 0.25-0.83 | .01 | ||
| Favorable/intermediate | 47 | 1 | ||
| Unfavorable | 29 | 0.46 | ||
| IPSS | 0.311-1.15 | .12 | ||
| Int-2 or lower | 45 | 1 | ||
| High | 30 | 0.60 | ||
| Therapy related | 0.25-1.20 | .13 | ||
| No | 43 | 1 | ||
| Yes | 28 | 0.55 | ||
| Induction regimen | 1.24-6.90 | .01 | ||
| Other (7+3 or PNA) | 37 | 1 | ||
| IDAC | 64 | 2.91 |
| Variable . | ORR, % . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| Age, y | 0.28-0.78 | <.01 | ||
| <65 | 48 | 1 | ||
| ≥65 | 34 | 0.47 | ||
| Cytogenetic risk | 0.25-0.83 | .01 | ||
| Favorable/intermediate | 47 | 1 | ||
| Unfavorable | 29 | 0.46 | ||
| IPSS | 0.311-1.15 | .12 | ||
| Int-2 or lower | 45 | 1 | ||
| High | 30 | 0.60 | ||
| Therapy related | 0.25-1.20 | .13 | ||
| No | 43 | 1 | ||
| Yes | 28 | 0.55 | ||
| Induction regimen | 1.24-6.90 | .01 | ||
| Other (7+3 or PNA) | 37 | 1 | ||
| IDAC | 64 | 2.91 |
| Variable . | Median OS, mo . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Cytogenetic risk | 0.99-2.06 | .06 | ||
| Favorable/intermediate | 15.6 | 1 | ||
| Unfavorable | 8.1 | 1.43 | ||
| Disease status at HMA failure | 0.95-2.24 | .09 | ||
| Stable | 13.6 | 1 | ||
| Progressive | 10.5 | 1.46 |
| Variable . | Median OS, mo . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Cytogenetic risk | 0.99-2.06 | .06 | ||
| Favorable/intermediate | 15.6 | 1 | ||
| Unfavorable | 8.1 | 1.43 | ||
| Disease status at HMA failure | 0.95-2.24 | .09 | ||
| Stable | 13.6 | 1 | ||
| Progressive | 10.5 | 1.46 |
Logistic regression multivariate analysis model for multivariate analysis of overall response and Cox model for multivariate analysis of OS after induction therapy, including all covariates with a potential outcome on response in univariate analysis (P value in univariate <.15). Cytogenetic risk was evaluated per MDS IPSS classification.
OR, odds ratio.
Factors that significantly decreased survival in univariate analysis included adverse cytogenetic risk by IPSS, and progression of disease at HMA failure. There was a trend that patients with lower-risk MDS at initiation of HMA had an improved outcome, but this did not reach statistical significance (P = .19; supplemental Figure 2). There was no evidence that a progression to AML was worse than other forms of progression per International Working Group 2006 (see supplemental analyses). As seen in Table 3, both adverse cytogenetics and progression at the time of HMA failure had a detrimental impact on OS and had a borderline significance in the adjusted multivariate model.
Response and survival after induction chemotherapy in AML
For the AML group, 46 (78%) died and 13 (22%) were alive at last follow-up. Median duration of follow-up was 12.9 months (range, 2.1-54 months). The ORR to induction chemotherapy was 32% (n = 19, 95% CI [20.1-43.9]); 8-week mortality was 15% (n = 20, 95% CI [5.9-24.1]), and median OS was 6.2 months (95% CI [2.4-10]). The CIR was 46% at 1 year and 100% at 2 years (Table 2).
Among the chemotherapy groups in AML, patients receiving 7+3 had a trend toward improved median OS (7+3, 8 months; IDAC, 6.9 months; and PNA, 4.4 months; P = .06) (Table 2). There was no difference in the ORR, 8-week mortality, median CIR, or the percentage of responders bridging to allo-SCT among the regimens (Table 2; Figure 1B; supplemental Figure 1). We evaluated the impact of the addition of anthracycline in the subgroup of AML patients not treated with 7+3 (ie, IDAC and PNA groups). In univariate analysis, the ORR was 19% without vs 31% with anthracyclines (P = .6) but the OS was 3.4 months vs 10.7 months (P = .05) for patients treated without or with anthracyclines, respectively.
In univariate analysis of response in AML patients, adverse cytogenetic risk and use of anthracyclines trended toward significance with P < .15. However, none of the previously mentioned variables reached significance in the logistic regression model (Table 4). In the Cox regression model, survival was significantly shortened by progression of disease at the time of HMA failure (hazard ratio [HR] 2.66; 95% CI [1.2-6.0]; P = .02) and prolonged by the use of anthracyclines (HR 0.37; 95% CI [0.2-0.8]; P = .01) (Table 4).
