Key Points
Treatment with a BRAF and MEK inhibitor can achieve a sustained response in BRAFV600-mutant Langerhans cell histiocytosis.
Detection of plasma BRAFV600-mutant circulating tumor DNA is a promising biomarker for monitoring disease activity in this entity.
Introduction
Langerhans cell histiocytosis (LCH) is a rare CD1a+ malignancy derived from myeloid progenitor cells.1 It can affect any organ, but it affects mostly the skeleton. Signal proteins in the mitogen-activated protein kinase (MAPK) pathway (or RAS-RAF-MEK-ERK pathway) are frequently mutated in LCH and lead to the constitutive activation of this oncogenic pathway. Activating BRAF mutations at the V600E position are present in ∼50% of cases, and mutations in MAP2K1/MEK1 encoding MEK are found in 19% of cases. These mutations are mutually exclusive.2,3
There are multiple reports of the successful treatment of BRAFV600-mutant LCH with BRAF inhibitors (vemurafenib and dabrafenib) as monotherapy. A response rate of 61.5% was reported with vemurafenib in a phase 2 trial involving patients with BRAFV600-mutant LCH and Erdheim-Chester disease (ECD; a CD1a– non-Langerhans histiocytic disorder characterized by a BRAFV600E mutation in 50% of cases).4 In BRAFV600-mutant melanoma, dual blockade of the MAPK pathway using a BRAF plus MEK inhibitor results in higher efficacy and less toxicity compared with monotherapy with a BRAF inhibitor (ie, combination therapy eliminated the incidence of BRAF inhibitor–induced palmoplantar hyperkeratosis and secondary skin malignancies, including skin papilloma, keratoacanthoma, and cutaneous squamous cell carcinoma).5-7 The potential of combined BRAF-MEK inhibition has not been fully explored in BRAF-mutant histiocytoses.
Quantitative assessment of plasma BRAFV600-mutant circulating tumor DNA (ctDNA) offers a reliable noninvasive method for diagnosing and monitoring patients with BRAFV600-mutant melanoma during treatment with BRAF-MEK inhibitors.8 It has been proposed as a complementary diagnostic tool in BRAFV600-mutant histiocytoses.9 Plasma BRAFV600-mutant ctDNA has been investigated in case series of LCH as a monitoring tool during treatment with chemotherapy, in which a decrease in ctDNA correlated with disease response.10,11
Case description
We report the case of an adult patient with LCH who was treated with the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor). Serial measurements of the copy number of plasma BRAFV600E-mutant ctDNA reflected the response to therapy, early relapse after treatment interruption, and successful retreatment.
Methods
Quantification of BRAFV600-mutant ctDNA was performed by using blood collected at baseline and at multiple time points during treatment. Blood samples were processed and analyzed as reported previously.8 Fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) was performed for metabolic staging. Amplicon sequencing of exons 11 and 15 of the BRAF gene was performed on the tissue sample. Next-generation sequencing analysis was performed twice to achieve a mean of 500 reads for each exon. The patient provided written informed consent by using a local medical ethics committee-approved form.
Results and discussion
A 41-year-old woman was diagnosed in August 2009 with central diabetes insipidus. Imaging of the pituitary gland was compatible with hypophysitis. An extensive work-up was not suggestive for autoimmune disease, sarcoidosis, or histiocytosis. She was treated with desmopressin.
In July 2014, she was admitted because of pyrexia, fatigue, shoulder pain, and elevated C-reactive protein and erythrocyte sedimentation rate. An extensive workup including FDG-PET/CT imaging showed multiple hypermetabolic bone lesions (Figure 1), pulmonary interlobulary septal thickening, and perirenal fatty inflammation. Biopsy of a bony lesion showed cells with irregular nuclei, multinucleated giant cells, and tissular infiltration of eosinophils. Immunohistochemistry showed CD1a positivity. The diagnosis of LCH was established. Next-generation sequencing revealed the presence of an activating BRAFV600E mutation.
