Key Points
NF-κB and Akt regulate human monocyte into macrophage differentiation; p38 MAPK and PTGS2 promote the generation of suppressive macrophage.
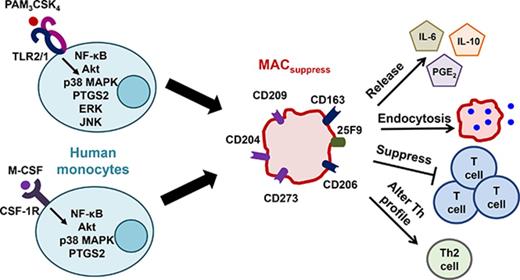
The Toll-like receptor 2/1 agonist PAM3 induces human monocytes to mature into immunosuppressive macrophages in vitro and in vivo.
Abstract
Human monocytes differentiate into either proinflammatory or immunosuppressive macrophages in response to distinct stimuli. Results show that the Toll-like receptor 2/1 agonist PAM3 replicates the ability of macrophage colony-stimulating factor (M-CSF) to induce the preferential generation of immunosuppressive macrophages in vitro, an activity confirmed by in vivo studies of rhesus macaques. By comparing the gene expression pattern of monocytes treated with M-CSF vs PAM3, the pathways regulating macrophage maturation were identified. NF-κB and Akt were found to play a central role in the overall process of monocyte into macrophage differentiation. Pathways regulated by p38 MAPK and PTGS2 biased this process toward the generation of immunosuppressive rather than proinflammatory macrophages. ERK and JNK contribute to PAM3- but not M-CSF–driven monocyte maturation. These findings clarify the mechanisms underlying the generation of immunosuppressive macrophages and support the use of PAM3 in the treatment of autoimmune and inflammatory diseases.
Introduction
Macrophages are a phenotypically and functionally diverse population of cells that play key roles in maintaining immune surveillance and tissue homeostasis.1 Bone marrow–derived monocytes differentiate into macrophages in response to signals provided by cytokines, cellular metabolites, and microbial products.2,3 Macrophages have historically been categorized into classically activated M1-like and alternatively activated M2-like subtypes.4,5 M1-like macrophages support inflammatory processes that protect the host from microbial infection and aid in the elimination of tumors.6,7 M2-like macrophages promote T helper 2 (Th2) immunity, suppress ongoing inflammatory responses, and facilitate tissue repair/remodeling.3,8 Recent work indicates that the phenotypic and functional heterogeneities of macrophages are not fully captured by the M1/M2 classification scheme.9 This work will therefore refer to human monocytes induced to mature into proinflammatory macrophages by treatment with granulocyte-macrophage colony-stimulating factor (GM-CSF), Toll-like receptor (TLR) agonists, and/or interferon-γ (IFN-γ) as MACinflam, whereas those that maturate into immunosuppressive macrophages after treatment with M-CSF (CSF-1) or other agents will be referred to as MACsuppress.10-13
The development and/or persistence of chronic inflammatory diseases, autoimmunity, and cancer have been linked to dysregulation in the balance between MACinflam and MACsuppress.1,5 Murine studies show that adoptively transferred MACsuppress protect against the development of type 1 diabetes, alleviate the symptoms of systemic lupus erythematosus and multiple sclerosis, ameliorate the severity of colonic and renal inflammations, and prevent graft rejection.14-19 These findings have fueled interest in therapies that can bolster the generation of MACsuppress in patients.
Although TLR agonists typically support the generation of MACinflam,3,20 we found that monocytic myeloid-derived suppressor cells (a minor subpopulation of monocytes) matured into MACsuppress when stimulated with the TLR2/1 agonist Pam(3)CSK(4) (PAM3).21 TLR2 is expressed at high levels on the surface of human monocytes, where in combination with TLR1 and 6 it mediates the recognition of endogenous (eg, heat-shock proteins, extracellular matrix components) and exogenous (components of mycoplasma, fungus, and bacteria) ligands.22-25 TLR2 stimulation helps protect the host from infection and may reduce inflammation by antagonizing IFN-γ signaling and promoting the production of interleukin-10 (IL-10).26,27
These observations led us to compare the effect of PAM3 vs M-CSF on the maturation of human monocytes. Conventional CD14+/HLA-DR+ monocytes treated with PAM3 (but not other TLR agonists) generated MACsuppress that were phenotypically and functionally similar to those produced by M-CSF. Microarray analyses coupled with gene/pathway inhibition studies identified the NF-κB complex and Akt as being central to the process of monocyte maturation into both MACinflam and MACsuppress. In contrast, p38 MAPK and PTGS2 were uniquely associated with the generation of MACsuppress, whereas the extracellular signal-regulated kinase 1/2 (ERK1/2) and JNK signaling pathways were activated after PAM3- but not M-CSF–dependent monocyte polarization. The in vivo activity of PAM3 was verified in rhesus macaques, where treatment increased the frequency of MACsuppress in the peripheral circulation and supported IL-10 production.
Methods
Reagents
FSL-1, lipopolysaccharide (LPS) B5, MPLA-SM, PAM3, peptidoglycan (PGN) BS, and R848 were obtained from Invivogen (San Diego, CA) and human recombinant M-CSF and IFN-γ from Miltenyi Biotec (Auburn, CA). Most antibodies were purchased from Biolegend (San Diego, CA). Celecoxib was purchased from Sigma Aldrich (St. Louis, MO); FR122047 from Cayman Chemical (Ann Arbor, MI); PD98059, perifosine, SP600125, SB203580, and celastrol from Invivogen; IL-6 (6708), M-CSF (26730), anti–M-CSF (26730), and anti–IL-6 (BAF206) from R&D Systems (Minneapolis, MN).