Multivariate analyses models for response and survival after induction chemotherapy in AML patients experiencing HMA failure
| Variable . | ORR, % . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| Cytogenetic risk | 0.16-1.54 | .20 | ||
| Favorable/intermediate | 37.5 | 1 | ||
| Unfavorable | 21.1 | 0.42 | ||
| Anthracyclines | 0.57-10.37 | .23 | ||
| No | 18.8 | 1 | ||
| Yes | 37.2 | 2.43 |
| Variable . | ORR, % . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| Cytogenetic risk | 0.16-1.54 | .20 | ||
| Favorable/intermediate | 37.5 | 1 | ||
| Unfavorable | 21.1 | 0.42 | ||
| Anthracyclines | 0.57-10.37 | .23 | ||
| No | 18.8 | 1 | ||
| Yes | 37.2 | 2.43 |
| Variable . | Median OS, mo . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Therapy related | 0.66- 2.90 | .39 | ||
| No | 7.8 | 1 | ||
| Yes | 4.4 | 1.4 | ||
| Cytogenetic risk | 0.79-2.9 | .21 | ||
| Favorable/intermediate | 7.9 | 1 | ||
| Unfavorable | 4.5 | 1.5 | ||
| Prior induction | 0.74-3.14 | .25 | ||
| No | 7.8 | 1 | ||
| Yes | 3.7 | 1.5 | ||
| Disease status at HMA failure | 1.19-5.98 | .02 | ||
| Stable | 7.9 | 1 | ||
| Progressive | 4.0 | 2.66 | ||
| Use of anthracyclines | 0.17-0.82 | .01 | ||
| No | 3.7 | 1 | ||
| Yes | 8 | 0.37 |
| Variable . | Median OS, mo . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Therapy related | 0.66- 2.90 | .39 | ||
| No | 7.8 | 1 | ||
| Yes | 4.4 | 1.4 | ||
| Cytogenetic risk | 0.79-2.9 | .21 | ||
| Favorable/intermediate | 7.9 | 1 | ||
| Unfavorable | 4.5 | 1.5 | ||
| Prior induction | 0.74-3.14 | .25 | ||
| No | 7.8 | 1 | ||
| Yes | 3.7 | 1.5 | ||
| Disease status at HMA failure | 1.19-5.98 | .02 | ||
| Stable | 7.9 | 1 | ||
| Progressive | 4.0 | 2.66 | ||
| Use of anthracyclines | 0.17-0.82 | .01 | ||
| No | 3.7 | 1 | ||
| Yes | 8 | 0.37 |
Logistic regression multivariate analysis model and Cox model for multivariate analysis of OS after induction therapy, including all covariates with a potential outcome on response in univariate analysis (P value in univariate <.15). Cytogenetic risk was evaluated per AML UK MRC classification.
Impact of allogeneic transplantation
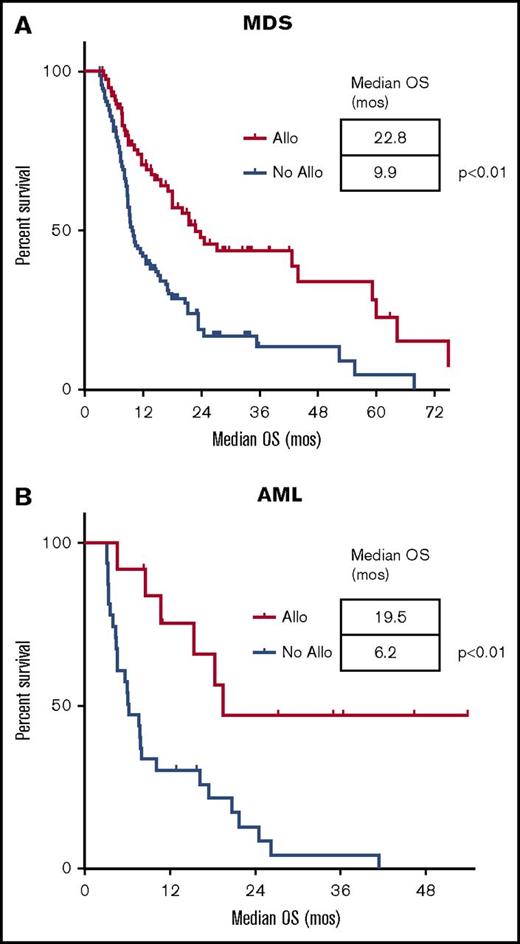
Allogeneic transplantation was performed in 26% of the patients (n = 95), and their characteristics are described in supplemental Table 2. Of these, 40% (51/126) of responding patients in MDS and 42% (8/19) of responding patients in AML were able to bridge to transplant. The majority of transplantations used reduced-intensity conditioning regimens (73%) with unrelated donors (53%). The median time to allogeneic transplantation calculated from the start of induction was 3 months. In a landmark analysis performed at 3 months after intensive chemotherapy, allotransplanted patients had an improved OS vs nontransplanted patients in MDS of 23 months vs 10 months (P < .01) and AML of 20 months vs 6 months (P < .01), respectively (Figure 2). We did not observe any survival plateau in the MDS cohort.
