Key Points
A novel TLR2 agonist was identified that stimulates the release of G-CSF, IL-6, and monocyte chemoattractant protein 1.
This agonist increases neutrophil numbers in a primate model of neutropenia.
Abstract
Neutropenia is a common consequence of radiation and chemotherapy in cancer patients. The resulting immunocompromised patients become highly susceptible to potentially life-threatening infections. Granulocyte colony-stimulating factor (G-CSF) is known to stimulate neutrophil production and is widely used as a treatment of chemotherapy-induced neutropenia. A small-molecule G-CSF secretagogue without a requirement for refrigerated supply chain would offer a more convenient and cost-effective treatment of chemotherapy-induced neutropenia. Bacterial lipopeptides activate innate immune responses through Toll-like receptor 2 (TLR2) and induce the release of cytokines, including G-CSF, from macrophages, monocytes, and endothelial. Pam2CSK4 is a synthetic lipopeptide that effectively mimics bacterial lipoproteins known to activate TLR2 receptor signaling through the TLR2/6 heterodimer. Substrate-based drug design led to the discovery of GSK3277329, which stimulated the release of G-CSF in activated THP-1 cells, peripheral blood mononuclear cells, and human umbilical vein endothelial cells. When administered subcutaneously to cynomolgus monkeys (Macaca fascicularis), GSK3277329 caused systemic elevation of G-CSF and interleukin-6 (IL-6), but not IL-1β or tumor necrosis factor α, indicating a selective cytokine-stimulation profile. Repeat daily injections of GSK3277329 in healthy monkeys also raised circulating neutrophils above the normal range over a 1-week treatment period. More importantly, repeated daily injections of GSK3277329 over a 2-week period restored neutrophil loss in monkeys given chemotherapy treatment (cyclophosphamide, Cytoxan). These data demonstrate preclinical in vivo proof of concept that TLR2 agonism can drive both G-CSF induction and subsequent neutrophil elevation in the cynomolgus monkey and could be a therapeutic strategy for the treatment of chemotherapy-induced neutropenia.
Introduction
Neutropenia, a disorder characterized by reduction in white blood cell count (primarily neutrophils), is a common consequence of radiation and chemotherapy treatment in cancer patients. The resulting immunocompromised patients become highly susceptible to potentially life-threatening infections.1
Granulocyte colony-stimulating factor (G-CSF) is known to stimulate neutrophil production and is widely used as a treatment of chemotherapy-induced neutropenia.2-5 However, recombinant G-CSF has significant manufacturing costs and requires refrigerated storage and a cold-supply chain, issues that are particularly limiting in developing countries. A small-molecule G-CSF secretagogue without these issues would offer a more convenient and cost-effective treatment of chemotherapy-induced neutropenia, particularly in emerging countries.
G-CSF is secreted by several cell types, including vascular endothelial cells,6 fibroblasts,7 and most notably cells of the monocyte/macrophage lineage (reviewed in Demetri and Griffin8 ). These cells secrete G-CSF in response to a variety of factors, including tumor necrosis factor α (TNF-α),7 interleukin-1 (IL-1),6,9 and the Toll-like receptor (TLR) agonist lipopolysaccharide (LPS).10,11
TLRs are a family of pattern recognition receptors that participate in innate immune responses by distinguishing pathogens from the commensal microbiome. TLRs recognize cell-surface components commonly expressed by bacteria, viruses, fungi, and protozoa.12 Among the TLRs, TLR2 recognizes the widest range of bacterial cell surface products including lipopolysaccharides, lipoproteins, lipoarabinomannans, glycosylphosphatidylinositol, glycoproteins, and peptidoglycans.13 Among these, lipopeptides appear to be the most robust activators of TLR2 signaling mediated by heterodimeric complexes with either TLR1 or TLR6.14
TLR2 is expressed on CD14+ monocytes,15 as well as vascular endothelial cells.16,17 Human umbilical vein endothelial cells (HUVECs) constitutively express TLR2 and TLR4 and produce IL-6 in response to LPS.18 In general, activation of these receptors triggers the release of cytokines, chemokines, and adaptive immune responses with some degree of selectivity imparted by the types of pathogens encountered.19 Interestingly, LPS can induce the release of cytokines, including G-CSF, from macrophages, monocytes, and endothelial cells.20 Thus, TLR2 agonists derived from LPS such as Pam2CSK4 are in use as adjuvants for vaccines,21,22 and the related agonist, Pam3CSK4, can facilitate engraftment of allogenic hematopoietic stem cells similar to G-CSF–induced engraftment.23
Pam2CSK4 and Pam3CSK4 are synthetic lipopeptides that effectively mimic bacterial lipoproteins known to activate TLR2 receptor signaling and, like LPS, behave as G-CSF secretagogues by inducing the release of G-CSF from bone marrow–derived macrophages.20 Small-molecule TLR2/4 agonists have been identified,24 and others have reported the discovery of small molecules that selectively activate TLR2/TLR1 pairing.25-27
These data prompted us to identify a small-molecule TLR2 agonist suitable to test the hypothesis that TLR2 receptor agonism would induce G-CSF and, in turn, reverse chemotherapy-induced neutropenia in vivo. Substrate-based drug design led to the discovery of GSK3277329, a more drug-like truncated analog of Pam2CSK4. In this report, we present the characterization of GSK3277329, which stimulates the release of G-CSF both in vitro and in vivo, induces neutrophil production in vivo, and reverses chemotherapy-induced neutropenia in nonhuman primates.
