Key Points
Accurate GZL diagnosis remains challenging, with >60% of patients with presumed GZL having the diagnosis reclassified on consensus review.
Treatment with DLBCL-based therapy appears most effective for GZL (including R-CHOP); however, new therapies are needed to improve outcomes.
Abstract
Gray zone lymphoma (GZL) is described as sharing features with classical Hodgkin lymphoma (cHL) and diffuse large B-cell lymphoma (DLBCL). However, there remains complexity in establishing diagnosis, delineating prognosis, and determining optimum therapy. Sixty-eight cases diagnosed as GZL across 15 North American academic centers were evaluated by central pathology review to achieve consensus. Of these, only 26 (38%) were confirmed as GZL. Morphology was critical to GZL consensus diagnosis (eg, tumor cell richness); immunohistochemistry showed universal B-cell derivation, frequent CD30 expression, and rare Epstein-Barr virus (EBV) positivity (CD20+, 83%; PAX5+, 100%; BCL6+, 20%; MUM1+, 100%; CD30+, 92%; EBV+, 4%). Forty-two cases were reclassified: nodular sclerosis (NS) cHL, n = 27 (including n = 10 NS grade 2); lymphocyte predominant HL, n = 4; DLBCL, n = 4; EBV+ DLBCL, n = 3; primary mediastinal large BCL n = 2; lymphocyte-rich cHL and BCL–not otherwise specified, n = 1 each. GZL consensus-confirmed vs reclassified cases, respectively, more often had mediastinal disease (69% vs 41%; P = .038) and less likely more than 1 extranodal site (0% vs 25%; P = .019). With a 44-month median follow-up, 3-year progression-free survival (PFS) and overall survival for patients with confirmed GZL were 39% and 95%, respectively, vs 58% and 85%, respectively, for reclassified cases (P = .19 and P = .15, respectively). Interestingly, NS grade 2 reclassified patients had similar PFS as GZL consensus-confirmed cases. For prognostication of GZL cases, hypoalbuminemia was a negative factor (3-year PFS, 12% vs 64%; P = .01), whereas frontline cyclophosphamide, doxorubicin, vincristine, and prednisone ± rituximab (CHOP±R) was associated with improved 3-year PFS (70% vs 20%; P = .03); both factors remained significant on multivariate analysis. Altogether, accurate diagnosis of GZL remains challenging, and improved therapeutic strategies are needed.
Introduction
Gray zone lymphoma (GZL) is an uncommon neoplasm initially described in 2005.1 B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and classical Hodgkin lymphoma (cHL), GZL was first recognized in the World Health Organization (WHO) classification as a distinct entity in 2008.2
Despite advances in immunophenotyping, molecular diagnostics, and more exact morphology-based discrimination, the diagnosis of GZL remains complex. In addition, initially described as primarily involving the mediastinum,1,3,4 further analyses have elucidated both primary mediastinal and nonmediastinal (systemic) clinical disease presentations.5,6 Regardless of presentation, patients with GZL have comparatively inferior survival rates compared with either cHL or DLBCL.5,7-10 Thus, accurate diagnosis is imperative, and development of new and improved therapies is critically needed.
A recent multicenter retrospective study described 112 GZL cases diagnosed and treated in 19 North American academic centers.5 However, central pathologic review was not conducted. To better understand the diagnostic challenges and to delineate useful morphologic and immunohistochemical features that may facilitate accurate diagnosis of GZL, a comprehensive study with detailed centralized pathologic review and associated analyses of clinical data was undertaken.
Methods
For the current study, we obtained 73 cases previously diagnosed as GZL across 15 US and Canadian academic medical centers for centralized pathologic review. Institutional review boards of all participating institutions approved the study. Slides were identified from each center (ie, full sections/tumor blocks) and submitted centrally. All cases were subjected to central pathology review by a panel of 5 hematopathologists (M.P., S.P., J.A.F., J.H., and E.S.J.). Each case was reviewed at the multiheaded scope and discussed until consensus for morphologic and immunohistochemical features and diagnosis was reached. Criteria for GZL diagnosis followed the 2016 WHO classification.11
Only cases diagnosed as GZL at initial presentation were considered; nearly all of the original cases had been examined by an academic hematopathologist. From 73 cases, 5 were excluded for insufficient material for morphologic evaluation or insufficient number of neoplastic cells represented. These 5 cases included 2 splenectomies with only rare scattered neoplastic cells, bone marrow biopsy with extensive fibrosis and rare crushed neoplastic cells, gastrointestinal tract biopsy with a few neoplastic cells seen in the lamina propria, and a minimal soft tissue fragment with rare neoplastic cells. The remaining 68 cases were evaluated on the basis of hematoxylin and eosin sections of routinely fixed and paraffin-embedded material. Immunostained slides from the referring institutions were available for review; additional stains were performed in selected cases at Tufts Medical Center and at Massachusetts General Hospital.
The recommended immunohistochemical panel considered adequate to diagnose GZL was performed in all cases and included CD20, CD79a, PAX5, MUM1, CD30, CD15, CD3, and Epstein-Barr virus (EBV) (EBV-encoded small RNA) in situ hybridization. This staining panel was decided before the central pathology review, and it was determined to be the most efficient and minimum panel of stains to perform on all cases. BOB.1 and OCT2 were also performed in a minority of cases. Beyond a tumor cell immunoprofile, diagnostic morphologic criteria included tumor cell density, fibrosis, necrosis, and the microenvironment. Immunohistochemical staining intensity was scored on a semiquantitative basis by comparing staining intensity of neoplastic cells with reactive cells on the scale 1 to 3 (weak, moderate, strong). Extent of staining was scored on a scale of 1 to 4 (1 = 1%-25%; 2 = 25%-50%; 3 = 50%-75%; 4 = >75% of neoplastic cells). Staining was considered satisfactory if background T and/or B cells were deemed positive.