Preparation of human monocytes
Elutriated monocytes were obtained from healthy volunteers after written consent in an institutional review board–approved protocol (National Institutes of Health, Bethesda, MD). Cells were cultured overnight in RPMI 1640 supplemented with 2% fetal calf serum (both from Lonza, Walkersville, MD), 2 mM of glutamine, and 25 mM of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (both from Invitrogen, Carlsbad, CA). Elutriated monocytes were stained with fluorochrome-conjugated antibody (Ab) against CD14 and HLA-DR. CD14brightHLA-DR+ monocytes were then purified by FACSAria II sorting (BD Biosciences).
In vitro differentiation
Purified monocytes were cultured with 500 ng/mL of M-CSF, 500 ng/mL of IFN-γ, 1 µg/mL of PAM3, 1 µg/mL of PGN, 10 ng/mL of FSL-1, 1 µg/mL of LPS, 1 µg/mL of MPLA, or 3 µg/mL of R848 for 3 to 5 days. These reagent concentrations were optimized in preliminary experiments. In some experiments, 25 µg/mL of neutralizing anti–M-CSF, 2.5 µM of celastrol (NF-κB inhibitor), 10 µM of celecoxib (PTGS2 inhibitor), 50 nM of FR122047 (PTGS1 inhibitor), 1 µM of PD98059 (ERK1/2 inhibitor), 5 µM of perifosine (Akt inhibitor), 10 µM of SP600125 (JNK inhibitor), 10 µM of SB203580 (p38 MAPK inhibitor), or dimethyl sulfoxide (vehicle control) were added to cultures for 5 days.
Analysis of cell surface and intracellular activation markers
Cultured monocytes were incubated on ice with Fc Block (Biolegend) for 15 minutes and stained with fluorescein-conjugated Ab for 30 minutes. For intracellular staining, cells were treated with Fix & Perm Medium A (Invitrogen) at room temperature for 15 minutes and then with Ab in Medium B for 20 minutes. Cells were washed in phosphate-buffered saline containing 2% bovine serum albumin and analyzed using an LSRFortessa (BD Biosciences).
T-cell isolation, proliferation, and polarization
Lymphocytes were obtained from matched monocyte donors by centrifugation over Histopaque-1077 (Sigma Aldrich) at 400 g for 30 minutes. The cells were washed, cultured for 3 days, and sort purified using a Naive CD4 Isolation Kit II (Miltenyi Biotec).
A total of 105 carboxyfluorescein diacetate succinimidyl ester–labeled naive CD4 T cells were mixed with 105 Human T-Activator Dynabeads (Thermo Fisher Scientific, Waltman, MA) plus 2 × 105 monocytes from the same donor (previously stimulated with 1 µg/mL of PAM3 or 500 ng/mL of M-CSF for 2 days). The carboxyfluorescein diacetate succinimidyl ester content of cells identified by staining with anti-CD4 Ab was determined on day 5 using an LSRFortessa. To detect T-cell polarization, 5 × 104 stimulated monocytes were mixed with 105 naive CD4 T cells from the same donor in the presence of 5 µg/mL of anti-CD3 and 2 µg/mL of anti-CD28 (eBioscience) Ab for 4 to 5 days. Cells were then stained with surface marker–specific Abs and analyzed by LSRFortessa.
Endocytosis assay
A total of 105 monocytes stimulated with PAM3 or M-CSF were incubated with 50 µg/mL of Anionic Alexa Fluor 488 dextran (MW 3000; Thermo Fisher Scientific) for 45 minutes in 200 µL of media at 37°C or 4°C. The rate of uptake was assessed using the LSRFortessa.
Cytokine enzyme-linked immunosorbent assay
Immunol 2HB microtiter plates (Thermo Fisher Scientific) were coated with anticytokine Ab and then blocked with phosphate-buffered saline containing 2% bovine serum albumin. Serially diluted standards and supernatants were added followed by biotinylated anticytokine Abs (R&D Systems), phosphatase-streptavidin (BD Biosciences), and K-Gold PNPP Substrate (Neogen Corporation, Lexington, KY). PGE2 (Enzo Life Sciences, Farmingdale, NY) and IL-12 (R&D Systems) enzyme-linked immunosorbent assays were performed according to manufacturer instructions. Optical density was measured using a SpectraMax M5 Microplate Reader and SoftMax Pro Acquisition and Analysis software (both Molecular Devices, Sunnyvale, CA).
Microarray analysis of gene expression
A total of 106 fluorescence-activated cell sorted monocytes were stimulated with 1 µg/mL of PAM3 or 500 ng/mL of M-CSF for 4 hours. Total RNA was extracted using an Rneasy Mini Kit (Qiagen, Valencia, CA), amplified using an Amino Allyl Messeage Amp II aRNA Kit (Thermo Fisher Scientific) and then transcribed into antisense amplified RNA (asRNA) using T7 RNA polymerase labeled with Cy5 monoreactive dye (GE Healthcare and Life Sciences, Pittsburgh, PA). Reference samples (Stratagene, San Diego, CA) were labeled in parallel with Cy3. asRNA was labeled with Cy3 or Cy5 dissolved in dimethyl sulfoxide and purified using an Rneasy MinElute Kit (Qiagen).
Cy3-labeled reference and Cy5-labeled sample asRNAs were combined, heat denaturated for 2 minutes at 98°C, mixed with hybridization solution, and incubated on human microarrays (Microarrays Inc., Huntsville, AL) for 18 hours at 42°C. The arrays were washed and scanned using an Axon scanner equipped with GenePix software 5.1 (Molecular Devices). Data were analyzed using the microarray database of the Center for Information Technology BioInformatics and Molecular Analysis Section and National Cancer Institute Center for Cancer Research. Upregulated genes were mapped into regulatory networks using Ingenuity Pathway Analysis (IPA) software (Qiagen). Accession code in Gene Expression Omnibus repository: GSE99609.