Materials and methods
Chemistry
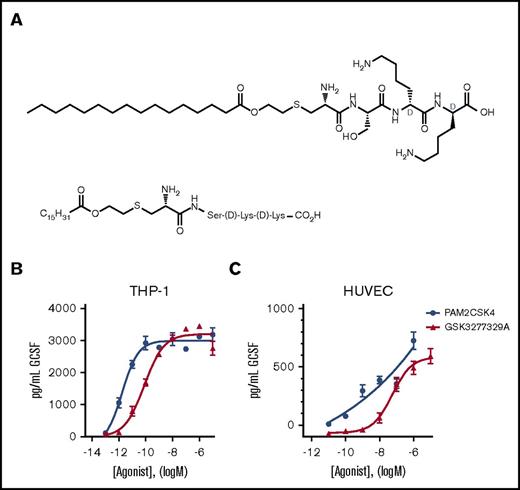
Lead optimization focused on substrate-based drug design using Pam2CSK4 as the starting point; details of this work will be the subject of a future disclosure (including relevant chemical syntheses). These efforts lead to the discovery of GSK3277329 (see Figure 1 for chemical structure), a monolipid tetrapeptide that potently stimulates G-CSF release in vitro and has suitable pharmacokinetic properties for evaluating the mechanism and tolerability of TLR2 agonism in vivo. Synonyms for GSK3277329 include (2R,5R,8S,11R)-11-amino-2,5-bis(4-aminobutyl)-8-(hydroxymethyl)-4,7,10,17-tetraoxo-16-oxa-13-thia-3,6,9-triazadotriacontan-1-oic acid, 2-(hexadecanoyloxyl)ethyl](d-lysyl-d-lysylseryl)cysteinyl, and Pam-Cys-Ser-D-Lys-D-Lys.
Structure and in vitro activity of GSK3277329. (A) Chemical structure of GSK3277329. (B-C) TLR2 agonists stimulate G-CSF release in a concentration-dependent manner in PMA-differentiated human THP-1 cells (n = 2 replicates) (B) and HUVECs (GSK329 n = 6 replicates, PAM2CSK4 n = 2 replicates) (C). Graphs show mean ± standard error of the mean (SEM). Error bars are not shown if the error is smaller than the symbol size.
Structure and in vitro activity of GSK3277329. (A) Chemical structure of GSK3277329. (B-C) TLR2 agonists stimulate G-CSF release in a concentration-dependent manner in PMA-differentiated human THP-1 cells (n = 2 replicates) (B) and HUVECs (GSK329 n = 6 replicates, PAM2CSK4 n = 2 replicates) (C). Graphs show mean ± standard error of the mean (SEM). Error bars are not shown if the error is smaller than the symbol size.
Cell culture
Pooled HUVECs were obtained from Lonza. Cells were grown in complete EGM-2 media. THP-1 cells were differentiated for 3 days with 1 mM PMA (phorbol 12-myristate 13-acetate). After 3 days, media with PMA was removed, and cells were treated with agonists for 20 to 24 hours. In order to avoid precipitation of the compounds shown, which have low aqueous solubility, compounds were stepwise diluted as follows for cell culture: 10 mM compound stocks in 100% dimethyl sulfoxide (DMSO) were diluted 1:25 in modified vehicle (21% DMSO, 79% sterile H2O, with 1% fetal bovine serum [FBS]) followed by 1:10 serial dilution in vehicle (25% DMSO, 75% sterile H2O, with 1%FBS). Once diluted compounds were prepared, final addition of compounds was done at 1:40 addition onto cells/media with 1% FBS. G-CSF was measured in cell supernatant with the human G-CSF Quantikine ELISA kit (R&D Systems, Minneapolis, MN). The enzyme-linked immunosorbent assay kit is a quantitative sandwich enzyme immunoassay and was run according to kit instructions. Half-maximal effective concentration (EC50) values were determined by GraphPad Prism software.
Animals
Animal studies were conducted in accordance with the protocols of the WuXi AppTec Institutional Animal Care and Use Committee according to the guidelines established by Association for Assessment and Accreditation of Laboratory Animal Care. Male Macaca fascicularis (Hainan Jingang Laboratory Animal Co., Haikou, Hainan Province, China) weighing 4 to 7 kg were randomly assigned to treatment groups based on body weight. All animals were acclimated by subcutaneous injection of saline for 7 days prior to compound evaluation to reduce stress-induced changes in circulating neutrophil counts. At the end of the study, animals were observed for 14 days. Vehicle-treated animals were returned to the colony. All cyclophosphamide-treated animals were killed at the end of the study.
Compound dosing
Cyclophosphamide (Cytoxan; JiangSu ShengDi Pharmaceuticals Co.) was prepared as a solution in saline at 20 mg/mL. Cyclophosphamide was administered at 60 mg/kg IV (3 mL/kg). GSK3277329 and Pam2CSK4 was formulated in saline and dosed subcutaneously at 1 mL/kg. Doses ranged from 1 to 15 mg/kg. Recombinant human G-CSF (Neupogen, Amgen Manufacturing) at 10 μg/mL was prepared by diluting the bulk G-CSF (300 μg/mL) with saline and dosed at 1 mL/kg.
Serum analyte determinations
For serum sample processing for cytokine, 1.5 mL blood was collected in a polypropylene tube and remained at room temperature for at least 1 hour before centrifugation. Samples were centrifuged (3000g for 15 minutes at 2°C to 8°C), and then serum was divided into 3 aliquots (0.15 mL per aliquot) and transferred to prelabeled polyethylene microcentrifuge tube. Plasma samples were obtained by collecting 0.5 mL blood in a tube containing potassium (K2) EDTA (8 μL, 0.5 M) on wet ice. Samples were centrifuged (3000g for 10 minutes at 2°C to 8°C) immediately. The plasma samples were stored in polypropylene microcentrifuge tubes at −60°C until analysis. Plasma levels of GSK3277329 were determined by liquid chromatography-tandem mass spectrometry. Whole blood (2.0 mL) was collected in a tube containing potassium (K2) EDTA for hematology counts. Plasma G-CSF/IL-6 levels were determined with Luminex technology and followed the methodology provided by the vendor (ProcartaPlexTM Multiplex Immunoassay, catalog no. EPX010-40420-901 [buffer kit]; EPX010-42001-901 [G-CSF]; EPX010-36053-901 [IL-6], lot no. 108679000 [buffer kit]; 114433005 [G-CSF]; 110880008 [IL-6]). Reagents were purchased from eBioscience.