Clinical data
Information on patient and diagnostic features, staging investigations, and treatment administered was provided by the patients’ institutional providers. Receipt of radiation therapy was not considered an event or progression. Therapy was not predetermined and was given at the discretion of the treating physician. Bulky disease was defined as any mass lesion 10 cm or larger, and primary refractory disease was defined as lack of at least a partial response to frontline therapy or progressive disease documented less than 6 months from initial partial or complete response. In addition, response was defined according to revised and current criteria (eg, including fluorodeoxyglucose–positron emission tomography).12,13
Statistical analysis
Characteristics were compared using χ2 test for categorical variables and Wilcoxon rank-sum for medians. Covariates were collected and comprised the data set on which univariate analyses for progression-free survival (PFS) and overall survival (OS) were performed. PFS was calculated from the date of diagnosis to date of death or disease relapse/progression. OS was computed from the date of diagnosis to the date of death. Patients without PFS or OS events were censored at the time of last clinical follow-up. Survival analyses were performed regardless of the amount or length of therapy received. Three-year PFS and OS rates were estimated through Kaplan-Meier method,14 whereas survival differences were assessed using the log-rank test. Univariate associations between clinical and laboratory factors and survival were derived using the Cox proportional hazards model.15 Variables with a P ≤ .10 in univariate analyses were entered into the multivariate Cox proportional hazards model in a stepwise fashion.16 Hazard ratios (HRs) and their 95% confidence intervals (CIs) were reported. All statistical analyses were conducted with SAS v9.2 (SAS Institute Inc., Cary, NC).
Results
Consensus classification
Among the 68 cases that were examined on consensus review, the original diagnosis of GZL was confirmed in only 26 cases (38%). Clinical descriptions of these bona fide GZL cases are detailed in Table 1. The remaining 42 cases (62%) were reclassified as depicted in supplemental Table 1. Morphologic and immunohistochemical examples of a GZL case and 2 reclassified cases are shown in Figure 1A-C.
Baseline characteristics: GZL consensus confirmed cases
| Characteristics . | n (%) . |
|---|---|
| Patient characteristics | |
| Median age | 37 y (range, 19-72 y) |
| Sex | |
| Male | 17 (62) |
| Female | 9 (38) |
| Performance status | |
| 0-1 | 24 (88) |
| 2-4 | 2 (8) |
| NA | 1 (4) |
| B symptoms | |
| Yes | 9 (35) |
| No | 13 (50) |
| NA | 4 (15) |
| Albumin | |
| Normal | 7 (27) |
| Low | 14 (54) |
| NA | 5 (19) |
| Hemoglobin | |
| Decreased | 15 (58) |
| Normal | 10 (38) |
| NA | 1 (4) |
| LDH | |
| Normal | 9 (35) |
| Elevated | 15 (58) |
| NA | 2 (7) |
| Disease characteristics | |
| Mediastinal involvement/disease | 18 (69) |
| Bone marrow involvement | |
| Absent | 25 (96) |
| Present | 0 (0) |
| NA | 1 (4) |
| Extranodal sites present | |
| ≤1 | 25 (96) |
| >1 | 0 (0) |
| NA | 1 (4) |
| Stage | |
| I/II | 17 (65) |
| III/IV | 8 (31) |
| NA | 1 (4) |
| Bulky disease | |
| Yes | 8 (31) |
| No | 16 (62) |
| NA | 2 (7) |
| IPI | |
| 0-2 | 17 (65) |
| 3-5 | 5 (19) |
| NA | 4 (16) |
| IPS | |
| 0-1 | 9 (35) |
| 2-3 | 9 (35) |
| 4-6 | 2 (7) |
| NA | 6 (23) |
| Characteristics . | n (%) . |
|---|---|
| Patient characteristics | |
| Median age | 37 y (range, 19-72 y) |
| Sex | |
| Male | 17 (62) |
| Female | 9 (38) |
| Performance status | |
| 0-1 | 24 (88) |
| 2-4 | 2 (8) |
| NA | 1 (4) |
| B symptoms | |
| Yes | 9 (35) |
| No | 13 (50) |
| NA | 4 (15) |
| Albumin | |
| Normal | 7 (27) |
| Low | 14 (54) |
| NA | 5 (19) |
| Hemoglobin | |
| Decreased | 15 (58) |
| Normal | 10 (38) |
| NA | 1 (4) |
| LDH | |
| Normal | 9 (35) |
| Elevated | 15 (58) |
| NA | 2 (7) |
| Disease characteristics | |
| Mediastinal involvement/disease | 18 (69) |
| Bone marrow involvement | |
| Absent | 25 (96) |
| Present | 0 (0) |
| NA | 1 (4) |
| Extranodal sites present | |
| ≤1 | 25 (96) |
| >1 | 0 (0) |
| NA | 1 (4) |
| Stage | |
| I/II | 17 (65) |
| III/IV | 8 (31) |
| NA | 1 (4) |
| Bulky disease | |
| Yes | 8 (31) |
| No | 16 (62) |
| NA | 2 (7) |
| IPI | |
| 0-2 | 17 (65) |
| 3-5 | 5 (19) |
| NA | 4 (16) |
| IPS | |
| 0-1 | 9 (35) |
| 2-3 | 9 (35) |
| 4-6 | 2 (7) |
| NA | 6 (23) |
LDH, lactate dehydrogenase; NA, not available.
