Key Points
Serum albumin and cholesterol levels predict survival in primary myelofibrosis, independent of each other and contemporary risk models.
The cachexia index, determined by serum albumin and cholesterol levels, might further refine current prognostic models in myelofibrosis.
Introduction
Constitutional symptoms, defined as the presence of weight loss that is >10% of baseline in the year before diagnosis or unexplained excessive sweats or fever persisting for at least a month1 is a consistent risk variable in current prognostic models in primary myelofibrosis (PMF). Other prognostic models include the International Prognostic Scoring System (IPSS),1 Dynamic IPSS (DIPSS),2 DIPSS-plus,3 and the recently unveiled mutation-enhanced IPSS (MIPSS70 and MIPSS70-plus).4 It is currently assumed that aberrant expression of inflammatory cytokines is the underlying pathology responsible for constitutional symptoms in PMF and is also the target for Janus kinase 2 (JAK2) inhibitor activity.5 In other words, constitutional symptoms in PMF reflect the hypercatabolic/cachectic state of the disease; the latter might also be assessed by using laboratory biomarkers of cachexia.6,7 Accordingly, we focused on serum albumin and cholesterol levels because of their known susceptibility to the effects of myelofibrosis-associated inflammatory cytokines and their global applicability.8-11 The phenomenon of markedly decreased cholesterol levels in myelofibrosis has long been recognized,12 and its prognostic relevance has been highlighted in multiple abstracts.13-16 More recent reports have suggested a similar scenario with serum albumin.17,18 In this study, we used both serum cholesterol and albumin levels to develop a new cachexia index for PMF that might further enhance prognostication in PMF.
Methods
After approval from the Mayo Clinic review board, study patients were selected from institutional databases. PMF was diagnosed according to World Health Organization criteria.19 Patient histories were retrospectively reviewed to identify patients with available information on serum albumin or cholesterol levels collected at time of diagnosis or first referral to our institution. To minimize drug-induced confounding of serum lipid levels, patients receiving statins or other lipid-lowering drugs were excluded from the study. Standard statistical tests were used to determine the significance of associations. Survival data were prepared by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazards regression models were applied for multivariable analysis. Covariates for univariable and multivariable analyses were selected on the basis of previous knowledge of their prognostic significance; a step-wise method was used with a backward elimination probability threshold of 0.1. We considered all variables currently included in DIPSS, DIPSS-plus, and MIPSS70-plus. Receiver operating characteristic (ROC) plots were used to determine serum cholesterol and albumin levels that were prognostically most discriminative in PMF; we made no assumptions regarding the relationship between cachexia and serum levels of cholesterol or albumin and simply used ROC analysis to determine discrimination points after confirming prognostic relevance of serum albumin and cholesterol levels as continuous variables.
Results and discussion
In all, 1109 consecutive patients with PMF were reviewed. Serum albumin level was recorded in 484 patients, and total cholesterol (TC) was recorded in 374; 179 patients had information on both cholesterol and albumin levels. Use of lipid-lowering drugs, including statins, was documented in 125 patients, including 29 patients with information on both parameters, warranting their exclusion from further analysis. After patients receiving lipid-lowering drugs were excluded, 414 patients were evaluable for serum albumin, 318 for TC, and 150 for both.
Table 1 lists presenting clinical and laboratory characteristics stratified by ROC-determined cutoff values for serum albumin (4.3 g/dL) and TC (122 mg/dL); the reference range for serum albumin level at our institution was 3.5 to 5 g/dL, and a TC level of <200 mg/dL was considered desirable (ie, normal), whereas TC levels of 200 to 239 mg/dL were considered borderline high and TC levels ≥240 mg/dL were considered high. Table 1 shows that patients with serum albumin levels <4.3 g/dL were older and universally displayed prognostically adverse clinical features and higher DIPSS/DIPSS-plus risk distribution; by contrast, serum albumin level did not correlate with cytogenetic or mutation profiles. Patients with serum TC <122 mg/dL were also more likely to display adverse features and higher-risk disease. In addition, there was a preponderance of males, and significant clustering occurred with the presence of JAK2 and ASXL1 mutations and the absence of CALR type 1–like mutation.