Statistical analysis
Statistical significance was determined by analysis of variance (ANOVA) and post hoc tests as noted by using the GraphPad Prism software.
Results
In vitro assessment
Pam2CSK4 is a synthetic diacylated lipopeptide that signals through TLR2. This agonist was evaluated in human peripheral blood mononuclear cells, PMA-differentiated THP-1 cells, and HUVECs to compare activation of the TLR2 receptor on mononuclear, monocytic, and endothelial cells. Pam2CSK4 induced G-CSF release from all 3 cell types, with mean EC50 values of 0.002 nM and 0.04 nM for human THP-1 and HUVECs, respectively. Pam2CSK4 is a bilipid acylated hexapeptide with a large molecular weight and poor physicochemical properties, which lead to difficulty in handling. Therefore, an effort was made to make lipopeptide truncates that were more drug-like, in particular reducing the molecular weight while still potently affecting G-CSF release. Thus, GSK3277329, a small synthetic monoacylated lipopeptide (Figure 1), was made that stimulated G-CSF release with slightly reduced potency compared with Pam2CSK4 in PMA-differentiated THP-1 with a mean EC50 value of 0.08 nM. Relative to THP-1 cells, its potency on HUVECs was significantly reduced (EC50 value, 2 µM) (Figure 1). The cells of mononuclear and monocytic lineage secreted significantly more G-CSF than vascular endothelial cells in response to TLR2 activation. Undifferentiated THP-1 cells did not respond to the TLR2 agonists (data not shown).
In vivo pharmacokinetics of GSK3277329
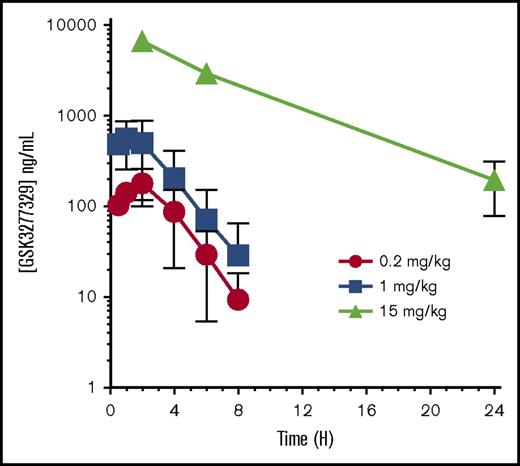
Pharmacokinetic evaluation of GSK3277329 in M fascicularis after subcutaneous injection revealed a dose-dependent exposure up to 15 mg/kg (Figure 2). Peak exposure occurred ∼1 hour after injection (Table 1). At 15 mg/kg, circulating levels were maintained above 800 nM (196 ng/mL) over the 24-hour sampling period. These levels were well above the 1 to 10 nanomolar EC90 concentration values needed to stimulate G-CSF release in monocytic cells (THP-1).
Pharmacokinetics of GSK3277329 in M fascicularis. Compound levels were measured in plasma at the indicated times after subcutaneous injection of 0.2, 1, and 15 mg/kg. Mean ± SEM of 2 animals per group at 0.2 and 1 mg/kg and 3 animals for the 15-mg/kg dose group are shown. Error bars are not shown if the error is smaller than the symbol size. H, hours.
Pharmacokinetics of GSK3277329 in M fascicularis. Compound levels were measured in plasma at the indicated times after subcutaneous injection of 0.2, 1, and 15 mg/kg. Mean ± SEM of 2 animals per group at 0.2 and 1 mg/kg and 3 animals for the 15-mg/kg dose group are shown. Error bars are not shown if the error is smaller than the symbol size. H, hours.
Pharmacokinetic characteristics of GSK3277329 in M fascicularis after subcutaneous administration
| Nominal dose . | 0.2 mg/kg (n = 2) . | 1 mg/kg (n = 2) . | 15 mg/kg (n = 3) . |
|---|---|---|---|
| Cmax, ng/mL | 184 (130, 237) | 615 (779, 450) | 6707 ± 1509 |
| Tmax, h | 1.5 (1.0, 2.0) | 0.75 (1.0, 0.5) | 2 |
| t1/2, h | 1.21 (1.13, 1.29) | 1.48 (1.96, 1.00) | 4.35 ± 0.639 |
| AUC0-inf, ng × h/mL | 662 (425, 899) | 2004 (3080, 928) | 44 181 ± 10 251 |
| Nominal dose . | 0.2 mg/kg (n = 2) . | 1 mg/kg (n = 2) . | 15 mg/kg (n = 3) . |
|---|---|---|---|
| Cmax, ng/mL | 184 (130, 237) | 615 (779, 450) | 6707 ± 1509 |
| Tmax, h | 1.5 (1.0, 2.0) | 0.75 (1.0, 0.5) | 2 |
| t1/2, h | 1.21 (1.13, 1.29) | 1.48 (1.96, 1.00) | 4.35 ± 0.639 |
| AUC0-inf, ng × h/mL | 662 (425, 899) | 2004 (3080, 928) | 44 181 ± 10 251 |
Means of 2 animals reported for 0.2- and 1-mg/kg dosed animals. Individual values are listed in parentheses. Means and SD values are reported for the 15-mg/kg dose.
AUC, area under the curve; Cmax, maximum serum concentration; inf, infinity; t1/2, half-life; Tmax, time taken to reach maximum concentration.