Consensus pathology. (A) Case example of consensus GZL diagnosis (B-cell lymphoma with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma). Sheetlike proliferation of tumor cells resembling PMBL is seen in the top left panel. Focally, collections of eosinophils are noted (top right). The tumor cells are strongly positive for CD20, CD30, and CD15 (bottom). (B) Reclassified case: cHL NS2. Representative images from 2 cases of cHL NS2 are shown. (Top) Characteristic histology with a nodular growth pattern, fibrous bands, and a sheetlike growth of lacunar cells. (Bottom) Immunohistochemical stains from a second case show the nodular growth pattern with strong staining for CD20, but also positivity for CD30 and focal staining for CD15. (C) Reclassified case: primary mediastinal large B cell lymphoma. Diffuse proliferation of large neoplastic cells is seen (top left). The neoplastic cells have abundant clear cytoplasm and vesicular nuclei with basophilic nucleoli (top right). They are strongly, uniformly positive for CD20, with dim expression of CD30, and are negative for CD15 (bottom). (D) Synopsis of the immunohistochemical staining results from consensus confirmed GZL cases. The bar graph illustrates immunohistochemical staining for markers CD20, CD79a, PAX5, MUM1, CD30, CD15, CD3, and in situ hybridization for EBV (EBV-encoded small RNA [EBER]). For immunohistochemical studies, staining was considered to be positive if it had intensity of 2 or more (on +1 to +3 scale) and was distributed in more than 25% of neoplastic cells. Cases with weak/negative expression are not included in this graph. Hematoxylin and eosin staining, original magnification ×400 (A, B [top right panel], and C [top panels]) and ×200 (B [top left panel]). Immunohistochemical staining, original magnification ×400 (all other panels).
Consensus pathology. (A) Case example of consensus GZL diagnosis (B-cell lymphoma with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma). Sheetlike proliferation of tumor cells resembling PMBL is seen in the top left panel. Focally, collections of eosinophils are noted (top right). The tumor cells are strongly positive for CD20, CD30, and CD15 (bottom). (B) Reclassified case: cHL NS2. Representative images from 2 cases of cHL NS2 are shown. (Top) Characteristic histology with a nodular growth pattern, fibrous bands, and a sheetlike growth of lacunar cells. (Bottom) Immunohistochemical stains from a second case show the nodular growth pattern with strong staining for CD20, but also positivity for CD30 and focal staining for CD15. (C) Reclassified case: primary mediastinal large B cell lymphoma. Diffuse proliferation of large neoplastic cells is seen (top left). The neoplastic cells have abundant clear cytoplasm and vesicular nuclei with basophilic nucleoli (top right). They are strongly, uniformly positive for CD20, with dim expression of CD30, and are negative for CD15 (bottom). (D) Synopsis of the immunohistochemical staining results from consensus confirmed GZL cases. The bar graph illustrates immunohistochemical staining for markers CD20, CD79a, PAX5, MUM1, CD30, CD15, CD3, and in situ hybridization for EBV (EBV-encoded small RNA [EBER]). For immunohistochemical studies, staining was considered to be positive if it had intensity of 2 or more (on +1 to +3 scale) and was distributed in more than 25% of neoplastic cells. Cases with weak/negative expression are not included in this graph. Hematoxylin and eosin staining, original magnification ×400 (A, B [top right panel], and C [top panels]) and ×200 (B [top left panel]). Immunohistochemical staining, original magnification ×400 (all other panels).
GZL cases
All 26 GZL cases were of B-cell derivation (ie, positive for ≥1 B-cell marker: CD20, CD79a, and/or PAX5). In general, neoplastic cells in the confirmed GZL cases were abundant, often occurring in sheets, with tumor cells usually being large with centroblastic or immunoblastic appearance and a high degree of pleomorphism. Most were strongly CD20 positive (21/26; 81%), whereas other B-cell markers were variable (Figure 1D). Detailed immunohistochemistry data are described in supplemental Table 1. MUM1 was available for evaluation in 22/26 cases and was consistently strong (scores 3 or 4) in all but 1 case (ie, 96% strong MUM1 positivity), with moderate staining in the other case. In addition to strong CD20 positivity, most cases were CD30+ (24/26, 92%), including 4 CD20− cases. These 4 latter cases did not qualify for the diagnosis of cHL, in part based on a lack of morphologic criteria characteristic for cHL; most notably, absence of a more extensive inflammatory background and cytomorphology resembling primary mediastinal large B-cell lymphoma (PMBL) with strong PAX5 staining. CD15 was scored as positive (ie, at least 2+) in 11/26 cases (42%); in 5 cases (19%), CD15 was strongly expressed in all neoplastic cells along with strong CD30 coexpression. CD3 was positive in bystander T cells only. Only 1 GZL case (4%) was EBV+. There was no morphologic and/or immunophenotypic difference between GZL primarily occurring in the mediastinum or involving peripheral sites (data not shown).
Reclassified cases
Altogether, 42 cases originally diagnosed as GZL were reclassified. The majority of these reclassified cases (27/42; 64%) were interpreted by the panel as cHL NS, which included a subset of cases (10/27) interpreted as cHL NS, grade 2 (cHL NS2), also referred to historically as cHL NS with lymphocyte depletion.17,18 Most of these cases had strong CD20 expression and relatively weaker CD30 expression (supplemental Table 2). Four cases were reclassified as nodular lymphocyte-predominant Hodgkin lymphoma. In contrast to cHL and GZL, neoplastic cells of nodular lymphocyte predominant Hodgkin lymphoma had a germinal center cell immunophenotype (ie, were BCL-6 positive) and arose in altered follicles with admixed small CD20+ B-cells. Four of the reclassified cases were DLBCL NOS, and 2 were diagnosed as PMBL. EBV-driven lymphoid proliferations can morphologically and immunophenotypically overlap with cHL; 3 EBV-positive DLBCL cases were originally interpreted as GZL.