In vivo studies of rhesus macaques
All rhesus macaque (Macaca mulatta) experiments were approved by the White Oak Consolidated Animal Care and Use Committee and conducted at the US Food and Drug Administration White Oak animal facility in accordance with the Guide for the Care and Use of Laboratory Animals (eighth edition). Groups were weight and sex matched (n = 6 per group). Animals were anesthetized with ketamine (5-10 mg/kg), shaved, and then injected subcutaneously with 2 mg of PAM3 or sterile saline solution (Quality Biological, Gaithersburg, MD) in 1 mL using a 30-g needle. Blood samples and skin biopsies were obtained under ketamine (5-10 mg/kg) and xylazine (0.5-1 mg/kg) anesthesia.
Skin biopsies obtained from the sites of injection were placed in TRIzol reagent, mixed with Zirconia Beads (Biospec Products, Bartlesville, OK), and shredded by glass bead friction using the Precellys 24 Cryolys system (Bertin Technologies, Paris, France).
RNA was purified and reverse transcribed into complementary DNA (cDNA) using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The quantitative reverse transcription polymerase chain reaction reactions for quantifying IL-10 and tumor necrosis factor α (TNFα) were conducted by adding Universal master mix (Applied Biosystems) with a 1/20th volume of cDNA per reaction using a Viia7 reverse transcription polymerase chain reaction system (Applied Biosystems).
Statistical analysis
Significance was determined using 2-sided unpaired Student t tests or Bonferroni-corrected multiple comparison tests (GraphPad Software Inc., La Jolla, CA).
Results
Effect of TLR agonists on human monocytes
Human macrophages are broadly classified into 2 functionally distinct populations: proinflammatory and immunosuppressive.4,5 Both populations express the 25F9 surface marker, but only MACsuppress coexpress high levels of the mannose receptor CD206 or the scavenger receptor CD163.3,28,29 The conventional route to generating human MACsuppress involves treating monocytes with M-CSF.10-12 In contrast, monocytes stimulated with most TLR ligands mature into MACinflam.20
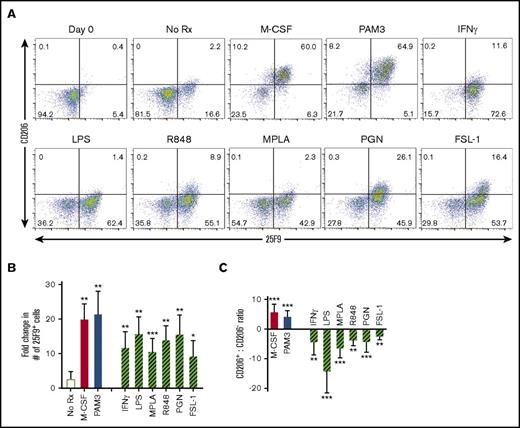
Building on the observation that PAM3 induces monocytic myeloid-derived suppressor cells to mature into MACsuppress, the response of fluorescence-activated cell sorter–purified human monocytes to that TLR agonist was assessed. When cultured in medium alone for 3 days, <20% of CD14+/HLA-DR+ monocytes survived, and of those, <20% differentiated into macrophages (Figure 1A-B). Survival was significantly improved by the addition of M-CSF or agonists targeting TLR1, 2, 4, 6, 7, or 8 (P < .01; Figure 1B). PAM3 was unique among the TLR agonists in its ability to mimic the activity of M-CSF and induce monocytes to differentiate into MACsuppress characterized phenotypically by the expression of 25F9+, CD163+, and CD206+ (Figure 1; supplemental Figure 1). By comparison, monocytes stimulated via TLR4 (LPS or MPLA), TLR7/8 (R848), or IFN-γ generated MACinflam characterized by the expression of 25F9+ but neither CD206 nor CD163 (P < .01; Figure 1A,C).
TLR agonists and M-CSF induce CD14+HLA-DR+human monocytes to differentiate into macrophages. Monocytes from healthy volunteers were stimulated in vitro with optimized concentrations of M-CSF (500 ng/mL), PAM3 (1 µg/mL), IFN-γ (500 ng/mL), LPS (1 µg/mL), MPLA (1 µg/mL), R848 (3 µg/mL), PGN (1 µg/mL), or FSL-1 (10 ng/mL). Samples stimulated for 3 (n = 2) or 5 (n = 4) days yielded similar patterns of macrophage polarization. (A) Representative dot plots exemplifying changes in 25F9 and CD206 expression. (B) Fold change in the number of MACsuppress (solid bars) or MACinflam (cross-hatched bars) present at the end of culture (mean ± standard deviation of 5-6 independently studied donors per data point). (C) Ratio of CD206+ to CD206− 25F9+ macrophages in the samples described in panel B. *P < .05, **P < .01, ***P < .001 vs unstimulated cultures. Rx, treatment.
TLR agonists and M-CSF induce CD14+HLA-DR+human monocytes to differentiate into macrophages. Monocytes from healthy volunteers were stimulated in vitro with optimized concentrations of M-CSF (500 ng/mL), PAM3 (1 µg/mL), IFN-γ (500 ng/mL), LPS (1 µg/mL), MPLA (1 µg/mL), R848 (3 µg/mL), PGN (1 µg/mL), or FSL-1 (10 ng/mL). Samples stimulated for 3 (n = 2) or 5 (n = 4) days yielded similar patterns of macrophage polarization. (A) Representative dot plots exemplifying changes in 25F9 and CD206 expression. (B) Fold change in the number of MACsuppress (solid bars) or MACinflam (cross-hatched bars) present at the end of culture (mean ± standard deviation of 5-6 independently studied donors per data point). (C) Ratio of CD206+ to CD206− 25F9+ macrophages in the samples described in panel B. *P < .05, **P < .01, ***P < .001 vs unstimulated cultures. Rx, treatment.