In vivo stimulation of cytokines and neutrophils
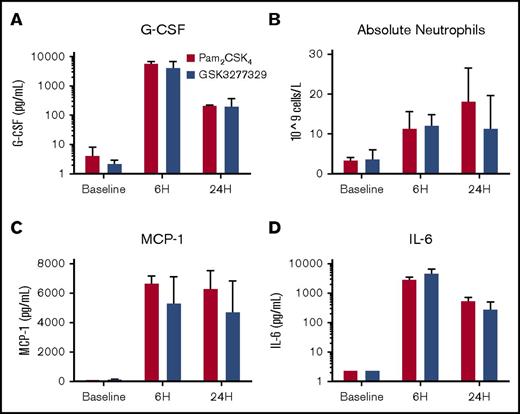
A single dose of Pam2CSK4 or the monoacylated lipopeptide GSK3277329 increased serum G-CSF, monocyte chemoattractant protein 1 (MCP-1), IL-6, and neutrophils in the circulation of normal M fascicularis (Figure 3). The level of induction of the cytokines (4.2 vs 5.9 ng/mL for G-CSF) as well as the neutrophils (12.1 × 109 vs 11.4 × 109 cells/L) was similar between GSK3277329 and Pam2CSK4, respectively. TNF-α and IL-1β levels were not changed by either of these TLR2 agonists (data not shown).
Acute effects of TLR2 agonists on cytokine production and white blood cell counts in M fascicularis. (A-D) After a single subcutaneous injection of either Pam2CSK4 (red bars, 1-mg/kg dose) or GSK3277329 (blue bars, 15-mg/kg dose), circulating levels of G-CSF (A), MCP-1 (C), and IL-6 (D) and absolute neutrophil counts (B) were measured at either 6 or 24 hours after injection. Bars represent mean ± SEM (3 animals per group). Error bars are not shown for baseline IL-6 values, because the values shown are at the limit of quantification level. ANOVA analysis shows a significant effect of treatment on these graphed parameters (P < .05).
Acute effects of TLR2 agonists on cytokine production and white blood cell counts in M fascicularis. (A-D) After a single subcutaneous injection of either Pam2CSK4 (red bars, 1-mg/kg dose) or GSK3277329 (blue bars, 15-mg/kg dose), circulating levels of G-CSF (A), MCP-1 (C), and IL-6 (D) and absolute neutrophil counts (B) were measured at either 6 or 24 hours after injection. Bars represent mean ± SEM (3 animals per group). Error bars are not shown for baseline IL-6 values, because the values shown are at the limit of quantification level. ANOVA analysis shows a significant effect of treatment on these graphed parameters (P < .05).
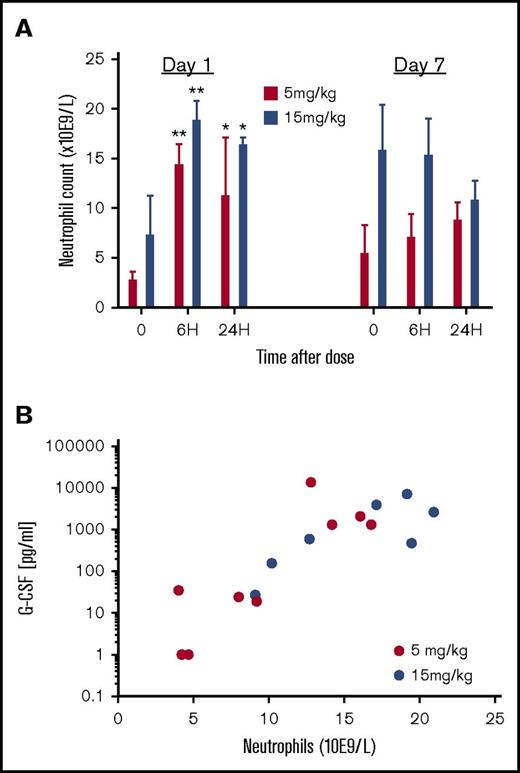
Repeat daily administration of GSK3277329 in normal animals maintained elevated neutrophils measured at 7 days (Figure 4A). A significant and durable neutrophil increase (>10 × 109 cells/L) was observed at 15 mg/kg, although the lower dose of 5 mg/kg was less effective. Increasing levels of serum G-CSF suggests a correlation (r2 = 0.369) with the elevated number of circulating neutrophils from both dosing groups but, due to the small sample size, was not significant (Figure 4B).
Effect of repeat dosing of GSK3277329 (5 and 15 mg/kg subcutaneous injection once a day) on circulating neutrophil counts and the correlation with G-CSF levels in M fascicularis. (A) Neutrophil counts before, 6 hours after, and 24 hours after the first dose of GSK3277329 and the seventh dose. Significant difference compared with predose group (t = 0) determined by 2-way ANOVA followed by Sidak’s post hoc test (*P < .05, **P < .01). (B) Correlation plot between circulating G-CSF levels and neutrophil counts measured 6 hours after GSK3277329 administration, grouping all animals from both dosing groups and samples collected after the first and seventh dose (3 animals per dose group).
Effect of repeat dosing of GSK3277329 (5 and 15 mg/kg subcutaneous injection once a day) on circulating neutrophil counts and the correlation with G-CSF levels in M fascicularis. (A) Neutrophil counts before, 6 hours after, and 24 hours after the first dose of GSK3277329 and the seventh dose. Significant difference compared with predose group (t = 0) determined by 2-way ANOVA followed by Sidak’s post hoc test (*P < .05, **P < .01). (B) Correlation plot between circulating G-CSF levels and neutrophil counts measured 6 hours after GSK3277329 administration, grouping all animals from both dosing groups and samples collected after the first and seventh dose (3 animals per dose group).