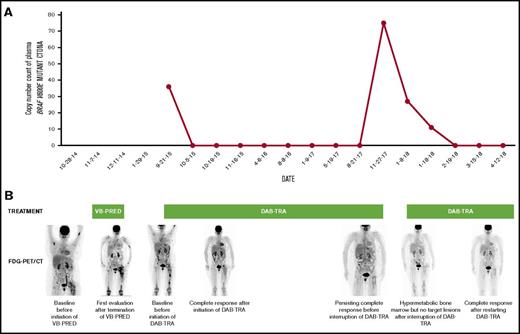
Longitudinal disease monitoring with plasmaBRAFV600E-mutant ctDNA and FDG-PET/CT during treatment with vinblastine-prednisone (VB-PRED) and dabrafenib-trametinib (DAB-TRA). (A) Longitudinal disease monitoring by quantitative assessment of the copy number of BRAFV600E-mutant ctDNA within the plasma. BRAFV600E-mutant DNA becomes undetectable during treatment with DAB-TRA but is detectable when treatment is interrupted. When treatment is resumed, BRAFV600E-mutant DNA copy numbers decrease and become undetectable after 6 weeks of treatment. (B) FDG-PET maximal intensity projection imaging. Sites of disease activity are revealed by more intense (black color) FDG focal uptake in the skeleton. Upon treatment with dabrafenib-trametinib, FDG uptake is normalized but recurs discreetly after interruption of therapy.
Longitudinal disease monitoring with plasmaBRAFV600E-mutant ctDNA and FDG-PET/CT during treatment with vinblastine-prednisone (VB-PRED) and dabrafenib-trametinib (DAB-TRA). (A) Longitudinal disease monitoring by quantitative assessment of the copy number of BRAFV600E-mutant ctDNA within the plasma. BRAFV600E-mutant DNA becomes undetectable during treatment with DAB-TRA but is detectable when treatment is interrupted. When treatment is resumed, BRAFV600E-mutant DNA copy numbers decrease and become undetectable after 6 weeks of treatment. (B) FDG-PET maximal intensity projection imaging. Sites of disease activity are revealed by more intense (black color) FDG focal uptake in the skeleton. Upon treatment with dabrafenib-trametinib, FDG uptake is normalized but recurs discreetly after interruption of therapy.
She received first-line therapy between November and December 2014 (delay at patient’s discretion) with vinblastine 6 mg/m2 and prednisone 40 mg during 6 weeks; therapy was poorly tolerated. FDG-PET/CT imaging performed 2 months after initiation of treatment showed a resolution of the lung and kidney lesions but new bone lesions appeared. A biopsy of a bone lesion showed no signs of LCH, and close follow-up was provided. In September 2015, she presented again with hip pain and constitutional symptoms. A new FDG-PET/CT scan showed relapse of the bone, kidney, and lung lesions.
Treatment with dabrafenib 150 mg twice per day and trametinib 2 mg once per day was started. Trametinib was initiated to reduce dabrafenib-related toxicity (hyperkeratosis and potential induction of secondary skin tumors). Baseline blood plasma BRAFV600E-mutant ctDNA count was detected at a level of 36 BRAFV600E copies/mL (Figure 1).
Her symptoms improved within days. A follow-up FDG-PET/CT scan after 2 months of treatment showed a near-total metabolic response. Plasma ctDNA became undetectable during treatment. The patient experienced mild treatment-related toxicity (subpyrexia, arthralgia, and fatigue) that was managed with a dose reduction to 100 mg twice per day of dabrafenib and 1 mg once per day of trametinib. In November 2017, she reported therapeutic noncompliance because of fatigue. Although plasma ctDNA was detected (75 copies/mL), treatment was interrupted in mutual agreement with the patient because of a persisting radiologic response.