TLR2 forms homodimers that recognize PGN and heterodimers with TLR6 and TLR1 that respond to FSL-1 and PAM3, respectively.23 This analysis was therefore broadened to examine the response of human monocytes to FSL-1 and PGN. Similar to LPS and R848, these agents stimulated purified monocytes to preferentially mature into 25F9+, CD163−, and CD206− macrophages (Figure 1B-C). Because PAM3 was unique in preferentially generating MACsuppress from human monocytes, subsequent experiments focused on confirming this activity and identifying the underlying mechanism.
Effect of PAM3 is independent of M-CSF
If PAM3 triggered the production of M-CSF, its ability to induce human monocytes to mature into MACsuppress would be explained. This possibility was examined by adding neutralizing anti–M-CSF Ab to monocyte cultures. As expected, anti–M-CSF significantly inhibited M-CSF–driven macrophage differentiation (P < .001; supplemental Figure 2A). In contrast, inclusion of anti–M-CSF had no effect on cultures stimulated with PAM3, consistent with the absence of M-CSF in supernatants from PAM3-treated cultures (supplemental Figure 2B).
Phenotypic comparison of macrophages generated by PAM3 vs M-CSF
To more fully characterize the macrophages generated by PAM3 and M-CSF, detailed phenotypic studies were performed. Both agonists had similar effects on the expression of the monocyte surface markers CD11b and CD14 and the activation markers CD68 and CD40 (Figure 2). Both agents increased the frequency of cells expressing those markers by 60% to 80% and their median fluorescence intensity by five- to 25-fold when compared with unstimulated controls (P < .01; Figure 2B-C). PAM3 and M-CSF also stimulated 60% to 80% of cells to upregulate expression of the macrophage differentiation markers CD273 (PD-L2), CD204 (SR-A), and CD209 (DC-SIGN; Figure 2B), although the magnitude of this effect was ∼twofold higher for monocytes cultured with M-CSF (P < .001; Figure 2C). These molecules are linked to the function of MACsuppress; CD273 is a ligand for PD1 that plays a critical role in macrophage-mediated suppression of T-cell proliferation, whereas CD204 and CD209 regulate endocytic activity.30-32 The expression of CD16 (FcγRIII, a receptor for opsonin-dependent phagocytosis) also differed between M-CSF– vs PAM3-generated macrophages, with significantly fewer of the latter upregulating this receptor (<40% vs >60%; P < .01; Figure 2B).
Phenotype of PAM3 and M-CSF macrophages. Purified monocytes were stimulated in vitro for 5 days. Representative histogram (A), percentage (B), and fold change (C) in median fluorescence intensity (MFI) of cells expressing each surface marker (mean ± standard deviation of 6 independently studied donors per group). Bars show the effect of stimulation with PAM3 (blue), M-CSF (red), or medium alone (open green). **P < .01, ***P < .001 vs unstimulated cultures; ++P < .01, +++P < .001 PAM3 vs M-CSF cultures.
Phenotype of PAM3 and M-CSF macrophages. Purified monocytes were stimulated in vitro for 5 days. Representative histogram (A), percentage (B), and fold change (C) in median fluorescence intensity (MFI) of cells expressing each surface marker (mean ± standard deviation of 6 independently studied donors per group). Bars show the effect of stimulation with PAM3 (blue), M-CSF (red), or medium alone (open green). **P < .01, ***P < .001 vs unstimulated cultures; ++P < .01, +++P < .001 PAM3 vs M-CSF cultures.
Functional activity of macrophages generated by PAM3 vs M-CSF
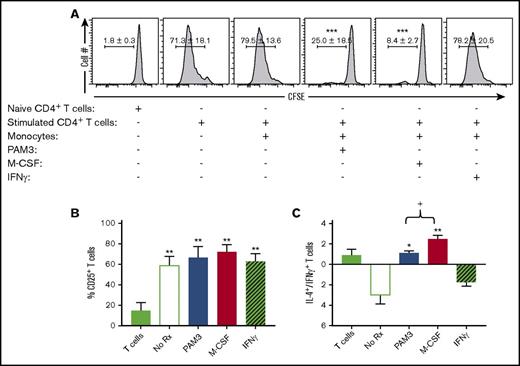
The functional activity of MACsuppress includes their ability to inhibit the proliferation of activated T cells, support the differentiation of naive CD4 into IL-4–secreting Th2 cells, and clear debris via endocytosis.3 Each of these activities was examined in PAM3- and M-CSF–generated macrophages. The macrophages generated by both agents significantly inhibited the proliferation of autologous T cells (Figure 3A). In contrast, MACinflam generated by treatment with IFN-γ and untreated monocytes had no such effect. Suppressive activity was not due to M-CSF or PAM3 directly affecting T cells, because their proliferation was only reduced when monocytes were present (data not shown).