Effect of GSK3277329 in a neutropenia model
Previous reports have indicated that recombinant human G-CSF administration could reverse chemotherapy-induced neutropenia in primates.28 Having demonstrated stimulation of G-CSF and subsequent elevation of neutrophils, we set out to evaluate the therapeutic potential of the TLR2 agonist GSK3277329 in chemotherapy-induced neutropenia in M fascicularis. In this model, neutropenia was induced with cyclophosphamide administered (IV route) at 60 mg/kg once a day for 2 days. Three days after the last cyclophosphamide dose, GSK3277329 (3 mg/kg) or human recombinant G-CSF (10 μg/kg) was given daily for 14 days. The 3-mg/kg dose of GSK3277329 was chosen because in a pilot study, as little as 1 mg/kg could double neutrophil numbers in normal animals. The 10-μg/kg dose of G-CSF was chosen based on previous reports where a robust neutrophil response was observed.28
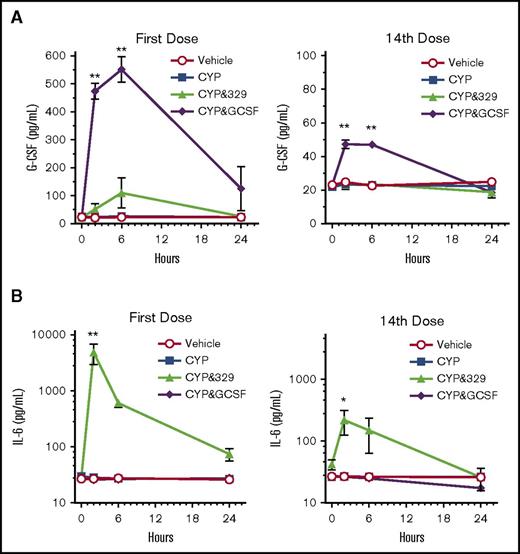
Because a single dose of GSK3277329 could increase circulating G-CSF and IL-6 protein levels (Figure 3), these cytokines were also evaluated following once-daily repeat dosing in neutropenic animals. Circulating cytokine levels were determined at 0, 2, 6, and 24 hours after the dosing on days 1 and 14 (Figure 5).
The effect of a TLR2 agonist or G-CSF on IL-6 or G-CSF levels in cyclophosphamide-treated M fascicularis. (A) Circulating G-CSF levels in M fascicularis (n = 3 per group) after vehicle, cyclophosphamide (CYP), or CYP plus GSK3277329 (3 mg/kg) or CYP plus G-CSF (10 µg/kg) treatment. (B) Circulating IL-6 levels after vehicle, CYP, or CYP plus GSK3277329 (3 mg/kg) or CYP plus G-CSF (10 µg/kg) treatment. Samples were taken at the indicated times after the first or 14th dose. Mean ± SEM values are plotted. No error bars are shown if the error is smaller than the height of the symbol. Significant differences against the vehicle and CYP groups at each time point using 2-way ANOVA with Tukey’s post hoc test are noted by asterisks (**P < .0001, *P < .005).
The effect of a TLR2 agonist or G-CSF on IL-6 or G-CSF levels in cyclophosphamide-treated M fascicularis. (A) Circulating G-CSF levels in M fascicularis (n = 3 per group) after vehicle, cyclophosphamide (CYP), or CYP plus GSK3277329 (3 mg/kg) or CYP plus G-CSF (10 µg/kg) treatment. (B) Circulating IL-6 levels after vehicle, CYP, or CYP plus GSK3277329 (3 mg/kg) or CYP plus G-CSF (10 µg/kg) treatment. Samples were taken at the indicated times after the first or 14th dose. Mean ± SEM values are plotted. No error bars are shown if the error is smaller than the height of the symbol. Significant differences against the vehicle and CYP groups at each time point using 2-way ANOVA with Tukey’s post hoc test are noted by asterisks (**P < .0001, *P < .005).
G-CSF levels were transiently increased by GSK3277329 after the first dose (Figure 5A). However, by the end of the treatment period (after the 14th dose), GSK3277329 did not increase G-CSF levels. Interestingly, human G-CSF–treated animals also had lower G-CSF at the end of the study, suggesting a G-CSF clearance mechanism. This finding is consistent with reports that neutrophils can clear G-CSF from the circulation.29 Thus, as the number of neutrophils increase, so G-CSF is more rapidly cleared. Similarly, GSK3277329 also induced IL-6 more robustly at the beginning of the study than at the end of the treatment period (Figure 5B). Cyclophosphamide with or without G-CSF had no impact on circulating IL-6 levels.
