Key Points
VWF attenuates primed T-cell proliferation and memory B-cell differentiation.
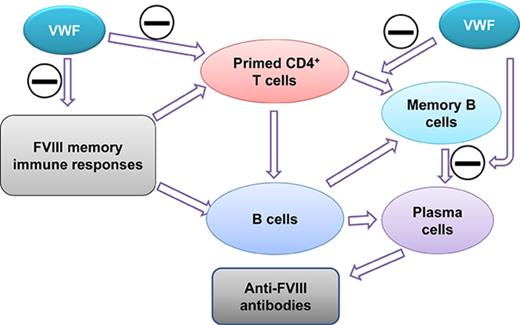
VWF mitigates FVIII memory responses in FVIIInull mice.
Abstract
Immune tolerance induction (ITI) with aggressive infusion of factor VIII (FVIII) is the current strategy used to eradicate FVIII inhibitors and restore normal FVIII pharmacokinetics in inhibitor patients. Whether the use of FVIII products containing von Willebrand factor (VWF) will affect the efficacy of ITI is still controversial. In this study, we explored the impact of VWF on FVIII memory immune responses in hemophilia A (HA) mice. A T-cell proliferation assay and cytokine profile analysis were used to study FVIII-primed CD4+ T cells. When CD4+ T cells from primed FVIIInull mice were restimulated with recombinant human FVIII (rhF8) plus recombinant human VWF (rhVWF) in vitro, the percentages of daughter CD4+ T cells were significantly decreased compared with the groups cultured with rhF8 only. Levels of interferon-γ and interleukin 10 were significantly lower in the rhF8 plus rhVWF groups than in the rhF8 groups. When memory B-cell pools from primed FVIIInull mice were cultured with rhF8 with or without rhVWF to induce differentiation of memory B cells into antibody-secreting cells (ASCs), the number of ASCs was significantly lower in the rhF8 plus VWF group than in the rhF8 group. When memory B-cell pools were transferred into NSGF8KO mice followed by rhF8 immunization with or without rhVWF, the titers of anti-F8 inhibitors and total immunoglobulin G were significantly higher in the rhF8 group than in the rhF8 plus rhVWF group, with an average difference of 2.23- and 2.04-fold. Together, our data demonstrate that VWF attenuates FVIII memory immune responses in HA mice.
Introduction
Von Willebrand factor (VWF) is a carrier protein for factor VIII (FVIII), protecting FVIII from protease degradation.1-4 Deficiency of FVIII results in hemophilia A (HA), a genetic bleeding disorder that is currently treated with protein replacement therapy using either plasma-derived FVIII (pdFVIII) or recombinant human FVIII (rhF8).5 Development of inhibitory antibodies, referred to as inhibitors, against FVIII is a significant complication that occurs in 30% of severe HA patients after protein replacement therapy.6 These inhibitors will neutralize functional FVIII activity, rendering FVIII protein replacement therapy less effective.7-9 Treatment of inhibitor patients is one of the biggest challenges in hemophilia care. Immune tolerance induction (ITI) with aggressive infusion of high-dose FVIII is the only proven strategy used to eradicate the persisted inhibitors and restore normal FVIII pharmacokinetics in some inhibitor patients.10-12 Whether using FVIII products containing VWF will affect the FVIII immune response in human patients and facilitate ITI has been debated for decades.13-19
Evidence suggests that VWF protects FVIII from endocytosis by human dendritic cells and presentation to T cells in vitro.20 It has been shown that infusion of pdFVIII, which contains VWF, results in reduced anti–FVIII antibody formation in HA mice.21,22 However, using FVIIInull mice to study the impact of human VWF (hVWF) on immune responses to human FVIII (hF8) could be problematic owing to the potential for antigenic competition.23 A recent study19 from a multicenter, randomized, and open-label clinical trial demonstrated that the incidence of inhibitor development was significantly higher in HA patients treated with rhF8 than in those treated with pdFVIII, but some concerns were raised15 regarding that study, such as the short follow-up period. Our previous studies demonstrated that VWF affects FVIII synthesis, storage, and accessibility to neutralization by inhibitors.24,25 VWF has a dose-dependent protective effect on FVIII from inhibitor inactivation.26 The interaction between VWF and FVIII is critical in maintaining the clinical efficacy of platelet-derived FVIII gene therapy in HA mice in the presence of inhibitors.27 How VWF affects FVIII immune responses in HA with a setting of preexisting anti-FVIII immunity is still uncertain and is not fully understood.