Landmark analysis of OS for patients undergoing allogeneic stem cell transplantation vs no transplantation. In MDS (A) and AML (B). Survival is expressed in months after induction. The landmark analysis was set 3 months after induction. Each tick mark represents a censored patient. (A-B) Significantly longer median survival for patients with MDS and AML treated with allogeneic transplantation when compared with those not undergoing transplantation. Allo, allogeneic transplantation.
Landmark analysis of OS for patients undergoing allogeneic stem cell transplantation vs no transplantation. In MDS (A) and AML (B). Survival is expressed in months after induction. The landmark analysis was set 3 months after induction. Each tick mark represents a censored patient. (A-B) Significantly longer median survival for patients with MDS and AML treated with allogeneic transplantation when compared with those not undergoing transplantation. Allo, allogeneic transplantation.
Outcome prediction after HMA failure: application of the MDS consortium predictive model
In order to identify a subgroup of patients who may have a better benefit from induction chemotherapy, we evaluated the recently presented scoring system developed by the Cleveland group on behalf of the MDS consortium for patients with HMA failure.30 A subgroup of 133 patients with available data was included in this analysis. There was a trend for increased ORR in the low-score vs high-score group (45% vs 32%, respectively; P = .11) and for increased OS in the low-score vs high-score group (8.6 months vs 6.1 months, respectively; P = .06; supplemental Figure 3).
Discussion
Our work, based on a large cohort of patients with MDS and AML experiencing HMA failure and treated with induction chemotherapy, allowed us to refine our understanding of the impact and role of intensive regimens in these settings. In patients with MDS prior to initiation of HMA, treatment with an intermediate- to high-dose cytarabine-based regimen led to a superior response rate and a greater percentage of responders undergoing allogeneic stem cell transplantation. There was however no definitive benefit in OS. However, it seems that a long-term survival benefit was only seen in patients treated with IDAC (Figure 1), including in the allotransplanted population (Figure 2; supplemental Figure 4). In patients with AML, with respect to the smaller number of patients, there was no clear benefit of 1 type of induction regimen, but the use of anthracycline seems more critical than in MDS patients. Our results support the use of induction chemotherapy as a reasonable platform to treat younger patients with MDS and AML after HMA failure. However, induction chemotherapy seems to have a limited impact on survival without subsequent allogeneic transplantation, and only 26% of patients in the MDS and AML cohort were able to be transplanted. In addition, in this study, several prognostic factors were identified to help define the group of patients that may benefit from this approach.
There are several limitations to our work. The use of a retrospective study design is prone to selection bias. However, this type of study would be challenging to conduct prospectively. In addition, the different chemotherapy regimens and practice patterns (including the choice of first-line HMA vs induction) across countries and centers may have increased the heterogeneity of our data. We attempted to control for this by systematically adjusting for region (United States vs Europe) in our multivariate analyses and by categorizing regimens based on the first induction regimen used. Considering our analysis of outcome, several variables were not included in the final dataset, as the number of patients with available information was too low: genomolecular profiling and the depth of response (CR vs CRi) were only available in the most recent years,31,32 and the potential role of comorbidities on induction mortality.33-36
For MDS, we were able to demonstrate a superior response rate and ability to bridge to allo-SCT with IDAC. The 8-week mortality also seems improved with the use of IDAC regimen in univariate analyses. It is important to acknowledge that patients treated with IDAC were significantly younger as compared with the other groups of patients. On the other hand, it is interesting that the IDAC group also had the highest proportion of heavily pretreated patients, with nearly 20% of them having received a prior transplantation. This likely contributed to the lack of benefit observed in survival and CIR. Altogether, these results suggest that IDAC may be a better platform for induction chemotherapy after HMA failure in MDS. Of note, we did not observe a strong impact of the addition of anthracyclines, opening the opportunity of testing new agents in this setting.