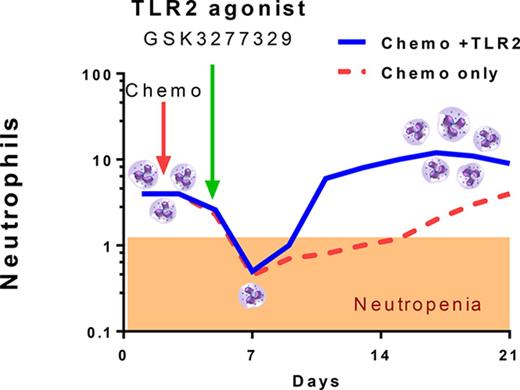
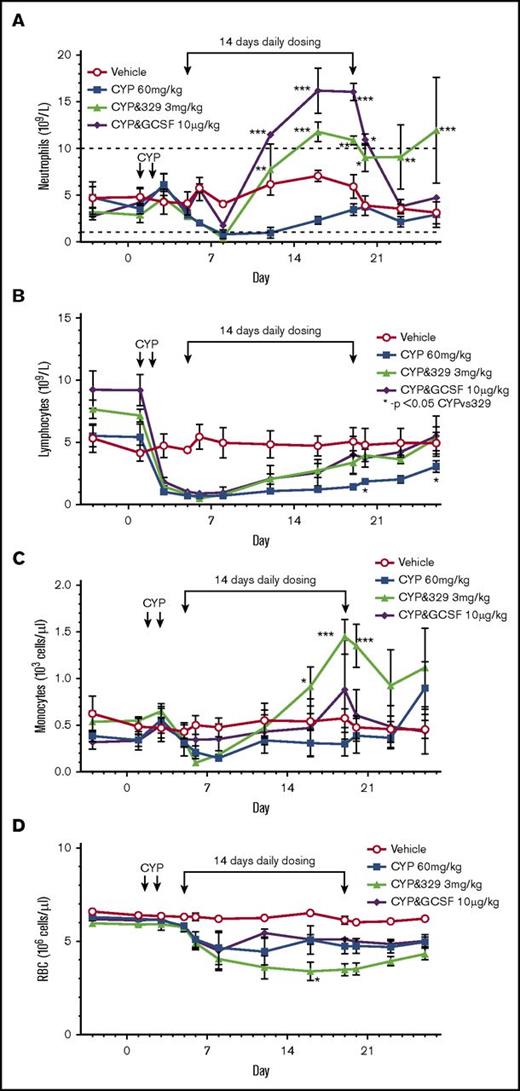
Cyclophosphamide induced severe neutropenia and reduced neutrophil numbers to <1 × 109 cells/L. The normal range of neutrophil numbers are between 1 and 10 ×109 cells/L. Within 7 days, both treatments reversed the chemotherapy-induced neutropenia (>7 × 109cells/L and 11 × 109 cells/L for GSK3277329 and G-CSF, respectively), and by approximately day 10 of dosing, the neutrophil counts were significantly above normal vehicle-treated levels and well above cyclophosphamide-only–treated animals (Figure 6). Although GSK3277329 restored neutrophils after 1 week of treatment, it was unable to blunt the initial chemotherapy-induced decline. In comparison, G-CSF also attenuated the initial decrease of neutrophils, which is consistent with the ability of G-CSF to demarginate neutrophils from the bone marrow and periphery into circulation.30,31 Neutrophil counts returned to normal levels (∼3-4 × 109 cells/L) once G-CSF administration was stopped. Interestingly, GSK3277329 sustained the levels of neutrophils (≥9 × 109 cells/L) for at least 6 days following cessation of treatment. After the washout period, 2 animals had neutrophil levels returning to the normal range (5.1 and 7.5 × 109 cells/L), whereas 1 animal still had elevated counts (23.2 × 109 cells/L). Consistent with the neutrophil counts, total lymphocyte numbers were reduced by cyclophosphamide and recovery was accelerated by both G-CSF and GSK3277329.
Effect of a TLR2 agonist or G-CSF on chemotherapy-induced neutropenia. Symbols indicate mean ± SEM values. Error bars are not shown if the error is smaller than the size of the symbol. (A-D) Neutrophil (A), lymphocyte (B), monocyte (C), and red blood cell (D) counts were measured in M fascicularis treated with vehicle, cyclophosphamide (CYP), or CYP plus GSK3277329 (3 mg/kg) or CYP plus G-CSF (10 µg/kg) (n = 3 per group). The normal range of neutrophil numbers between 1 and 10 × 109 cells/L is noted by dashed lines (A). GSK3277329 or G-CSF were dosed daily for 14 days as indicated by the linked arrows. The first dose received was on day 5, and the last dose on day 19. Significant differences vs the CYP group were determined by 2-way ANOVA followed by Dunnett’s multiple comparisons post-hoc test (*P < .05, **P < .01, ***P < .005).
Effect of a TLR2 agonist or G-CSF on chemotherapy-induced neutropenia. Symbols indicate mean ± SEM values. Error bars are not shown if the error is smaller than the size of the symbol. (A-D) Neutrophil (A), lymphocyte (B), monocyte (C), and red blood cell (D) counts were measured in M fascicularis treated with vehicle, cyclophosphamide (CYP), or CYP plus GSK3277329 (3 mg/kg) or CYP plus G-CSF (10 µg/kg) (n = 3 per group). The normal range of neutrophil numbers between 1 and 10 × 109 cells/L is noted by dashed lines (A). GSK3277329 or G-CSF were dosed daily for 14 days as indicated by the linked arrows. The first dose received was on day 5, and the last dose on day 19. Significant differences vs the CYP group were determined by 2-way ANOVA followed by Dunnett’s multiple comparisons post-hoc test (*P < .05, **P < .01, ***P < .005).
Two out of the 3 animals treated with GSK3277329 presented with reduced appetite, reduced activity, elevated body temperature on days 14 to 19, and some swelling at the injection site several days after treatment was initiated (Table 2). Animals treated with G-CSF also demonstrated some lethargy but did not have injection site reactions, reduced appetite, or changes in body temperature. All clinical signs resolved after cessation of treatment with GSK3277329.