Patient characteristics
Detailed clinical information was available for 25 of the 26 consensus-confirmed GZL cases, which is detailed in Table 1. There was a male predominance (male:female, 1.9:1), and the median age of these patients with GZL was 37 years, with only 4/25 (16%) being older than 60 years. Performance status was overall good, whereas a majority of patients presented with anemia, elevated lactate dehydrogenase, and hypoalbuminemia. No patients had evidence or more than 1 site of extranodal disease or bone marrow involvement, and nearly 70% had primary mediastinal presentation, with the remaining patients having no evidence of mediastinal disease. Approximately two-thirds of patients had early-stage disease, with one-third having bulky disease (defined as >10 cm). For clinical prognostic scoring, only a minority of patients had high International Prognostic Index or International Prognostic Score.
Clinical differences between primary mediastinal GZL vs nonmediastinal (systemic) GZL from the consensus-confirmed cases included age (ie, 35 years vs 51 years, respectively; P = .05), anemia at diagnosis (44% vs 87%, respectively; P = .08), stage I/II disease at diagnosis (89% vs 46%, respectively; P = .03), and presence of bulk disease (44% vs 0, respectively; P = .06).
Clinical features of the cases reclassified on consensus review are depicted in supplemental Table 3. Male predominance was more pronounced (ie, 3:1) in reclassified cases compared with consensus GZL cases. Among reclassified cases, median age was 44 years, and 22% of these patients were older than 60 years. In addition, fewer patients among the reclassified cases had primary mediastinal disease (41% vs 69%; P = .038), whereas a higher proportion had involvement of more than 1 extranodal site (25% vs 0; P = .019). A majority of patients with reclassified lymphomas had advanced-stage disease (ie, 56%) compared with GZL consensus cases; however, this was not significant (P = .12).
Treatment
Among GZL confirmed cases, the most common chemotherapy regimen given was cyclophosphamide, doxorubicin, vincristine, and prednisone ± rituximab (CHOP±R) therapy, given to 17/25 (68%) of patients; 15 of these 17 patients received rituximab with CHOP. Six of 25 patients with GZL received doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), 2 with rituximab, and 2 patients received dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab (DA-EPOCH-R). Overall response rate (ORR) among all patients with GZL was 60% with a complete remission rate of 56% (ORR by therapy: ABVD±R, 33%; CHOP±R, 71%; EPOCH-R, 50%); 8% had stable disease, and 32% had progressive disease/primary refractory. Among 12 patients with GZL who had relapsed or refractory disease, 9 proceeded to salvage therapy followed by autologous hematopoietic stem cell transplant.
CHOP was also the most common therapeutic regimen for the reclassified cases given to 17/36 (47%) patients (16/17 with rituximab). Ten patients with reclassified lymphomas received ABVD (none with rituximab), whereas 4 received DA-EPOCH-R; 2 bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procarbazine, and prednisone (BEACOPP); and 1 patient received ifosfamide, carboplatin, and etoposide (ICE). All the latter 7 patients received concurrent rituximab. Among data available on 9 patients reclassified to cHL NS2, 4 received ABVD±R, 4 CHOP-R, and 1 DA-EPOCH-R. ORR for reclassified cases was 67% with 56% complete remission (ORR by therapy: ABVD±R, 60%; CHOP±R, 65%; EPOCH-R, 75%; other: 100%); 31% had primary refractory disease. Among 18 reclassified patients who had relapsed or refractory disease, 12 had salvage therapy and hematopoietic stem cell transplant (n = 9 autologous and n = 3 allogeneic).
Survival
With a median follow-up of 44 months (range, 8-120 months), the 3-year PFS and OS for GZL consensus patients were 39% and 95%, respectively (Figure 2). This compared with 3-year PFS and OS for reclassified patients of 58% and 85%, respectively (P = .19 and P = .15, respectively). Among the histologies within the reclassified group, cHL NS2 appeared to have inferior PFS as compared with cHL and DLBCL (Figure 2), with outcomes more similar to consensus-confirmed GZL cases.
Survival. The 3-year (A) PFS of 25 patients with GZL compared with 36 reclassified lymphoma cases was 39% and 58%, respectively (P = .19), and corresponding 3-year (B) OS was 95% and 85%, respectively (P = .15). Kaplan-Meier curves comparing PFS (C) and OS (D) for cHL NS2 vs cHL vs DLBCL; 3-year PFS rates were 43%, 65%, and 67% (P = .67), respectively, and 3-year OS rates were 83%, 88%, and 71%, respectively (P = .80). The outcome (E) for patients with GZL based on CD30 expression was 3-year PFS of 83% for Neg-1 vs 34% for 2-3 on immunohistochemistry; (F) the 3-year PFS for patients with hypoalbuminemia vs normal albumin were 64% vs 12% (P = .01). Kaplan-Meier curves (G) for patients who received CHOP±R therapy for frontline therapy vs not; 3-year PFS was 70% vs 20%, respectively (P = .03). The 2 latter findings persisted on multivariable Cox regression analysis.
Survival. The 3-year (A) PFS of 25 patients with GZL compared with 36 reclassified lymphoma cases was 39% and 58%, respectively (P = .19), and corresponding 3-year (B) OS was 95% and 85%, respectively (P = .15). Kaplan-Meier curves comparing PFS (C) and OS (D) for cHL NS2 vs cHL vs DLBCL; 3-year PFS rates were 43%, 65%, and 67% (P = .67), respectively, and 3-year OS rates were 83%, 88%, and 71%, respectively (P = .80). The outcome (E) for patients with GZL based on CD30 expression was 3-year PFS of 83% for Neg-1 vs 34% for 2-3 on immunohistochemistry; (F) the 3-year PFS for patients with hypoalbuminemia vs normal albumin were 64% vs 12% (P = .01). Kaplan-Meier curves (G) for patients who received CHOP±R therapy for frontline therapy vs not; 3-year PFS was 70% vs 20%, respectively (P = .03). The 2 latter findings persisted on multivariable Cox regression analysis.