Since the anti-FVIII immune response is a CD4+ T-cell–dependent humoral response, in the current study, we investigated (1) how VWF affects FVIII-primed CD4+ T-cell proliferation and cytokine production ex vivo, (2) how VWF affects FVIII-specific differentiation of memory B cells into antibody-secreting cells (ASCs) and anti–FVIII antibody production ex vivo, and (3) how VWF affects FVIII-memory immune responses in vivo using a HA mouse model. Our results demonstrate that VWF attenuates FVIII-primed CD4+ T-cell proliferation and mitigates differentiation of memory B cells into ASCs and anti–FVIII antibody production both in vitro and in vivo.
Materials and methods
Mice
FVIII knockout (FVIIInull, F8KO) mice used in this study were on a 129/SV × C57BL/6 mixed genetic background. Animals were generated by targeted disruption of exon 17 of the FVIII gene28 and originally obtained from Haig Kazazian at the University of Pennsylvania School of Medicine. NSGF8KO mice29 were generated by our laboratory through crossing the FVIIInull gene into the immunocompromised NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) background that lacks mature T and B cells.30 All mice were maintained in pathogen-free microisolator cages at the animal facilities operated by the Medical College of Wisconsin. Animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Isoflurane or ketamine was used for anesthesia.
Antibodies
Anti–mouse CD4, anti–mouse T-cell receptor (TCR) β, anti–mouse CD44, anti–mouse CD62L, anti-mouse CD25, anti–mouse Foxp3, anti-CD8, and anti–B220 antibodies were purchased from eBioscience (San Diego, CA). Anti-mouse CD138 antibody was purchased from BD Biosciences (Franklin Lakes, NJ).
Inhibitor model development
FVIIInull mice were immunized intravenously via retro-orbital injection with rhF8 (Xyntha, Pfizer Inc., New York, NY) at a dosage of 50 U/kg per week for 4 weeks to induce anti-FVIII immune responses. One week after the last immunization, blood samples were collected and inhibitor titers were determined by Bethesda assay as described in our previous report24 to confirm inhibitory antibodies developed in immunized animals. rhF8-sensitized animals were used for further studies as described below.
T-cell proliferation assay
Forty-eight hours before animals were sacrificed for spleen isolation, mice were boosted with 50 U/kg rhF8 one more time. Splenocytes were isolated and labeled with CellTrace Violet using the CellTrace Violet Cell Proliferation Kit (Life Technologies, Carlsbad, CA). Four and a half million cells per well were cultured in flat-bottom 96-well plates (BD Falcon, Franklin Lakes, NJ) with 300 µL complete RPMI 1640 media containing various doses of rhF8 with or without 1 U/mL recombinant human VWF (rhVWF; Baxalta, Bannockburn, IL) in duplicate for 96 hours. Ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO) and albumin (ALB; Sigma) were used as unrelated antigen controls. After culture, cells were collected and stained with anti–CD4 and anti–TCR β antibodies to identify CD4+ T cells. 7-Amino-actinomycin (BD Biosciences) staining was used to exclude dead cells. In some experiments, cells were also stained with anti–CD44 and anti–CD66L antibodies for central memory cells or effector cells or anti–CD25 and anti–Foxp3 antibodies for regulatory T (Treg) cells. Cells were analyzed by LSRII flow cytometry (BD Biosciences), and data were analyzed using FlowJo software (FlowJo, Ashland, OR). Conditioned media was collected for cytokine profile analysis using the Bio-Plex cytokine assay (Bio-Rad, Hercules, CA) following the protocol provided by the manufacturer.