PAM3- and M-CSF–stimulated monocytes suppress T-cell proliferation and alter the generation of Th1 vs Th2 cells. Autologous T cells stimulated with anti-CD3/CD28 were added to purified monocytes activated as described in Figure 2. (A) T-cell proliferation was monitored by carboxyfluorescein diacetate succinimidyl ester dilution on day 5 (mean ± standard deviation of 4-5 independently studied donors). Percentage of CD25+ T cells (B) and IL-4– vs IFN-γ–expressing T-cell ratio (C) on day 7 (mean ± standard error of the mean of 4-5 independently studied donors). *P < .05, **P < .01, ***P < .001 vs stimulated T cells plus monocytes; +P < .05 PAM3 vs M-CSF cultures.
PAM3- and M-CSF–stimulated monocytes suppress T-cell proliferation and alter the generation of Th1 vs Th2 cells. Autologous T cells stimulated with anti-CD3/CD28 were added to purified monocytes activated as described in Figure 2. (A) T-cell proliferation was monitored by carboxyfluorescein diacetate succinimidyl ester dilution on day 5 (mean ± standard deviation of 4-5 independently studied donors). Percentage of CD25+ T cells (B) and IL-4– vs IFN-γ–expressing T-cell ratio (C) on day 7 (mean ± standard error of the mean of 4-5 independently studied donors). *P < .05, **P < .01, ***P < .001 vs stimulated T cells plus monocytes; +P < .05 PAM3 vs M-CSF cultures.
MACsuppress drive naive T cells to mature into IL-4–secreting Th2 lymphocytes, whereas MACinflam induce them to mature into IFN-γ–secreting Th1 cells.1 Consistent with this distinction, IFN-γ–generated MACinflam triggered autologous T cells to mature into Th1 cells, whereas PAM3- or M-CSF–generated MACsuppress supported the generation of Th2 cells (Figure 3B-C). These differences are described in Figure 3C, which shows the ratio of IL-4– to IFN-γ–secreting CD25 T cells in culture (P < .05).
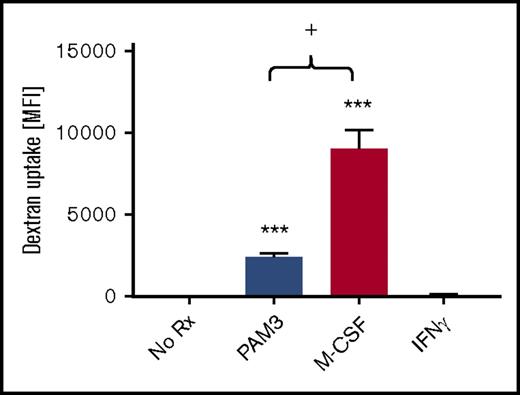
Eliminating pathogens and cell debris is another important function of tissue-resident MACsuppress.29,33 The recognition of these targets is mediated by scavenger, C-type lectin, and Fc receptors.1 The ability of macrophages generated by PAM3 vs M-CSF to internalize dextran particles (a model of receptor-mediated endocytosis) was therefore evaluated. PAM3-treated macrophages manifested significantly greater endocytic activity than unstimulated monocytes or MACinflam (generated by IFN-γ treatment of monocytes; Figure 4). However, M-CSF–generated macrophages phagocytosed nearly twofold more labeled dextran particles than PAM3-treated cells (P < .05; Figure 4), consistent with their greater expression of receptors associated with endocytosis (Figure 2).
Endocytic activity of PAM3- and M-CSF–generated monocytes. Purified monocytes were stimulated in vitro for 5 days and then incubated with 50 µg/mL of labeled dextran particles for 45 minutes at 4°C or 37°C. Uptake represents the difference in fluorescence intensity at 4°C vs 37°C for each stimulant (mean ± standard deviation of 5-6 independently studied donors per group). **P < .01, ***P < .001 vs unstimulated cultures; +P < .05 PAM3 vs M-CSF cultures.
Endocytic activity of PAM3- and M-CSF–generated monocytes. Purified monocytes were stimulated in vitro for 5 days and then incubated with 50 µg/mL of labeled dextran particles for 45 minutes at 4°C or 37°C. Uptake represents the difference in fluorescence intensity at 4°C vs 37°C for each stimulant (mean ± standard deviation of 5-6 independently studied donors per group). **P < .01, ***P < .001 vs unstimulated cultures; +P < .05 PAM3 vs M-CSF cultures.
Regulatory networks used during PAM3- and M-CSF–driven monocyte differentiation
These results indicate that both PAM3 and M-CSF induce monocytes to preferentially mature into MACsuppress. To gain insight into the regulatory pathway(s) underlying this differentiation process, microarray analyses were performed. CD14+/HLA-DR+ monocytes were purified from 5 donors and cultured in medium, PAM3, or M-CSF. Gene expression was evaluated after 4 hours, a time point previously found to be optimal for detecting the pathways activated by TLR stimulation using IPA software.21,34,35
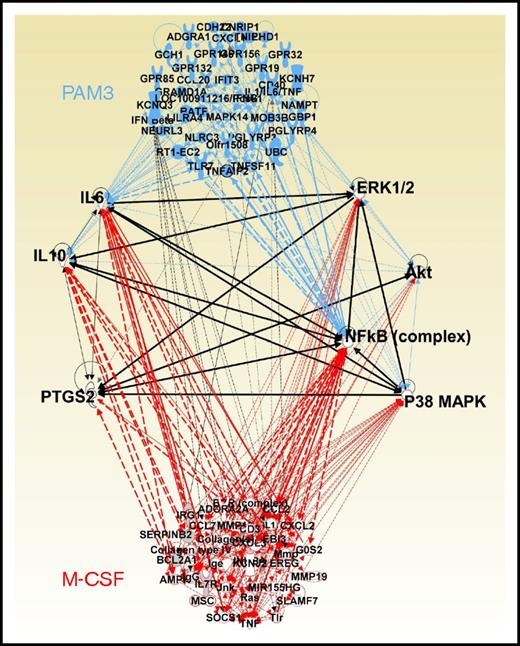
IPA predicted that regulatory networks involving the NF-κB complex, MAPK, ERK, Akt, and PTGS2 (COX-2) were central to both PAM3- and M-CSF–driven monocyte maturation. Stimulation of these networks was associated with the upregulation of messenger RNA (mRNA) encoding prostaglandins and cytokines (including IL-6 and IL-10 but not IL-12 or TNFα; Figure 5). To examine these predictions, the cytokine content of monocyte-stimulated culture supernatants was measured. IL-6 levels increased by >40-fold and IL-10 levels by > fourfold after treatment with either PAM3 or M-CSF (P < .05 vs unstimulated controls; Figure 6A). Similarly, levels of prostaglandin E2 (which is upregulated by PTGS2 in MACsuppress36,37 ) rose fourfold (P < .001), whereas these agents had no effect on proinflammatory mediators such as IL-12 or TNFα (Figure 6A; data not shown).