Clinical observations during the treatment period
| n = 3/group . | Appetite loss . | Hematuria . | Edema . | Malaise, low activity . | Injection site reaction . | Elevated body temperature . |
|---|---|---|---|---|---|---|
| Vehicle | 0 | 0 | 0 | 0 | 0 | 0 |
| CYP only | 1 | 0 | 1 | 0 | 0 | 0 |
| CYP + GSK3277329 | 1 | 1 | 2 | 2 | 2 | 2* |
| CYP + G-CSF | 0 | 0 | 0 | 3 | 0 | 0 |
| n = 3/group . | Appetite loss . | Hematuria . | Edema . | Malaise, low activity . | Injection site reaction . | Elevated body temperature . |
|---|---|---|---|---|---|---|
| Vehicle | 0 | 0 | 0 | 0 | 0 | 0 |
| CYP only | 1 | 0 | 1 | 0 | 0 | 0 |
| CYP + GSK3277329 | 1 | 1 | 2 | 2 | 2 | 2* |
| CYP + G-CSF | 0 | 0 | 0 | 3 | 0 | 0 |
Values reflect the number of animals in each group displaying a clinical observation. Appetite loss was defined as not consuming all food provided at daily feeding. Hematuria was noted if red discolored urine was seen. Edema was noted in animals with swollen eyelids or scrotum. Malaise was indicated if the animal did not show normal alert activity. Injection site reaction was noted if site was swollen and reddened for >24 hours. Elevated body temperatures were noted if animals presented with a temperature between 38°C and 40°C.
CYP, cyclophosphamide.
Noted on days 14 to 19 (see Figure 6).
To determine the minimal number of doses of GSK3277329 required to induce a sustained elevation of neutrophils, animals received a daily single dose of GSK3277329 for either 1, 3, or 7 consecutive days. GSK3277329 was administered 48 hours after the last cyclophosphamide treatment. Neutrophil counts were then measured 7 days after first dose of GSK3277329 or vehicle treatment in all groups (Figure 7). As expected, cyclophosphamide significantly reduced neutrophil counts to neutropenic levels (<1 × 109/L). Neither a single dose nor 3 consecutive daily doses of GSK3277329 had a sustained impact on neutrophil levels (<1 × 109/L in cyclophosphamide-treated animals sampled 7 days after the first dose. However, daily dosing of GSK3277329 for 7 consecutive days reversed the cyclophosphamide-induced neutropenia (3.5 × 109 cells/L).
Neutrophil counts in vehicle-treated animals or animals treated with 2 doses of cyclophosphamide. Cyclophosphamide (CYP)–treated animals received vehicle or 1, 3, or 7 daily doses of GSK3277329 (15 mg/kg) 2 days after the last cyclophosphamide dose. Neutrophil numbers were counted 7 days after the first dose. Bars represent mean ± SEM. *P < .05 vs vehicle by ANOVA and Dunnett’s multiple comparison post hoc test (n = 3 per group).
Neutrophil counts in vehicle-treated animals or animals treated with 2 doses of cyclophosphamide. Cyclophosphamide (CYP)–treated animals received vehicle or 1, 3, or 7 daily doses of GSK3277329 (15 mg/kg) 2 days after the last cyclophosphamide dose. Neutrophil numbers were counted 7 days after the first dose. Bars represent mean ± SEM. *P < .05 vs vehicle by ANOVA and Dunnett’s multiple comparison post hoc test (n = 3 per group).
Discussion
These studies describe the novel finding that activation of TLR2 receptors stimulates endogenous G-CSF release and consequently increase circulating neutrophil levels in nonhuman primates. More importantly, a small-molecule TLR2 agonist is also able to reverse neutropenia by durably restoring neutrophil levels in a chemotherapeutic setting. Previous studies in both animals and humans have shown that G-CSF first acutely demarginates the tissue resident neutrophils and mobilizes them from the bone marrow and periphery into the circulation for immediate response. Subsequently, it triggers accelerated maturation of precursor cells in the bone marrow for a sustained neutrophil response.30,31 Thus, G-CSF can reverse neutropenia rapidly and in a durable manner, leading to its widespread use for chemotherapy-induced neutropenia. However, as a biological agent, recombinant G-CSF has significant manufacturing costs and requires refrigerated storage and a cold-supply chain, making it rather inconvenient and unaffordable in several parts of the world, including developing countries. Therefore, a small-molecule G-CSF secretagogue without these issues could offer a treatment option for chemotherapy-induced neutropenia that is not only cost effective but also more convenient, particularly in emerging countries. This study then sought to identify and characterize a small-molecule agonist that could trigger the release of endogenous G-CSF in vivo and thus restore normal numbers of neutrophils in the circulation. Because activation of TLR can trigger cytokine release in general, including G-CSF,32 this mechanism was investigated for the ability to stimulate the neutrophil production in vivo in a chemotherapy-induced neutropenia setting.
This study demonstrated that TLR2 agonism stimulates the release of G-CSF from monocytic and endothelial cells in vitro as well as in cynomolgus monkeys in vivo. This is not unique to this novel TLR2 agonist (GSK3277329), as the TLR2 agonist Pam2CSK4 also caused a robust release of G-CSF in human peripheral blood mononuclear cells (data not shown) as well as in vivo. In addition to G-CSF, GSK3277329 also stimulated the release of IL-6 and MCP-1, but not TNF-α, in vivo. The lack of effect on TNF-α in cynomolgus monkeys contrasts with the effects of TLR2 agonists seen in dogs and mice.20,33 More importantly, GSK3277329 elevated neutrophil counts acutely in normal animals and restored circulating neutrophil numbers in cynomolgus monkeys that were neutropenic as a result of chemotherapy. At the same time, monocytes increased during the dosing schedule while red blood cells tended to be suppressed. It is possible that this compound may favor maturation of myeloid lineages over erythrocytes and would need to be investigated further.