Memory B-cell–mediated ELISPOT assay
Splenocytes from rhF8-immunized FVIIInull mice were used to prepare memory B-cell pools (CD138−) that contained memory B cells but were depleted of ASCs (CD138+ cells) following the protocol previously reported.31 In brief, a rat anti–mouse CD138 antibody was coated onto Dynabeads M-450 bound with sheep anti–rat immunoglobulin G (IgG) (Life Technologies). Splenocytes were incubated with CD138 antibody–conjugated Dynabeads at 4°C for 1 hour. CD138+ cells were depleted using a Magnet Particle Concentrator (Invitrogen, Oslo, Norway). To differentiate FVIII-specific memory B cells into ASCs, 1.5 × 106 CD138− cells/mL were cultured in 25-cm2 flasks (BD Biosciences) with completed RPMI 1640 media containing various doses of rhF8 with or without 1 U/mL rhVWF for 6 days. OVA and ALB were used as unrelated antigen controls. After culture, cells were harvested and resuspended in fresh completed RPMI 1640 media, and 100-μL serial dilutions of cells were seeded in rhF8-coated polyvinylidendifluorid-bottom 96-well MultiScreen-IP filtration plates (Millipore Corporation, San Francisco, CA). ASCs were detected followed the enzyme-linked immunospot (ELISPOT) assay procedures described in our previous report.32
In vivo memory immune response study
Splenocytes were harvested from rhF8-immunized FVIIInull mice, and CD138+ cells were depleted as described above. CD138− splenic cell pools were infused into NSGF8KO mice with a cell dose of 2 × 107 in 200 µL Dulbecco’s phosphate-buffered saline (Life Technologies) for each animal via retro-orbital IV injection. Each cell pool was transferred into sex-matched paired littermates without any preconditioning. Twenty-four hours after rhF8-primed CD138− splenic cell transfer, blood samples were collected for T- and B-cell analysis and for the antibody assay to confirm that immune cells, but not ASCs, were successfully transferred into NSGF8KO mice. Animals were then immunized with rhF8 at a dose of 50 U/kg with or without 50 U/kg rhVWF. One week after immunization, blood samples were collected following the procedures as previously described.33 Plasmas were isolated for the Bethesda assay to determine inhibitor titers and for the ELISA assay to determine anti-FVIII total IgG titers following protocols as previously reported.24,32,33 Total antibody titers were expressed as the highest dilution of plasma showing a positive result. Samples from NSGF8KO mice were used as a control. Leukocytes were isolated and analyzed by flow cytometry for CD4+, CD8+, and B cells. Untransfused NSGF8KO and wild-type C57BL6 mice were used as controls for fluorescence-activated cell sorting.
Statistical analysis
Data are presented as mean ± standard deviation. Statistical comparisons of data sets between experimental groups of rhF8 with or without rhVWF treatments were evaluated by the paired Student t test using SigmaPlot 13.0 (Systat Software, San Jose, CA). The Mann-Whitney U test was used to compare normalized data sets. A value of P < .05 was considered statistically significant.