Genes upregulated by PAM3 vs M-CSF and associated regulatory networks. Purified monocytes from 5 donors were stimulated for 4 hours as described in Figure 1. Their changes in gene expression were monitored by microarray. Upregulated genes were identified by comparison with untreated cells from the same donor using a stringency cutoff of P < .0001. All genes with regulatory interactions that could be mapped by IPA were then linked by network analysis. Critical networks are connected by black lines.
Genes upregulated by PAM3 vs M-CSF and associated regulatory networks. Purified monocytes from 5 donors were stimulated for 4 hours as described in Figure 1. Their changes in gene expression were monitored by microarray. Upregulated genes were identified by comparison with untreated cells from the same donor using a stringency cutoff of P < .0001. All genes with regulatory interactions that could be mapped by IPA were then linked by network analysis. Critical networks are connected by black lines.
Evaluation of IPA-predicted regulatory pathways and changes in protein production. Purified monocytes were stimulated in vitro for 5 days. (A) Fold increase in IL-6, IL-10, PGE2, and IL-12p70 in culture supernatants on day 5 (mean ± standard deviation [SD] of 7-8 independently studied donors). For unstimulated cultures, concentrations were: 45 pg/mL of PGE2 and 50 pg/mL of IL-12p70. Because IL-6 and IL-10 could not be detected in unstimulated cultures, the fold increase was calculated based on the sensitivity of the enzyme-linked immunosorbent assay, 10 pg/mL for IL-6 and 30 pg/mL for IL-10. (B-C) Effect of blocking NF-κB (2.5 µM of celastrol), Akt (5 µM of perifosine), p38 MAPK (10 µM of SB203580), ERK (1 µM of PD98059), JNK (10 µM of SP600125), PTGS2 (10 µM of celecoxib), or PTGS1 (50 nM of FR122047) on the generation of macrophages by including inhibitors during culture (mean ± SD of 4-6 independently studied donors per group). *P < .05, **P < .01, ***P < .001 vs unstimulated/control cultures. n.s., not significant.
Evaluation of IPA-predicted regulatory pathways and changes in protein production. Purified monocytes were stimulated in vitro for 5 days. (A) Fold increase in IL-6, IL-10, PGE2, and IL-12p70 in culture supernatants on day 5 (mean ± standard deviation [SD] of 7-8 independently studied donors). For unstimulated cultures, concentrations were: 45 pg/mL of PGE2 and 50 pg/mL of IL-12p70. Because IL-6 and IL-10 could not be detected in unstimulated cultures, the fold increase was calculated based on the sensitivity of the enzyme-linked immunosorbent assay, 10 pg/mL for IL-6 and 30 pg/mL for IL-10. (B-C) Effect of blocking NF-κB (2.5 µM of celastrol), Akt (5 µM of perifosine), p38 MAPK (10 µM of SB203580), ERK (1 µM of PD98059), JNK (10 µM of SP600125), PTGS2 (10 µM of celecoxib), or PTGS1 (50 nM of FR122047) on the generation of macrophages by including inhibitors during culture (mean ± SD of 4-6 independently studied donors per group). *P < .05, **P < .01, ***P < .001 vs unstimulated/control cultures. n.s., not significant.
To examine the regulatory pathways used during PAM3- and M-CSF–driven macrophage maturation, the effect of inhibiting each pathway on the generation of MACinflam and MACsuppress was monitored. Inhibiting either Akt or the NF-κB complex reduced the general process by which monocytes differentiate into macrophages by 70% to 90%, leaving the remaining viable monocytes as the dominant cell type in those cultures (note that the generation of both CD163+ and CD163− macrophages was inhibited [P < .01]; Figure 6B-C). By comparison, blockade of p38 MAPK or PTGS2 reduced the generation of CD163+ MACsuppress by 60% to 80% while increasing the frequency of CD163− MACinflam (P < .01; Figure 6B-C). As expected, negative controls (PTGS1 and vehicle alone) had no effect on PAM3- or M-CSF–induced monocyte differentiation (Figure 6B-C). These findings suggest that p38 MAPK and PTGS2 determine whether the macrophages generated via Akt- and NF-κB–dependent pathways become proinflammatory or immunosuppressive.
Microarray analysis further showed that MAP3K8 (Tpl2) was significantly upregulated in PAM3- but not M-CSF–stimulated cultures (Figure 5). Because Tpl2 regulates the expression of both ERK and JNK,38 the contribution of those proteins to monocyte maturation was examined. Inhibiting JNK or ERK significantly reduced PAM3-dependent generation of MACsuppress but had little effect on M-CSF–induced macrophage maturation (Figure 6B-C). JNK also blocked MACinflam maturation in PAM3-stimulated samples, suggesting that it has a central role in the general process of TLR2/1-dependent macrophage polarization (Figure 6B).