We noted that circulating G-CSF levels and neutrophil numbers were correlated, indicating that the novel TLR2 agonist GSK3277329 likely restored neutrophil numbers at least in part through the secretion of G-CSF. However, the amount of G-CSF that was induced by GSK3277329 was significantly less than the amount of G-CSF given exogenously to achieve the same levels of neutrophil production. Another important observation is that the neutrophil levels in circulation were elevated for several days beyond cessation of GSK3277329 treatment, whereas after G-CSF treatment, neutrophil levels dropped immediately after treatment discontinuation. These data suggest the possibility that other mechanisms in addition to the G-CSF release following TLR2 agonism with GSK3277329 could contribute to sustained neutrophil levels in circulation. Because GSK3277329 increased IL-6 and MCP-1 levels in vivo, and because these cytokines are also associated with increased neutrophil levels,34 it is possible that TLR2 stimulation achieves prolonged neutrophil elevation through these cytokines in addition to G-CSF or possibly other mechanisms yet to be identified. The increased IL-6 levels were likely significant, as 2 out of the 3 animals in that group also presented with elevated body temperatures (38°C-40°C) when circulating neutrophils and monocytes were elevated. Consistent with this notion, intranasal administration of the TLR2 agonist dipalmitoyl-S-glyceryl cysteine increased IL-6 and MCP-1 levels while boosting neutrophil counts and improving survival and resistance to influenza infection.34 Also, a TLR2 agonist induced increased numbers of neutrophils sufficiently to increase survival following lung infection.35 Small-molecule agonists of the TLR2/4 receptors have been described previously that stimulate cytokine release.36 For example, OM-174 reduces tumor progression and prolongs survival in combination with chemotherapy, presumably through TNF-α secretion and induction of inducible nitric oxide synthase expression by acting on both TLR4 and TLR2 receptors.24 Although this suggests that TLR2/4 agonists could be promising molecules to prolong survival in cancer patients receiving chemotherapy, the TLR2 agonist in our study did not induce TNF-α. This difference could be due to the selective engagement of the TLR2 receptors in heterodimeric complexes that results in distinct cytokine expression profiles and is likely a compound-specific property of GSK3277329. It is possible, however, that the mixed TLR2/4 agonists might similarly reverse chemotherapy-induced neutropenia. Nonetheless, a lack of generic cytokine response is desirable, because this mechanism does not appear to give rise to a cytokine storm. It should be noted that neutrophil counts were not measured >2 weeks after the dosing of GSK3277329. Because 2 out of the 3 animals on GSK3277329 had neutrophil levels in the normal range at the end of the experiment, continuing hyperneutrophilia is not expected. However, additional studies on the time course following washout would be needed to determine if neutrophil levels return to normal in all animals.
In patients undergoing chemotherapy, there is a loss of myeloid cells and thus an important source of G-CSF secreting cells. This might suggest limited efficacy of a mechanism that would target myeloid cells to release the G-CSF needed to drive neutrophil replenishment. However, vascular endothelial cells also have TLR2 receptors and have the capacity to secrete G-CSF. Human endothelial cells have been demonstrated as a source of G-CSF by the observation that IL-1β induces G-CSF secretion >200-fold in HUVECs.37 Furthermore, it has previously been demonstrated that LPS can induce G-CSF release from HUVECs as well.38 We now show that the TLR2 agonist GSK3277329 caused the release of G-CSF from myeloid THP-1 cells as well as endothelial HUVECs in vitro. Because GSK3277329 could induce G-CSF production and restore neutrophil levels in chemotherapy-induced neutropenia model in our study, it is likely that cells other than myeloid and possibly endothelial cells may have contributed to G-CSF release and are likely responsible for the more durable neutrophil response. It should be noted that monocyte cells also increased in number toward the end of the dosing window, which might also be an indirect factor contributing to the reversal of neutropenia. Although we did not identify the exact cell type that produced G-CSF in vivo, these data suggest that treatment with a TLR2 agonist is an effective means of increasing endogenous cytokine production, including G-CSF, by acting on multiple cell types and that TLR2 agonism is expected to support the generation of new neutrophils and benefit patients in a chemotherapeutic setting.
Although TLR2 agonists are in clinical use as vaccine adjuvants,25,39,40 activation of the TLR2 pathways chronically may also include adverse effects in vivo. A role for TLR2 in the CNS was shown by receptor deficiency causing behavioral alterations.41 It was shown that hypothalamic activation of TLR2 with the ligand Pam3CSK4 induced anorexia, hypoactivity, and hyperthermia in rats.42 Indeed, similar symptoms were observed in some of the animals in this study. In support of TLR2 mediating effects on appetite, it had been shown that TLR2-deficient mice consume more food and develop obesity.43 By extrapolation, TLR2 agonists would be expected to decrease appetite. These behaviors are consistent with malaise associated with response to a bacterial infection. Therefore, because it is likely that the behavioral events would manifest with chronic dosing in patients and limit long-term tolerability, developing this approach for the clinic must manage these potential effects in patients. Although we did not measure the levels of GSK32377329 in the brain, further optimization of this compound to limit or eliminate brain exposure could mitigate these behavioral and appetite issues.
In summary, these data demonstrate the preclinical in vivo proof of concept that TLR2 agonism can drive both G-CSF induction and subsequent neutrophil elevation to treat chemotherapy-induced neutropenia. Because the levels of G-CSF induced by this compound were less than the levels normally needed to significantly elevate neutrophil counts, this compound likely recruits additional mechanisms to reverse neutropenia in a sustained fashion. The results in this study further suggest that TLR2 activation may offer a more sustained treatment of neutropenia. However, treatment with this compound was also associated with tolerability issues, and these would need to be addressed before such compounds could be progressed to the clinic.
Acknowledgments
The authors thank Elizabeth M. Matthew for editorial support in preparing this manuscript.
This research was funded by GlaxoSmithKline PLC.
Authorship
Contribution: J.E.C., J.H.G., M.B., and J.J.F. performed experiments; N.J.L. analyzed results and made the figures; and N.J.L., M.P.D., J.M.A., J.G.E., M.C., A.O., and S.K. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors are employees of GlaxoSmithKline PLC.
Correspondence: Nicholas J. Laping, New Targets Incubator, Future Pipelines Discovery, GSK, 709 Swedeland Rd, King of Prussia, PA 19406; e-mail nicholas.j.laping@gsk.com.