Clinical and laboratory characteristics of 414 patients with primary myelofibrosis studied for serum albumin level and 318 patients studied for serum total cholesterol
| Characteristic . | Serum albumin <4.3 g/dL (n = 285), . | Serum albumin ≥4.3 g/dL (n = 129), . | P . | Serum total cholesterol <122 mg/dL (n = 119), . | Serum total cholesterol ≥122 mg/dL (n = 199), . | P . |
|---|---|---|---|---|---|---|
| n (%) . | n (%) . | n (%) . | n (%) . | |||
| Age, y | ||||||
| Median (range) | 67 (22-92) | 61 (19-84) | <.001 | 64 (26-86) | 61 (19-88) | .02 |
| >65 | 157 (55) | 44 (34) | <.001 | 53 (45) | 79 (40) | .4 |
| Males | 182 (64) | 71 (55) | .09 | 93 (78) | 108 (54) | <.001 |
| Hemoglobin <10 g/dL | 172 (60) | 42 (33) | <.001 | 68 (57) | 78 (39) | .002 |
| Requiring transfusion | 121 (43) | 29 (23) | <.001 | 48 (40) | 46 (23) | .001 |
| Leukocytes >25 × 109/L | 56 (20) | 10 (8) | .001 | 20 (17) | 26 (13) | .36 |
| Platelets <100 × 109/L | 87 (31) | 23 (18) | .006 | 29 (24) | 45 (23) | .72 |
| Circulating blasts ≥1% | 156 (55) | 44 (34) | <.001 | 73 (61) | 87 (44) | .002 |
| Constitutional symptoms | 113 (40) | 26 (20) | <.001 | 50 (42) | 45 (23) | <.001 |
| DIPSS risk distribution* | <.001 | <.001 | ||||
| High | 46 (16) | 2 (1.6) | 14 (12) | 13 (7) | ||
| Intermediate-2 | 126 (44) | 39 (30) | 66 (56) | 65 (33) | ||
| Intermediate-1 | 96 (34) | 54 (42) | 26 (22) | 76 (38) | ||
| Low | 17 (6) | 34 (26) | 13 (11) | 45 (23) | ||
| DIPSS-plus risk distribution (no. evaluable: 411 [albumin]; 316 [cholesterol])† | < .001 | <.001 | ||||
| High | 123 (43) | 23 (18) | 50 (42) | 50 (25) | ||
| Intermediate-2 | 109 (38) | 46 (36) | 46 (39) | 65 (33) | ||
| Intermediate-1 | 37 (13) | 29 (23) | 12 (10) | 41 (21) | ||
| Low | 15 (5) | 29 (23) | 11 (9) | 41 (21) | ||
| Driver mutations (no. evaluable: 277 [albumin]; 234 [cholesterol]) | .67 | .005 | ||||
| JAK2 | 122 (69) | 63 (64) | 64 (75) | 83 (56) | ||
| CALR type 1–like | 31 (17) | 21 (21) | 5 (6) | 34 (23) | ||
| CALR type 2–like | 6 (3) | 2 (2) | 3 (4) | 7 (5) | ||
| MPL | 7 (4) | 7 (7) | 3 (4) | 9 (6) | ||
| Triple-negative | 12 (7) | 6 (6) | 10 (12) | 16 (11) | ||
| Revised cytogenetic risk distribution (no. evaluable: 389 [albumin]; 303 [cholesterol])‡ | .14 | .12 | ||||
| Very high risk | 29 (11) | 6 (5) | 8 (7) | 5 (3) | ||
| Unfavorable | 43 (16) | 22 (18) | 14 (12) | 32 (17) | ||
| Favorable | 196 (73) | 93 (77) | 95 (81) | 149 (80) | ||
| ASXL1-mutated (no. evaluable: 201 [albumin]; 191 [cholesterol]) | 51 (40) | 20 (27) | .06 | 42 (58) | 39 (33) | <.001 |
| SRSF2-mutated (no. evaluable: 204 [albumin]; 191 [cholesterol]) | 21 (16) | 13 (17) | P = .89 | 13 (18) | 10 (8) | .06 |