One month later, she presented at the outpatient clinic with pyrexia, lethargy, and bone pains. Inflammatory blood parameters were increased, and the plasma ctDNA count was once more detected at a level of 27 copies/mL. FDG-PET/CT scans showed diffuse hypermetabolism of the bone marrow but no measurable lesions. Dabrafenib-trametinib was restarted at the previous dose level, and symptoms resolved quickly. The ctDNA count decreased again to an undetectable level up to the latest follow-up.
This case illustrates three interesting and promising concepts with respect to treatment and follow-up of patients with BRAFV600E-mutant LCH. First, this patient was treated with chemotherapy but progressed rapidly. Subsequent treatment with dabrafenib-trametinib led to immediate symptomatic relief and a metabolic response. There are several reports of treating BRAFV600-mutant LCH with BRAF inhibitor monotherapy. To the best of our knowledge, this is the first report of successful first-line treatment of BRAFV600E-mutant LCH with combined BRAF-MEK inhibition. Reversal of acquired resistance through a KRAS mutation to dabrafenib by adding on trametinib has been reported in ECD.12 A trial with dabrafenib-trametinib in BRAFV600-mutant ECD is underway (NCT02281760; Dabrafenib and Trametinib in People With BRAF V600E Mutation Positive Lesions in Erdheim Chester Disease).
Second, interrupting dabrafenib-trametinib treatment led to a rapid clinical and biological relapse, and response could be rapidly regained after restarting the same treatment. This suggests that BRAFV600E-mutant LCH is dependent on mutant BRAF for its malignant phenotype, but in our case, acquired resistance did not emerge over a long period of treatment. Almost immediate reactivation of the disease upon withdrawal of BRAF-MEK inhibition suggests that a small proportion of cells that remain dormant during therapy mediate a relapse when BRAF-MEK inhibition is withheld. However, retreating with BRAF-MEK inhibition can be a viable option, as illustrated by our case.
Third, monitoring plasma BRAFV600E-mutant ctDNA is a promising biomarker: disappearance of ctDNA in the plasma mirrors treatment response, and reappearance of ctDNA in plasma indicates disease activity and disease relapse. At baseline, our patient showed a positive count that quickly resolved to undetectable after initiating treatment and remained undetectable during treatment. During the period of therapeutic noncompliance and after interrupting treatment, we detected ctDNA, which corresponded with clinical deterioration and increased blood inflammatory parameters. After retreatment with dabrafenib and trametinib, the ctDNA count returned to undetectable, in concordance with clinical and biological evolution.
This case suggests that combined BRAF-MEK inhibition can achieve a sustained response in BRAFV600E-mutant LCH. Interrupting treatment could lead to a rapid relapse, but the response can be regained by restarting the same treatment. Detection of plasma BRAFV600E-mutant ctDNA is a promising biomarker for monitoring treatment response and as an indicator for relapse, potentially before the appearance of radiologically detectable lesions. These observations warrant further confirmation in a prospective study.
Acknowledgment
This research has been partially funded by a scientific grant from Kom op tegen Kanker.
Authorship
Contribution: G.A. treated the patient, wrote the article, and collected remarks from other authors; T.S. treated the patient, analyzed ctDNA samples, and reviewed the manuscript; K.F. treated the patient and reviewed the manuscript; H.E. performed metabolic imaging with FDG-PET/CT and provided images for the manuscript; and B.N. treated the patient and reviewed the manuscript.
Conflict-of-interest disclosure: G.A. received funding for travel, accommodations, and expenses from Merck Sharp & Dohme and Pfizer. T.S. served as consultant or advisor and served on the speakers’ bureau for Novartis and received funding for travel, accommodations, and expenses from GlaxoSmithKline and Novartis. B.N. received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche; served as a consultant or advisor for Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche; served on the speakers’ bureau for Novartis; received research funding from Merck KGaA, Novartis, and Pfizer; and received funds for travel, accommodations, and expenses from Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Gil Awada, Department of Medical Oncology, Universitair Ziekenhuis Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium; e-mail: gil.awada@uzbrussel.be.