Prognostication
We studied a multitude of pathologic and clinical variables for potential prognostication of outcome of patients with GZL. From the immunohistochemistry markers examined (ie, CD20, CD79a, PAX5, BCL6, MUM1, CD30, and CD15) and analyzing across several different cut-points among these markers, only strong PAX5 expression (ie, 3 vs ≤ 2: HR, 4.25; 95% CI, 1.09-16.60; P = .037) and moderate to strong expression of CD30 (ie, 2-3 vs ≤ 1: HR, 7.56; 95% CI, 1.000-58.83; P = .05) were associated with inferior PFS. Among clinical variables on univariate analyses (Table 2), serum albumin was significantly associated with patient outcome, and sex was of borderline significance (ie, male improved PFS). Examining the effect of treatment regimen on univariate analysis, use of CHOP±R was associated with improved PFS and receipt of radiotherapy was borderline; use of ABVD±R and DA-EPOCH-R predicted for inferior outcome, although only 2 patients with GZL were treated with the latter regimen in this series, and thus cannot be reliably assessed. Use of rituximab was not statistically significant on univariate analysis, but trended toward improved PFS (HR, 0.37; P = .08; 95% CI, 0.12-1.13).
Factors predictive of PFS (univariate analysis)
| . | 4-year PFS . | |||
|---|---|---|---|---|
| HR . | 95% HR confidence limits . | P . | ||
| Low . | High . | |||
| Baseline clinical factors | ||||
| Age (continuous) | 0.99 | 0.96 | 1.03 | .75 |
| Sex (male vs female) | 0.35 | 0.12 | 1.08 | .06 |
| B symptoms (yes vs no) | 2.47 | 0.75 | 8.16 | .13 |
| BMI (median) | 1.00 | 0.86 | 1.16 | .99 |
| ECOG performance status (2-4 vs 0-1) | 1.40 | 0.42 | 4.70 | .58 |
| Hemoglobin < 10.5 g/dL (no vs yes) | 0.79 | 0.24 | 2.61 | .70 |
| LDH (creased vs normal) | 1.71 | 0.48 | 6.16 | .40 |
| ESR (increased vs normal) | 1.77 | 0.16 | 19.63 | .63 |
| Albumin < 4.0 g/dL (yes vs no) | 5.06 | 1.11 | 23.19 | .03 |
| Primary mediastinal disease (yes vs no) | 0.42 | 0.14 | 1.26 | .12 |
| Bulky disease (yes vs no) | 0.33 | 0.07 | 1.53 | .15 |
| Stage at diagnosis (1-2 vs 3-4) | 0.80 | 0.25 | 2.63 | .72 |
| Prognostic score, IPI (continuous) | 1.28 | 0.63 | 2.61 | .49 |
| Prognostic score, IPS (continuous) | 1.49 | 0.65 | 3.42 | .34 |
| Treatment factors | ||||
| Rituximab (yes vs no) | 0.36 | 0.12 | 1.13 | .08 |
| ABVD (yes vs no) | 2.72 | 0.88 | 8.42 | .08 |
| CHOP (yes vs no) | 0.22 | 0.02 | 0.53 | .008 |
| EPOCH (yes vs no) | 6.55 | 1.28 | 33.45 | .02 |
| Consolidative radiotherapy (yes vs no) | 0.36 | 0.12 | 1.14 | .08 |
| . | 4-year PFS . | |||
|---|---|---|---|---|
| HR . | 95% HR confidence limits . | P . | ||
| Low . | High . | |||
| Baseline clinical factors | ||||
| Age (continuous) | 0.99 | 0.96 | 1.03 | .75 |
| Sex (male vs female) | 0.35 | 0.12 | 1.08 | .06 |
| B symptoms (yes vs no) | 2.47 | 0.75 | 8.16 | .13 |
| BMI (median) | 1.00 | 0.86 | 1.16 | .99 |
| ECOG performance status (2-4 vs 0-1) | 1.40 | 0.42 | 4.70 | .58 |
| Hemoglobin < 10.5 g/dL (no vs yes) | 0.79 | 0.24 | 2.61 | .70 |
| LDH (creased vs normal) | 1.71 | 0.48 | 6.16 | .40 |
| ESR (increased vs normal) | 1.77 | 0.16 | 19.63 | .63 |
| Albumin < 4.0 g/dL (yes vs no) | 5.06 | 1.11 | 23.19 | .03 |
| Primary mediastinal disease (yes vs no) | 0.42 | 0.14 | 1.26 | .12 |
| Bulky disease (yes vs no) | 0.33 | 0.07 | 1.53 | .15 |
| Stage at diagnosis (1-2 vs 3-4) | 0.80 | 0.25 | 2.63 | .72 |
| Prognostic score, IPI (continuous) | 1.28 | 0.63 | 2.61 | .49 |
| Prognostic score, IPS (continuous) | 1.49 | 0.65 | 3.42 | .34 |
| Treatment factors | ||||
| Rituximab (yes vs no) | 0.36 | 0.12 | 1.13 | .08 |
| ABVD (yes vs no) | 2.72 | 0.88 | 8.42 | .08 |
| CHOP (yes vs no) | 0.22 | 0.02 | 0.53 | .008 |
| EPOCH (yes vs no) | 6.55 | 1.28 | 33.45 | .02 |
| Consolidative radiotherapy (yes vs no) | 0.36 | 0.12 | 1.14 | .08 |
Bold values indicate factors that are statistically significant. The following variables had too few events to provide an accurate estimate for Cox overall survival models: bone marrow involved (yes vs no), creatinine at diagnosis (increased vs normal), more than 1 extranodal site (yes vs no).