| Characteristic . | Serum albumin <4.3 g/dL (n = 285), . | Serum albumin ≥4.3 g/dL (n = 129), . | P . | Serum total cholesterol <122 mg/dL (n = 119), . | Serum total cholesterol ≥122 mg/dL (n = 199), . | P . |
|---|---|---|---|---|---|---|
| n (%) . | n (%) . | n (%) . | n (%) . | |||
| Age, y | ||||||
| Median (range) | 67 (22-92) | 61 (19-84) | <.001 | 64 (26-86) | 61 (19-88) | .02 |
| >65 | 157 (55) | 44 (34) | <.001 | 53 (45) | 79 (40) | .4 |
| Males | 182 (64) | 71 (55) | .09 | 93 (78) | 108 (54) | <.001 |
| Hemoglobin <10 g/dL | 172 (60) | 42 (33) | <.001 | 68 (57) | 78 (39) | .002 |
| Requiring transfusion | 121 (43) | 29 (23) | <.001 | 48 (40) | 46 (23) | .001 |
| Leukocytes >25 × 109/L | 56 (20) | 10 (8) | .001 | 20 (17) | 26 (13) | .36 |
| Platelets <100 × 109/L | 87 (31) | 23 (18) | .006 | 29 (24) | 45 (23) | .72 |
| Circulating blasts ≥1% | 156 (55) | 44 (34) | <.001 | 73 (61) | 87 (44) | .002 |
| Constitutional symptoms | 113 (40) | 26 (20) | <.001 | 50 (42) | 45 (23) | <.001 |
| DIPSS risk distribution* | <.001 | <.001 | ||||
| High | 46 (16) | 2 (1.6) | 14 (12) | 13 (7) | ||
| Intermediate-2 | 126 (44) | 39 (30) | 66 (56) | 65 (33) | ||
| Intermediate-1 | 96 (34) | 54 (42) | 26 (22) | 76 (38) | ||
| Low | 17 (6) | 34 (26) | 13 (11) | 45 (23) | ||
| DIPSS-plus risk distribution (no. evaluable: 411 [albumin]; 316 [cholesterol])† | < .001 | <.001 | ||||
| High | 123 (43) | 23 (18) | 50 (42) | 50 (25) | ||
| Intermediate-2 | 109 (38) | 46 (36) | 46 (39) | 65 (33) | ||
| Intermediate-1 | 37 (13) | 29 (23) | 12 (10) | 41 (21) | ||
| Low | 15 (5) | 29 (23) | 11 (9) | 41 (21) | ||
| Driver mutations (no. evaluable: 277 [albumin]; 234 [cholesterol]) | .67 | .005 | ||||
| JAK2 | 122 (69) | 63 (64) | 64 (75) | 83 (56) | ||
| CALR type 1–like | 31 (17) | 21 (21) | 5 (6) | 34 (23) | ||
| CALR type 2–like | 6 (3) | 2 (2) | 3 (4) | 7 (5) | ||
| MPL | 7 (4) | 7 (7) | 3 (4) | 9 (6) | ||
| Triple-negative | 12 (7) | 6 (6) | 10 (12) | 16 (11) | ||
| Revised cytogenetic risk distribution (no. evaluable: 389 [albumin]; 303 [cholesterol])‡ | .14 | .12 | ||||
| Very high risk | 29 (11) | 6 (5) | 8 (7) | 5 (3) | ||
| Unfavorable | 43 (16) | 22 (18) | 14 (12) | 32 (17) | ||
| Favorable | 196 (73) | 93 (77) | 95 (81) | 149 (80) | ||
| ASXL1-mutated (no. evaluable: 201 [albumin]; 191 [cholesterol]) | 51 (40) | 20 (27) | .06 | 42 (58) | 39 (33) | <.001 |
| SRSF2-mutated (no. evaluable: 204 [albumin]; 191 [cholesterol]) | 21 (16) | 13 (17) | P = .89 | 13 (18) | 10 (8) | .06 |
Patients were stratified by ROC-determined cutoff points for albumin and total cholesterol. The values in bold indicate a significant P value (< .05).