Results
The impact of VWF on rhF8-primed CD4+ T cells
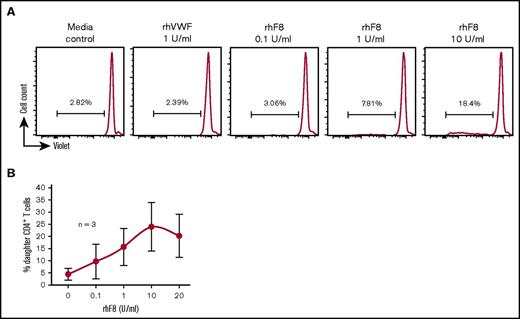
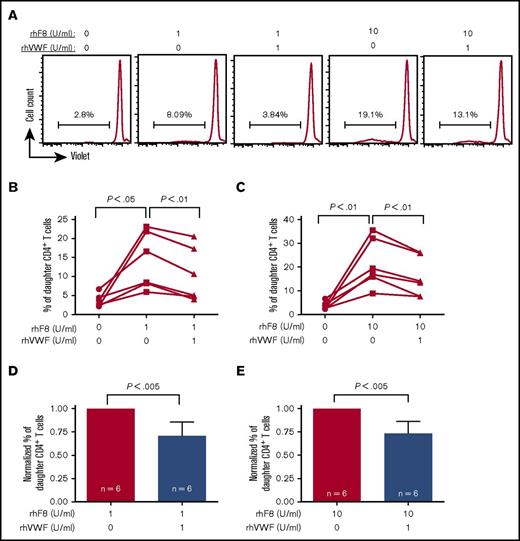
Since the FVIII immune response is CD4+ T-cell dependent, we first investigated how VWF affects FVIII-primed CD4+ T cells in response to FVIII restimulation. To address this question, we used a T-cell proliferation assay. After in vitro rhF8 restimulation without VWF for 96 hours, the proliferation of CD4+ T cells from rhF8-primed FVIIInull mice was FVIII specific and dose dependent (Figure 1A-B). The percentage of daughter (proliferated) CD4+ T cells (14.0% ± 7.5%) in the condition cultured with 1 U/mL of rhF8 was significantly higher than without rhF8 (3.7% ± 1.7%). The daughter cells further increased to 21.5% ± 10.3% when cells were incubated with 10 U/mL rhF8. However, when 1 U/mL rhVWF was added to the culture media in addition to rhF8, percentages of daughter CD4+ T cells were significantly decreased in both the 1 U/mL and 10 U/mL rhF8 treatment groups (10.4% ± 7.1% and 15.8% ± 8.4%, respectively) (Figure 2A-C). In the presence of rhVWF, T-cell proliferation decreased an average of 30% in the low-dose rhF8 (1 U/mL) group and 27% in the high-dose (10 U/mL) group (Figure 2D-E). Adding rhVWF and rhF8 simultaneously to the cells, briefly premixing rhVWF with rhF8, or preincubating of rhVWF with rhF8 for 1 hour did not make a significant difference in attenuating T-cell proliferation upon rhF8 restimulation (data not shown). As controls, when an unrelated antigen, OVA or ALB, was added to cell culture media in addition to rhF8, T-cell proliferation was not reduced (supplemental Figure 1).
rhF8 dose response on FVIII-sensitized CD4+T-cell proliferation. Splenocytes isolated from rhF8-immunized FVIIInull mice were labeled with CellTrace Violet and cultured with various doses of rhF8 or rhVWF for 96 hours. Concanavalin A was used as a positive control for T-cell proliferation. Cells were stained with anti–mouse CD4 and anti–mouse TCRβ antibodies and analyzed by flow cytometry for daughter CD4+ T cells. (A) Representative flow cytometry histograms. (B) rhF8 dose–response curve.
rhF8 dose response on FVIII-sensitized CD4+T-cell proliferation. Splenocytes isolated from rhF8-immunized FVIIInull mice were labeled with CellTrace Violet and cultured with various doses of rhF8 or rhVWF for 96 hours. Concanavalin A was used as a positive control for T-cell proliferation. Cells were stained with anti–mouse CD4 and anti–mouse TCRβ antibodies and analyzed by flow cytometry for daughter CD4+ T cells. (A) Representative flow cytometry histograms. (B) rhF8 dose–response curve.