In vivo activity of PAM3
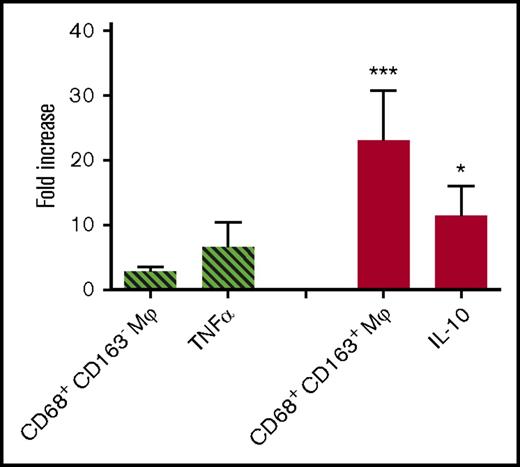
The in vivo response of humans to TLR agonists mirrors that of other primates (unlike the response of rodents, which differ from humans in their expression of and response to TLR stimulation).39-41 Rhesus macaques were therefore treated with 2 mg of PAM3, and the effect of subcutaneous treatment on circulating macrophages was monitored for 72 hours. Consistent with in vitro results derived from studies of purified human monocytes, the frequency of CD68+/CD163+ macrophages in the peripheral circulation of macaques treated with PAM3 increased nearly 20-fold compared with pretreatment levels or saline-treated controls (P < .001; Figure 7). PAM3 had no significant effect on the frequency of circulating CD68+/CD163− macrophages.
In vivo activity of PAM3. Rhesus macaques were injected subcutaneously with 2 mg of PAM3 or saline. Data show the maximum increase in the frequency of CD163− and CD163+ macrophages in the peripheral blood (mean + standard error of the mean of 6 animals per group) over 3 days. Skin from the site of injection was examined for changes in mRNA encoding the proinflammatory cytokine TNFα (cross-hatched bar) and the anti-inflammatory mediator IL-10 (solid bar) at 4 hours. *P < .05, ***P < .001 vs saline control.
In vivo activity of PAM3. Rhesus macaques were injected subcutaneously with 2 mg of PAM3 or saline. Data show the maximum increase in the frequency of CD163− and CD163+ macrophages in the peripheral blood (mean + standard error of the mean of 6 animals per group) over 3 days. Skin from the site of injection was examined for changes in mRNA encoding the proinflammatory cytokine TNFα (cross-hatched bar) and the anti-inflammatory mediator IL-10 (solid bar) at 4 hours. *P < .05, ***P < .001 vs saline control.
The site of PAM3 injection was examined for changes in mRNA levels encoding pro- and anti-inflammatory mediators. Consistent with in vitro results (Figure 6), IL-10 mRNA levels rose by >10-fold after PAM3 treatment, whereas there was no significant change in proinflammatory TNFα, NOS2, or IL-12 mRNA levels (Figure 7; data not shown).
Discussion
Manipulating the frequency of MACsuppress affects the host’s response to infection, cancer, and autoimmune disease.1,5,42 This work defines the regulatory pathways that mediate the maturation of human monocytes into MACsuppress. It further shows that PAM3 is unique among TLR agonists in stimulating monocytes to differentiate into MACsuppress rather than MACinflam. The resulting cells are characterized phenotypically by the expression of 25F9, CD206, and CD163 and functionally by their ability to mediate endocytosis, suppress the proliferation of activated T cells, and induce naive CD4 cells to mature into IL-4–secreting Th2 cells (Figures 1, 3, and 4; supplemental Figure 1). PAM3 stimulated monocytes to mature into MACsuppress as efficiently as M-CSF (Figure 1; supplemental Figure 1). However, M-CSF played no role in PAM3-induced macrophage polarization, because that cytokine was not present in PAM3-stimulated culture supernatants (supplemental Figure 2), and neutralizing M-CSF did not inhibit the generation of MACsuppress by PAM3 (supplemental Figure 2).
The phenotype and function of macrophages generated by PAM3 and M-CSF were compared (Figures 1-6). The 2 populations were similar with respect to their expression of most surface markers (although M-CSF more effectively upregulated receptors associated with endocytic activity; Figure 2). Functionally, the 2 populations shared the capacity to suppress T-cell proliferation and promote type-2 cytokine production by T cells (Figure 3), although M-CSF generated macrophages with greater endocytic activity (Figure 4).
The similarity between PAM3- and M-CSF–generated macrophages suggests that common regulatory pathways mediated the differentiation of monocytes into MACsuppress. IPA analysis of gene expression data showed that regulatory networks involving the NF-κB complex, PI3K-Akt, p38 MAPK/ERK/JNK, and PTGS2 were upregulated by both PAM3 and M-CSF (Figure 5). Some of these pathways were previously identified in studies of murine monocytes and human cell lines; however, their TLR expression and innate immune response patterns differed from those of primary human monocytes.43-45 Our work evaluated the effect of inhibiting key regulatory genes on the generation of MACsuppress in a physiologically relevant system. Blocking IκB kinase (an upstream regulator of all of NF-κB complexes) significantly reduced the generation of both CD163+ vs CD163− macrophages, indicating that NF-κB plays a central in the overall process of monocyte into macrophage differentiation (Figure 6B-C). Blocking Akt also suppressed the general process of monocyte differentiation (Figure 6B-C), consistent with evidence that the PI3K-Akt pathway is essential to the survival of monocyte-derived human macrophages.46 Although it would be of interest to define the role of each NF-κB subunit and Akt isoform in human monocyte differentiation, the absence of selective inhibitors of each isoform and subunit component currently prevents such a determination.