ECOG, Eastern Cooperative Oncology Group; ESR, erythrocyte sedimentation rate.
On Cox regression multivariable analysis, PAX5 lost statistical significance while increased CD30 expression was borderline in predicting inferior PFS (HR, 7.08; 95% CI, 0.88-57.27; P = .06) (Figure 2E). Among clinical variables, albumin was the only factor associated with patient survival (ie, hypoalbuminemia: PFS HR, 5.54; 95% CI, 1.16-26.42; P = .03), and receipt with CHOP±R for frontline therapy also remained significant when controlling for albumin and International Prognostic Index (PFS HR, 0.017; 95% CI, 0.001-0.319; P = .006), as also depicted via Kaplan-Meier curves in Figures 2F-G.
Discussion
The entity of GZL sharing features with both cHL and DLBCL was first formulated at a joint workshop on cHL,19 with a successive series published from the National Cancer Institute in 2005.1 It was subsequently included in the 2008 WHO classification of lymphoid neoplasms.1-3,10,20 A retrospective analysis of 112 GZL cases diagnosed and treated across 19 North American academic centers described clinical features and outcomes of GZL treated in the contemporary era, including the majority of cases presenting with systemic disease without mediastinal disease and PFS rates comparatively inferior compared with cHL or DLBCL.5 Further, there was a suggestion of improved outcomes with use of regimens typically used for DLBCL, rather than cHL. A critical limitation of this retrospective study, however, was the lack of centralized pathology review. Slides from the majority of these previously reported GZL cases were obtained, and centralized pathologic consensus review was undertaken, together with analyses of all associated clinical data. To the best of our knowledge, this is the largest clinical-pathologic consensus study conducted to date on GZL.
Remarkably, less than 40% of the original cases diagnosed as GZL in the recent multicenter retrospective study had this diagnosis confirmed on pathologic consensus review, with the majority of cases being reclassified to a different lymphoma diagnosis. Despite all original cases being diagnosed and treated at mostly large academic medical centers with excellent hematopathology expertise, this result underscores the modest interobserver agreement and continued complexity of pathologic diagnosis of GZL. Collectively, morphology remains a critical initial method in the diagnosis of GZL, with immunohistochemistry and in situ hybridization for EBV providing key diagnostic parameters. Other ancillary testing (eg, flow cytometry or molecular studies) does not contribute substantially to the diagnosis at this time. Furthermore, caution should be applied to tissue with limited material and limited number of neoplastic cells.
A spectrum of morphologies with features of cHL and PMBL can occur in GZL, and divergent morphologic areas may be seen within the same tumor specimen. An important morphologic feature of GZL is the abundance of tumor cells, often with confluent sheets of tumor cells. In general, the neoplastic cells in GZL occur in a background containing a paucity of inflammatory cells, although eosinophils, histiocytes, and small lymphocytes can be seen. Variable fibrosis can be present, including extensive coarse fibrosis as well as fine compartmentalizing fibrosis. Nuclear cytomorphology in GZL is an important feature because the neoplastic cell nuclei exhibit a broader range in size and shape, with more infrequent eosinophilic nucleoli, than the Hodgkin Reed Sternberg cells and variants of cHL.
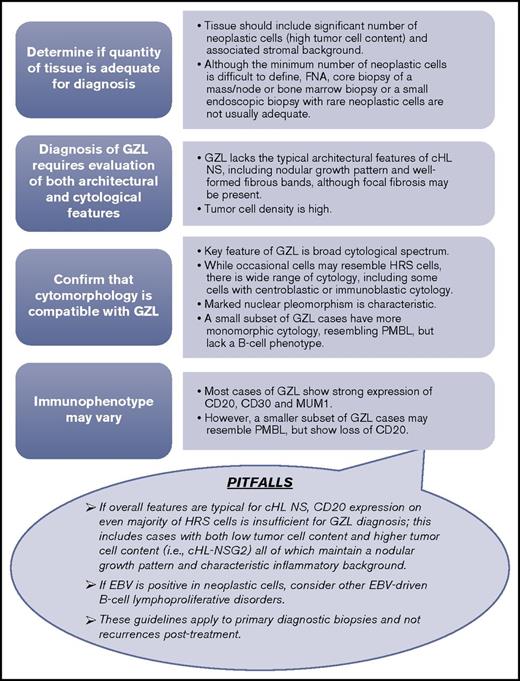
Similar to morphologic findings, the immunophenotype of GZL is variable with transitional and divergent patterns (ie, tumors with cHL-like morphology can exhibit classic DLBCL or PMBL immunophenotype, and vice versa). Tumors resembling cHL may show prominent CD20, weaker/absent CD30, and absent CD15, whereas tumors resembling PMBL are frequently strongly positive for CD30 and CD15 with negativity of CD20 and CD79a. All GZL cases were of B-cell derivation showing expression of at least 1 B-cell marker; CD20 was positive in all but 4 GZL cases, and all neoplastic cells in GZL invariably expressed MUM1. This feature, in conjunction with nuclear cytomorphology, can be helpful in highlighting neoplastic cell nuclei within the inflammatory or fibrotic background. CD30 positivity was a common finding and, if seen in conjunction with CD15 and PMBL morphology, should raise suspicion for GZL. Altogether, based on consensus opinion, the minimum immunohistochemical panel considered adequate for diagnosis should include CD20, PAX5, MUM1, CD30, CD15, and EBV by in situ hybridization; these recommendations, along with an advocated diagnostic algorithm, are detailed in Figure 3.
GZL diagnostic algorithm. The minimum diagnostic panel for workup of GZL should include B-cell markers (CD20 and PAX5), MUM1, CD30, and CD15. A broad panel of T-cell and cytotoxic markers is desirable to rule out anaplastic large cell lymphoma. EBV by in situ hybridization should also be a part of the diagnostic panel. (Top) Stepwise approach to the diagnostic evaluation of GZL. (Lower balloon) depicts potential pitfalls in the diagnostic evaluation of GZL to also consider. FNA, fine needle aspiration.