The impact of rhVWF on FVIII-sensitized CD4+T cell proliferation in response to rhF8 restimulation. Splenocytes isolated from rhF8-immunized FVIIInull mice were labeled with CellTrace Violet and cultured with a low (1 U/mL) or a high (10 U/mL) dose of rhF8 with or without 1 U/mL rhVWF for 96 hours. Cells were stained with anti–mouse CD4 and anti–mouse TCRβ antibodies and analyzed by flow cytometry for daughter CD4+ T cells. (A) Representative flow cytometry histograms. (B-C) The impact of rhVWF on CD4+ T cell proliferation in response to a low dose of rhF8 (B) and a high dose of rhF8 (C). The paired Student t test was used to compare data sets. (D-E) Normalized daughter cell data. The percentage of daughter CD4+ T cells in the condition without VWF was defined as 1. The Mann-Whitney U test was used to compare normalized data sets. These data demonstrate that VWF can attenuate FVIII-sensitized CD4+ T-cell proliferation in response to rhF8 restimulation.
The impact of rhVWF on FVIII-sensitized CD4+T cell proliferation in response to rhF8 restimulation. Splenocytes isolated from rhF8-immunized FVIIInull mice were labeled with CellTrace Violet and cultured with a low (1 U/mL) or a high (10 U/mL) dose of rhF8 with or without 1 U/mL rhVWF for 96 hours. Cells were stained with anti–mouse CD4 and anti–mouse TCRβ antibodies and analyzed by flow cytometry for daughter CD4+ T cells. (A) Representative flow cytometry histograms. (B-C) The impact of rhVWF on CD4+ T cell proliferation in response to a low dose of rhF8 (B) and a high dose of rhF8 (C). The paired Student t test was used to compare data sets. (D-E) Normalized daughter cell data. The percentage of daughter CD4+ T cells in the condition without VWF was defined as 1. The Mann-Whitney U test was used to compare normalized data sets. These data demonstrate that VWF can attenuate FVIII-sensitized CD4+ T-cell proliferation in response to rhF8 restimulation.
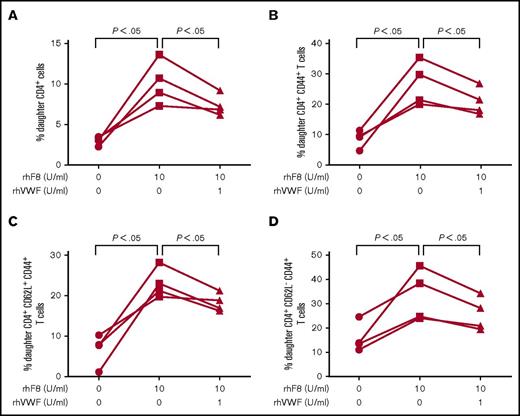
We also analyzed the proliferation of splenic CD4+ T cell subsets, including activated CD4+ T cells (CD4+CD44+), effector memory CD4+ T cells (CD4+CD44+CD62L−), central memory CD4+ T cells (CD4+CD44+CD62L+), and Treg cells from rhF8-primed FVIIInull mice in response to rhF8 restimulation in vitro. Similar to CD4+ T cells (Figure 3A), the percentages of daughter cells in the subpopulations of CD4+CD44+, CD4+CD44+CD62L−, and CD4+CD44+CD62L+ cells were significantly higher after rhF8 restimulation for 72 hours than in control groups with no rhF8 stimulation. However, the daughter cell percentages were significantly reduced when rhVWF was added to culture conditions (Figure 3B-D). We found that daughter cells in the rhF8 groups were from memory CD4 T cells. Effector memory cells proliferated faster than central memory cells after rhF8 restimulation (supplemental Figure 2), supporting the notion that effector memory T cells respond rapidly when encountering the same antigen.34-37 The percentage of total Treg cells and daughter cells significantly increased after rhF8 restimulation with or without rhVWF. However, there was no significant difference between the rhF8 plus rhVWF group and the rhF8-only group (supplemental Figure 3).