DIPSS uses 5 independent predictors of inferior survival: age >65 years, hemoglobin <10 g/dL, leukocytes >25 × 109/L, circulating blasts ≥1%, and constitutional symptoms.
DIPSS-plus includes all the DIPSS variables plus 3 more: red cell transfusion need, platelet count <100 × 109/L, and unfavorable karyotype.
Revised cytogenetic risk stratification: very high risk includes single or multiple abnormalities of −7, i(17q), inv(3)/3q21, 12p-/12p11.2, 11q–/11q23, +21, or other autosomal trisomies, not including +8/+9; favorable risk includes normal karyotype or sole abnormalities of 13q–, +9, 20q–, chromosome 1 translocation, or duplication or sex chromosome abnormality including –Y; unfavorable risk includes all other abnormalities.
ASXL1, additional sex combs–like 1; CALR, calreticulin; JAK2, Janus kinase 2; MPL, myeloproliferative leukemia virus oncogene; SRSF2, serine/arginine-rich splicing factor 2.
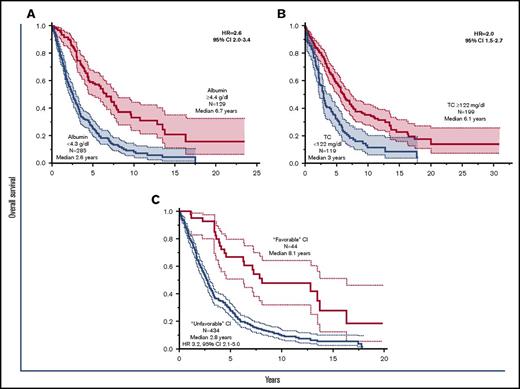
During age-adjusted univariable analysis, lower levels of serum albumin (P < .001), TC (P < .001), high-density lipoprotein (P < .001), and low-density lipoprotein (P < .001) cholesterol, but not triglycerides (P = .6), were associated with shortened survival; only the first two retained their significance on multivariable analysis. ROC analysis resulted in a TC level of 122 mg/dL and serum albumin level of 4.3 g/dL as the most optimal cutoff values for survival prediction (Figure 1A-B). Serum albumin level of <4.3 g/dL and TC level of <122 mg/dL both remained significant during multivariable analysis that included DIPSS, DIPSS-plus, or the revised cytogenetic risk stratification for PMF; their respective hazard ratios (HRs) and 95% confidence intervals (CIs) were 1.8 (95% CI, 1.2-2.8) and 1.8 (95% CI,1.2-2.8) with DIPSS (informative n = 150), 1.7 (95% CI, 1.1-2.6) and 1.7 (95% CI, 1.1-2.5) with DIPSS-plus (informative n = 149), and 2.8 (95% CI, 1.8-4.4) and 2.2 (95% CI, 1.4-3.4) with cytogenetic risk (informative n = 137). Additional multivariable analysis that included the individual components of DIPSS confirmed the interindependent prognostic contribution of serum albumin (P = .02) and TC (P < .001), whereas it resulted in loss of significance for constitutional symptoms (P = .86) and circulating blasts ≥1% (P = .89). The results were similar with DIPSS-plus risk variables, for which prognostic independence was confirmed for both albumin (P < .01) and TC (P < .01), as well as for unfavorable karyotype (P < .01), age >65 years (P < .01), leukocyte count >25 × 109/L (P < .01), and transfusion need (P = .03). All other DIPSS-plus variables lost significance with P values ranging from .34 to .70.
Survival data in primary myelofibrosis stratified by serum albumin and cholesterol levels. Survival data (A) among 414 patients with primary myelofibrosis studied for serum albumin level and (B) among 318 patients studied for serum total cholesterol (TC) level. (C) Shows 478 patients whose data were informative for a subthreshold level for either serum albumin or cholesterol (operationally categorized as unfavorable cachexia index) compared with those in whom both values were above the threshold levels (operationally categorized as favorable cachexia index).