GZL diagnostic algorithm. The minimum diagnostic panel for workup of GZL should include B-cell markers (CD20 and PAX5), MUM1, CD30, and CD15. A broad panel of T-cell and cytotoxic markers is desirable to rule out anaplastic large cell lymphoma. EBV by in situ hybridization should also be a part of the diagnostic panel. (Top) Stepwise approach to the diagnostic evaluation of GZL. (Lower balloon) depicts potential pitfalls in the diagnostic evaluation of GZL to also consider. FNA, fine needle aspiration.
Most of the reclassified cases fell into the category of cHL NS. Strong expression of CD20 on the neoplastic cells likely contributed the diagnosis of GZL in a large subset of these, but the low tumor cell content, Hodgkin Reed Sternberg cell morphology, and inflammatory background favored cHL NS. Notably, expression of CD20 in a tumor otherwise typical of cHL should not lead to a diagnosis of GZL. Moreover, the NS2 subtype of cHL was relatively frequently among reclassified cases. The NS2 variant of NSHL, also known as cHL NS with lymphocyte depletion, tracks back several decades to original work at the National Cancer Institute, and subsequently at the British National Lymphoma Investigation, where it was identified as a subset with aggressive behavior and poor prognosis in several studies.17,18,21,22 Although knowledge of various morphologic subtypes of cHL and historical perspective on nomenclature development are helpful in understanding different entities, this became less relevant with improved treatment,23 and the WHO does not currently mandate grading of cHL NS in routine clinical practice. However, it is important to be aware of the morphological spectrum within cHL NS to avoid misinterpretation of these cases. Biopsies diagnosed as cHL NS2 showed fibrosis with at least a partially nodular growth pattern and a high content of Hodgkin Reed Sternberg cells, often palisaded around areas of necrosis with frequent eosinophils and neutrophils. It should be noted that the extent of necrosis often tends to correlate with burden of neoplastic cells.24 cHL NS2 may resemble GZL in those cases showing confluent growth of lacunar cells in a relatively paucicellular fibrotic stroma.
Gene expression studies have shown common features between PMBL and cHL, including some common genetic findings among these tumors.25-27 Further studies showed similar genetic aberrations in GZL,10 although methylation profiling still revealed that cHL, GZL, and DLBCL clustered separately by principle component analysis.20 Biologic analyses to compare the cases of cHL NS2 and GZL were beyond the scope of the current study, in part as samples were limited from this large retrospective series drawn from many different centers. Interestingly, the relatively poor outcomes for patients with cHL NS2 here appeared similar to the patients with consensus GZL in this analysis (ie, 3-year PFS rates of 39% and 43%, respectively); however, the patients with cHL NS2 likely did not receive optimal HL-directed therapy, given the original diagnosis of GZL with DLBCL-directed therapy in the majority of those cases.
Clinically, the median age, stage, and incidence of bulk disease of the current cohort of patients with GZL consensus is similar to that of other recent reports of GZL.28 The majority of patients with GZL here also had early-stage disease, and 70% had primary mediastinal disease; a minority of patients had nonmediastinal, systemic disease, which is a change from the prior report,5 where this was the more common clinical presentation. However, it is still important to highlight that either clinical presentation may be encountered with GZL. Cases with consensus GZL without mediastinal disease were older both in the current study and prior published series.10 Regardless of clinical presentation, however, PFS appears to be inferior compared with DLBCL or cHL patient populations.
Therapy was fairly heterogeneous among the consensus GZL cases; however, patients were primarily treated according to either a cHL-like (eg, ABVD) or DLBCL-like (eg, CHOP±R) paradigm. Among all patients with GZL here, a significant minority of patients had primary refractory disease to frontline therapy. In addition, PFS was best for patients with GZL treated according to a DLBCL regimen. Further, CHOP±R appeared to be the optimal chemotherapy regimen in this analysis; however, definitive conclusions are tempered because of a small number of patients treated with other therapeutic regimens. A recent French series of GZL reported improved outcomes for patients treated with more intensive therapeutic regimens (ie, methylprednisolone, doxorubicin, cyclophosphamide, procarbazine, etoposide, bleomycin, vincristine (escBEACOPP) or doxorubicin, methylprednisolone, cyclophosphamide, bleomycin, vindesine.29 It is unclear whether these more dose-intensive regimens are needed for therapy of GZL. It should be highlighted that 3-year event-free survival rates for these regimens were 73% and 70%, respectively, which compares with the 71% PFS rate for patients who received CHOP±R in the current series. In both series, the majority of patients had early-stage disease. Nevertheless, the excellent OS in the current and other series, including good outcomes with second-line therapy for patients with GZL (with hematopoietic stem cell transplant in the majority of cases) suggests more intensive therapeutic platforms may play a role in the management of this disease.
Prognostically, increased PAX5 or moderate/strong CD30 expression was associated with inferior PFS in consensus patients with GZL. CD30 expression has been shown to be a favorable prognostic factor in DLBCL,30 although some series have shown conflicting data.31 In part, given that weak or absent CD30 staining was relatively uncommon in the current series, this observation warrants confirmation. We were not able to confirm the prognostic importance of CD15 staining as shown before.28 In addition, we did not test for the presence of tumor-infiltrating dendritic cells in this current analysis. Clinically, serum albumin was the only factor significantly associated with patient outcome with presence of hypoalbuminemia at diagnosis being associated with 5-fold increased risk for progression. This is a clinical factor that has been shown to be prognostic in HL as well as non-Hodgkin lymphomas.32-38 However, the small number of patients and relative heterogeneity of therapy limit conclusions regarding prognostication.