For patients with AML prior to initiation of HMA, the lower number of patients limits our conclusions. The use of HMA as a first-line regimen may select for a population of patients with worse functional status or worse cytogenetics at diagnosis, even if it is not completely clear from the patient’s characteristics in our cohort (supplemental Table 1). We found an improvement in survival with the use of anthracycline-based regimens and a trend toward longer survival with 7+3, but interestingly, no benefit of IDAC-based approaches (Tables 2 and 4). However, the 7+3 group had significantly fewer patients with prior induction and prior transplantation (supplemental Table 1). This could explain the improved survival and incidence of relapse in patients treated with 7+3 and anthracycline-containing regimens. There was no impact of type of regimen on response or ability to bridge to allogeneic stem cell transplantation (Table 2). The outcomes of our AML cohort were in general more favorable than other studies investigating intensive chemotherapy regimens after HMA failure. This is likely due to a relatively lower percentage of patients with secondary AML and a lower proportion of patients harboring unfavorable cytogenetic risk (supplemental Table 1).
Our study identified several prognostic factors useful for identifying patients that will most benefit from induction chemotherapy. In the MDS cohort, age >65, adverse cytogenetics, and use of non-IDAC–containing regimen were associated with decreased response. Adverse cytogenetics and progressive disease at time of HMA failure were associated with shorter survival but with only a trend significance in the multivariate analysis In AML, the use of a non-anthracycline–containing regimen and having progressive disease at time of HMA failure were associated with decreased survival. In our study, patients with MDS after HMA failure without any of the above risk factors for response or survival had a response rate of 72%, median OS of 27 months, and an upfront probability of allogeneic transplantation of 72%. These numbers compare favorably to what has been observed and what we can expect from most of the ongoing clinical trials not driven by a molecular definition of the disease (such as isocitrate dehydrogenase or Fms-like tyrosine kinase-3 mutations37,38 ). Finally, the Cleveland Clinic score was also not able to discriminate a group of patients with a better outcome even if there was a trend for an improved response rate and improved survival.
We strongly emphasize the need to prioritize clinical trial participation for all patients experiencing HMA failure, and we should consider using induction chemotherapy as a backbone for novel agent evaluation for patients able to tolerate this approach. In addition to the conventional chemotherapy regimens used in this study, recently developed induction agents already used in AML could be applied to these populations. For instance, CPX-351 showed a survival benefit for patients with secondary AML, and a third of the patients in these studies were previously treated with HMA.39 An additional analysis showed that 20% of our patient population would have qualified for the CPX-351 phase 3 (supplemental Data). We observed relatively similar induction mortality and survival as compared with what was observed in the control arm of the study. Other agents like Sapacitabine or vosaroxin may also be considered potential combination therapies. It also seems reasonable to offer induction as 1 of the potential comparator arms (including best supportive care) vs investigational agent in randomized trials if the eligibility criteria allow patients eligible for allogeneic transplantation. Considering allogeneic transplantation, our study demonstrates again that long-term survival can only be achieved in allotransplanted patients. As expected with a median age of our cohort of 64 years, the majority of the preparative regimens were reduced intensity conditioning. Minimizing tumor burden with induction chemotherapy is an effective strategy toward limiting the risk of relapse after transplantation, in particular with the increased use of haplo-identical transplantation.40-43 In MDS patients, we did not observe any survival plateau after allogeneic stem cell transplant. Besides illustrating the underlying differences in the tumor biology and microenvironment between AML and MDS, it also stresses the need for preemptive treatments after transplantation for these patients.
In summary, our study confirmed that intensive chemotherapy, and its different variations, may be considered a valid option in selected patients after HMA failure. Induction chemotherapy needs to be systematically integrated into a more global strategy, including allogeneic transplantation. Our work also highlights that intensive chemotherapy may be considered a potential platform to develop combination therapies with investigational agents, continuing the efforts of the hematologist community to improve the outcome of the patients experiencing HMA failure.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all the physicians and the medical staff of the participating centers that took care of the patients. The authors also thank the clinical research personnel that helped to compile data in the different centers (Najla Al-Ali, Alexandra Dormal, Christopher Goebel, and Rosa Sapena).
Authorship
Contribution: B.B. included patients, analyzed data, and wrote the manuscript; R.S.K., L.A., M.A.S., L.P., A.E.D., N.V., A.A., U.G., T.C., U.P., S.G., P.F. included patients, analyzed data, and contributed to the writing of the manuscript; T.P. designed research, included patients, analyzed data, and wrote the manuscript; and all authors read and agreed to the current version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Prebet, Section of Hematology, Department of Internal Medicine, Yale School of Medicine, 37 College St, Room #101, New Haven, CT 06511; e-mail: thomas.prebet@yale.edu.