The impact of rhVWF on subsets of FVIII-sensitized CD4+T-cell proliferation in response to rhF8 restimulation. Splenocytes isolated from rhF8-immunized FVIIInull mice were labeled with CellTrace Violet and cultured with rhF8 with or without rhVWF for 96 hours. Cells were stained with anti–mouse CD4, TCRβ, CD44, and CD62L and analyzed by flow cytometry. A subset of CD4+ T cells was gated and analyzed for daughter cells. (A) CD4+ T-cell proliferation. (B) Activated CD4+ (CD4+CD44+) T-cell proliferation. (C) Central memory (CD4+CD44+CD62L+) T cell proliferation. (D) Effector CD4+ (CD4+CD44+CD62L−) T-cell proliferation. The paired Student t test was used to compare data sets. These data demonstrate that VWF can attenuate FVIII-sensitized effector as well as central memory CD4+ T-cell proliferation in response to rhF8 restimulation.
The impact of rhVWF on subsets of FVIII-sensitized CD4+T-cell proliferation in response to rhF8 restimulation. Splenocytes isolated from rhF8-immunized FVIIInull mice were labeled with CellTrace Violet and cultured with rhF8 with or without rhVWF for 96 hours. Cells were stained with anti–mouse CD4, TCRβ, CD44, and CD62L and analyzed by flow cytometry. A subset of CD4+ T cells was gated and analyzed for daughter cells. (A) CD4+ T-cell proliferation. (B) Activated CD4+ (CD4+CD44+) T-cell proliferation. (C) Central memory (CD4+CD44+CD62L+) T cell proliferation. (D) Effector CD4+ (CD4+CD44+CD62L−) T-cell proliferation. The paired Student t test was used to compare data sets. These data demonstrate that VWF can attenuate FVIII-sensitized effector as well as central memory CD4+ T-cell proliferation in response to rhF8 restimulation.
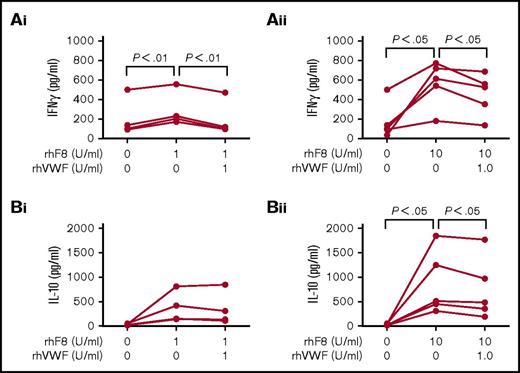
To further explore how VWF affects the FVIII immune response, we analyzed cytokine profiles in splenocyte culture supernatants. After rhF8 restimulation, the levels of interferon-γ (IFN-γ) significantly increased, but the levels were attenuated in the presence of rhVWF. The levels of IFN-γ were significantly lower in the groups cultured with rhF8 in the presence of rhVWF than in the groups cultured with rhF8 only, regardless of whether a high or low dose of rhF8 restimulation was used (Figure 4Ai-Aii). When the cells were treated with a high dose of 10 U/mL rhF8, IL-10 production was significantly increased compared with the group without rhF8 stimulation, and the level of IL-10 in the group treated with 10 U/mL rhF8 plus 1 U/mL rhVWF was barely significantly lower (P = .043) than that obtained from the group treated with 10 U/mL rhF8 only, but there was no statistically significant difference between groups treated with 1 U/mL rhF8 with or without VWF (Figure 4Bi-Bii). The levels of tumor necrosis factor α, IL-4, IL-5, and IL-12 were not significantly different between the groups cultured with rhF8 together with rhVWF and the rhF8 groups without VWF (data not shown).
Cytokine profile analysis. Splenocytes isolated from rhF8-immunized FVIIInull mice were cultured with rhF8 with or without rhVWF for 96 hours. The conditioned media was used to analyze the cytokine expression using the Bio-Plex cytokine assay. (Ai-Aii) Impact of VWF on IFN-γ expression in rhF8-sensitized splenocytes in response to rhF8 restimulation. (Bi-Bii) Impact of VWF on IL-10 expression in rhF8-sensitized splenocytes in response to rhF8 restimulation. The paired Student t test was used to compare data sets. These data demonstrate that VWF can attenuate IFN-γ production in FVIII-sensitized splenocytes in response to rhF8 restimulation.