We found that inhibiting p38 MAPK or PTGS2 selectively blocked the differentiation of human monocytes into CD163+ macrophages but enhanced the generation of CD163− macrophages (Figure 6B-C). These results confirm and extend those of murine studies documenting a role for the p38 MAPK pathway in the generation of MACsuppress47,48 but are inconsistent with data suggesting that p38 MAPK expression is required for the generation of MACinflam.49,50 Because p38 MAPK is an upstream mediator of PTGS2,51 current findings suggest that M-CSF and PAM3 use the p38 MAPK-PTGS2 axis to influence monocyte maturation. The expression of ERK, JNK, and MAP3K8 were upregulated in human monocytes treated with PAM3 but not M-CSF (Figure 6B-C). Studies in mice indicate that ERK and JNK are not required for M-CSF–dependent macrophage polarization,48 although ERK may play a role late in the differentiation process.52 Because ERK and JNK are secondary targets of MAP3K8,38 this constellation of findings suggests that their expression may be regulated in a concentration-dependent manner by MAP3K8.
MACsuppress have proven useful in the prevention/treatment of type 1 diabetes, systemic lupus erythematosus, multiple sclerosis, colon/renal inflammations, and graft rejection in murine models.14-19 Factors produced by MACsuppress (eg, IL-10 and IL-1RA) have similarly found use in the treatment of human autoimmune and inflammatory diseases.53 Although human monocytes differentiate into MACsuppress when treated with M-CSF,33 M-CSF also increases the frequency of circulating monocytes and thus can worsen tissue-specific inflammation.54,55 PAM3 does not have this adverse effect, suggesting it might be of therapeutic value. Although PAM3 is reported to induce the production of inflammatory mediators such as IL-1β,27 our studies of monocyte culture found this effect to be quite modest (IL-1β levels rose to 5.1 + 2.9 pg/mL; data not shown). Rhesus macaques (which reliably mirror the response of humans to TLR agonists) were used to evaluate the activity of PAM3 in vivo.39-41 PAM3 treatment induced a 20-fold increase in the frequency of circulating CD163+ macrophages and the local production of IL-10 but had no significant effect on CD163− macrophages, the production of inflammatory cytokines, or adverse effects other than swelling at the injection site (Figure 7).
This report establishes that PAM3 mimics the ability of M-CSF to support the maturation of human monocytes into MACsuppress. Analysis of the regulatory pathways employed during this process revealed that NF-κB and Akt were critical to the general process of monocyte into macrophage differentiation, p38 MAPK and PTGS2 influenced whether the resulting cells differentiated into proinflammatory or immunosuppressive macrophages, and ERK and JNK contributed to the generation of MACsuppress mediated by PAM3 but not M-CSF. Coupled with evidence that PAM3 has the same activity when tested in vivo in primates (where neither local nor systemic adverse effects were observed), current findings support the development of PAM3 for the treatment of inflammatory and autoimmune diseases.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE99609).
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Kathleen Noer and Roberta Matthai for their help in fluorescence-activated cell sorter purification of human monocytes.
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: D.B., D.T., and L.A.H. performed the experiments and analyzed results; D.B. and D.M.K. designed the research, analyzed results, and wrote the paper; and D.V. and D.M.K. supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dennis M. Klinman, Building 567 Room 205, Frederick National Laboratory for Cancer Research, Frederick, MD 21702; e-mail: klinmand@mail.nih.gov.


![Figure 6. Evaluation of IPA-predicted regulatory pathways and changes in protein production. Purified monocytes were stimulated in vitro for 5 days. (A) Fold increase in IL-6, IL-10, PGE2, and IL-12p70 in culture supernatants on day 5 (mean ± standard deviation [SD] of 7-8 independently studied donors). For unstimulated cultures, concentrations were: 45 pg/mL of PGE2 and 50 pg/mL of IL-12p70. Because IL-6 and IL-10 could not be detected in unstimulated cultures, the fold increase was calculated based on the sensitivity of the enzyme-linked immunosorbent assay, 10 pg/mL for IL-6 and 30 pg/mL for IL-10. (B-C) Effect of blocking NF-κB (2.5 µM of celastrol), Akt (5 µM of perifosine), p38 MAPK (10 µM of SB203580), ERK (1 µM of PD98059), JNK (10 µM of SP600125), PTGS2 (10 µM of celecoxib), or PTGS1 (50 nM of FR122047) on the generation of macrophages by including inhibitors during culture (mean ± SD of 4-6 independently studied donors per group). *P < .05, **P < .01, ***P < .001 vs unstimulated/control cultures. n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/26/10.1182_bloodadvances.2017011221/3/m_advances011221f6.jpeg?Expires=1769484778&Signature=3Ax17qFTu4DggKjoHUrwYKh~MpZ8BkIxMQr4L2lnCUDsfqOEXcxCSFfq17sKBKeT7XbUHUCjxha-K6fLwbeAFdaFWmJUgdomppZC2vdeeLZcHv6AlQ2oed7lJsp6WU67pRe5ug4cizOy1YpjU6YEznEFYYaLyfqeIJDBPgxXlQgI9xyWAUpODJUHR3U~FV3pZbmXq4hSf0oUirHahjTfmB4VQMzvNpWwj57pT8dHtvaRGF9ScU5nCzjZXlHSkwrgZWAGJvxTgDA4nEMTtcB75I3zhNcNaNpXyoKFWdT8ZtfCH2Vd41~q9j9MRLPkRQfu3Da--QzHrbzQTFD-8X2LKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)