In summary, the pathologic diagnosis of GZL remains challenging. Only 38% of cases were confirmed on central pathology review. High tumor cell content is very helpful when differentiating between GZL and cHL, the most common alternative diagnosis in this series. A spectrum of morphologies with cHL and PMBL can occur, and divergent morphologic areas can be seen within the same tumor. CD30 expression was common, whereas EBV positivity was rare. Accurate diagnosis is a prerequisite for selection of optimal treatment because current therapeutic strategies for cHL NS, the entity most commonly misdiagnosed as GZL, are different. The small number of patients and relative heterogeneity of therapy limit definitive delineation of optimal therapy for GZL; however, our results suggest that treatment with DLBCL-based regimens is most effective, including R-CHOP as well as DA-EPOCH-R, the latter as reported previously.28 Finally, we identified clinical factors that identified patients with GZL with divergent clinical outcomes. Continued analysis of clinical and pathological features is required to better identify the most important prognostic and biologic factors of GZL. In addition, rational targeted therapeutic agents should be studied in this disease.
Presented in part at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017; the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016; and the 10th International Symposium on Hodgkin Lymphoma, Cologne, Germany, 22-25 October 2016.
The full-text version of this article contains a data supplement.
Acknowledgment
The authors thank the institutional data managers and all local pathologists, treating physicians, and other providers.
Authorship
Contribution: M.P. designed research, performed research, analyzed data, and wrote the paper; S.P. performed research, analyzed data, and wrote the paper; J.A.F. performed research, analyzed data, and wrote the paper; J.H. performed research, analyzed data, and wrote the paper; H.C. performed research, analyzed data, and wrote the paper; J.A.K. analyzed data and wrote the paper; L.H.S. analyzed data and wrote the paper; T.F. analyzed data and wrote the paper; J.S.A. analyzed data and wrote the paper; A.K. analyzed data and wrote the paper; F.J.H.-I. analyzed data and wrote the paper; I.S.L. analyzed data and wrote the paper; O.W.P. analyzed data and wrote the paper; T.S.F. analyzed data and wrote the paper; J.W.F. analyzed data and wrote the paper; J.M.V. analyzed data and wrote the paper; K.A.B. analyzed data and wrote the paper; D.J. analyzed data and wrote the paper; B.W. analyzed data and wrote the paper; G.K.G. analyzed data and wrote the paper; R.D.G. performed research, analyzed data, and wrote the paper; E.S.J. designed research, performed research, analyzed data, and wrote the paper; and A.M.E. designed research, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.A.K. is Experimental Transplantation and Immunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD.
The current affiliation for G.K.G. is Hematopathology Section, Laboratory for Pathology, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Correspondence: Elaine S. Jaffe, Hematopathology Section, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, Building 10, Room 3s235, Bethesda, MD 20892-1500; e-mail: elainejaffe@nih.gov; and Andrew M. Evens, Division of Hematology/Oncology, Tufts Medical Center, 800 Washington St, Boston, MA 02111; e-mail: dramevens@mac.com.


![Figure 1. Consensus pathology. (A) Case example of consensus GZL diagnosis (B-cell lymphoma with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma). Sheetlike proliferation of tumor cells resembling PMBL is seen in the top left panel. Focally, collections of eosinophils are noted (top right). The tumor cells are strongly positive for CD20, CD30, and CD15 (bottom). (B) Reclassified case: cHL NS2. Representative images from 2 cases of cHL NS2 are shown. (Top) Characteristic histology with a nodular growth pattern, fibrous bands, and a sheetlike growth of lacunar cells. (Bottom) Immunohistochemical stains from a second case show the nodular growth pattern with strong staining for CD20, but also positivity for CD30 and focal staining for CD15. (C) Reclassified case: primary mediastinal large B cell lymphoma. Diffuse proliferation of large neoplastic cells is seen (top left). The neoplastic cells have abundant clear cytoplasm and vesicular nuclei with basophilic nucleoli (top right). They are strongly, uniformly positive for CD20, with dim expression of CD30, and are negative for CD15 (bottom). (D) Synopsis of the immunohistochemical staining results from consensus confirmed GZL cases. The bar graph illustrates immunohistochemical staining for markers CD20, CD79a, PAX5, MUM1, CD30, CD15, CD3, and in situ hybridization for EBV (EBV-encoded small RNA [EBER]). For immunohistochemical studies, staining was considered to be positive if it had intensity of 2 or more (on +1 to +3 scale) and was distributed in more than 25% of neoplastic cells. Cases with weak/negative expression are not included in this graph. Hematoxylin and eosin staining, original magnification ×400 (A, B [top right panel], and C [top panels]) and ×200 (B [top left panel]). Immunohistochemical staining, original magnification ×400 (all other panels).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/26/10.1182_bloodadvances.2017009472/3/m_advances009472f1.jpeg?Expires=1767726920&Signature=iyJBNjD5hIxtgexhDdqVLFtDpj4OvcE8PPvVme9zhP2tNWYcJr88A8KWvAMk50hK5UWtytKw0xKAf1YzBKYxZHGausi5FwIwDGfCJH-PdYbkJLm65HnFOe-ZRR6h4L3wvFNMeBpOi4IMeywBkh27wJ7j~nzRKVxiqLdEFPOt5tXwS9a3bzghenL53EHI4zbH9z5DDfqCSw5qxBzXf17UwfQdvl~JIADhN3yCz~OG-vfc8~Z6G2249uKY5FvBf-OO9KcXBE~atKiARvEUz08yCIElgqFeeeCD98iVHyJUMyFeVU5FAmaIoohE6nuv-c63W9m4zZSPwdUoKH5AHIRdCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)