Survival data in primary myelofibrosis stratified by serum albumin and cholesterol levels. Survival data (A) among 414 patients with primary myelofibrosis studied for serum albumin level and (B) among 318 patients studied for serum total cholesterol (TC) level. (C) Shows 478 patients whose data were informative for a subthreshold level for either serum albumin or cholesterol (operationally categorized as unfavorable cachexia index) compared with those in whom both values were above the threshold levels (operationally categorized as favorable cachexia index).
Serum albumin and cholesterol levels were consequently used to develop an operational cachexia index for PMF: a score of 0 was assigned when both albumin and TC levels were above the aforementioned ROC-determined cutoff levels and a score of 1 or 2 was assigned when 1 or both of the 2 laboratory parameters was below the cutoff level. The prognostic contribution of the PMF cachexia index was independent of DIPSS (no. evaluable = 150), DIPSS-plus (no. evaluable = 149), and the revised cytogenetic risk stratification (no. evaluable = 137). HRs with DIPSS were 1.9 (95% CI, 1.1-3.3) for cachexia index 1 and 3.3 (95% CI, 1.8-6.2) for cachexia index 2. With DIPSS-plus, HRs were 1.8 (95% CI, 1.1-3.2) for cachexia index 1 and 2.8 (95% CI, 1.5-5.3) for cachexia index 2. With the revised cytogenetic risk stratification, HRs were 2.0 (95% CI, 1.2-3.5) for cachexia index 1 and 5.8 (95% CI, 3.2-11.1) for cachexia index 2. To optimize its prognostic value and facilitate its incorporation into prognostic models, the cachexia index was subsequently considered as a binary variable with favorable (cachexia index 0) and unfavorable (cachexia index 1 or 2) categories (HR, 3.2; 95% CI, 2.1-5.0; Figure 1C). The prognostic contribution of unfavorable cachexia index was independent of DIPSS (P < .001), DIPSS-plus (P < .001), and the revised cytogenetic risk stratification (P < .001). The 2-tiered PMF cachexia index also remained significant against driver mutational status (no. evaluable = 334; P < .001), ASXL1 mutations (no. evaluable = 245; P = .01), SRSF2 mutations (no. evaluable 245; P < .001), and MIPSS70-plus (no. evaluable = 185; P = .03).
We have developed an operational cachexia index for PMF using 2 simple and widely available laboratory tests: serum albumin and serum cholesterol levels. Considered as a binary variable that is favorable (cachexia index 0) or unfavorable (cachexia index 1 and 2), the PMF cachexia index remained significant in the context of contemporary prognostic models, including those with cytogenetic and molecular information. Note that additional parameters other than serum albumin and cholesterol levels might need to be examined in the future for their additional contribution to cachexia index. Regardless, statistically optimal incorporation of cachexia index into prognostic models is likely to require its consideration as a binary variable (eg, favorable vs unfavorable cachexia index), regardless of the raw cachexia index scores. Again, note that our proposed determination of cachexia index was not based on PMF-specific disease features but on parameters that are commonly affected in a spectrum of malignant and nonmalignant diseases; that is, they are considered to be general indicators of level of cachexia and sickness. However, this fact does not undermine their value as prognostic biomarkers. Finally, the effect of treatment, especially using JAK2 inhibitors, on the PMF cachexia index should be prospectively evaluated in future studies, and the prognostic impact of its reversal should be assessed.
Authorship
Contribution: A.T. designed the study, contributed patients, helped abstract patient information, performed statistical analysis, and wrote the paper; A.D.P. and N.G. contributed patients and helped abstract patient information; R.P.K. was in charge of cytogenetic information; C.A.H. was in charge of information on pathology; T.L.L. was in charge of mutation analysis and interpretation; N.S., M.M., D.P., and M.N. helped abstract information from patient history; M.N. was in charge of obtaining serum lipid and albumin levels and also helped with statistical analysis; and all authors reviewed the manuscript and gave their approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayalew Tefferi, Division of Hematology, Department of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: tefferi.ayalew@mayo.edu.