Cytokine profile analysis. Splenocytes isolated from rhF8-immunized FVIIInull mice were cultured with rhF8 with or without rhVWF for 96 hours. The conditioned media was used to analyze the cytokine expression using the Bio-Plex cytokine assay. (Ai-Aii) Impact of VWF on IFN-γ expression in rhF8-sensitized splenocytes in response to rhF8 restimulation. (Bi-Bii) Impact of VWF on IL-10 expression in rhF8-sensitized splenocytes in response to rhF8 restimulation. The paired Student t test was used to compare data sets. These data demonstrate that VWF can attenuate IFN-γ production in FVIII-sensitized splenocytes in response to rhF8 restimulation.
Taken together, these results demonstrate that VWF significantly attenuates rhF8-primed CD4+ T cell proliferation and IFN-γ production in response to rhF8 restimulation.
The impact of VWF on FVIII-specific memory B cells in response to rhF8 restimulation in vitro
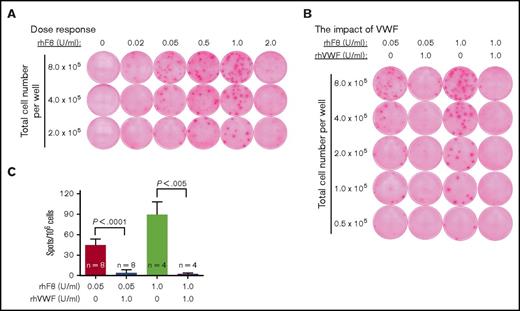
In a setting of preexisting anti-F8 immunity, how FVIII-specific memory B cells respond to FVIII restimulation and differentiate into ASCs is the critical pathway in terms of the clinical efficacy of FVIII infusion for the ITI protocol. To investigate how VWF affects memory B-cell differentiation upon FVIII restimulation, we used an ELISPOT-based assay. CD138− splenocytes from rhF8-primed FVIIInull mice were used as the source to prepare F8-specific memory B-cell pools. To stimulate the maturation of F8-specific memory B cells into ASCs, memory B-cell pools from primed FVIIInull mice were cultured with rhF8 with or without rhVWF for 6 days. After culture, newly formed ASCs were assessed by the ELISPOT assay. As shown in Figure 5A, a relatively low dose (0.05-1.0 U/mL) of rhF8 could efficiently induce memory B-cell differentiation and antibody production, but this was inhibited when the concentration of rhF8 was increased to 2.0 U/mL. When the cells from memory B-cell pool were cultured with 0.05 U/mL and 1.0 U/mL rhF8, there were 44.8 ± 8.9 and 89.5 ± 18.7 ASCs/106 cells, respectively. In contrast, there were only 3.9 ± 4.7 and 2.3 ± 1.5 ASCs/106 cells, respectively, after the cells were cultured with 0.05 U/mL and 1.0 U/mL rhF8 together with 1 U/mL rhVWF (Figure 5B-C). As controls, OVA and ALB neither stimulated rhF8-primed differentiation of memory B cells into ASCs and FVIII antibody production nor attenuated FVIII-specific memory B-cell differentiation and antibody production in response to rhF8 restimulation (supplemental Figure 4). These results demonstrate that FVIII-specific memory B-cell differentiation, when restimulated with rhF8, is attenuated in the presence of rhVWF.
Memory B-cell–mediated ELISPOT assay. Splenocytes were isolated from rhF8-immunized FVIIInull mice, and CD138+ plasma cells were depleted. The remaining cells (CD138−) were used as memory B-cell pools. CD138− memory B-cell pools were cultured with various doses of rhF8 with or without 1 U/mL rhVWF for 6 days to induce differentiation of memory B cells into ASCs. ASCs were determined by ELISPOT assay. Each spot represents a single ASC. (A) The rhF8 dose response on inducing differentiation of memory B cells into ASCs and anti–FVIII antibody production. (B) Representative ELISPOT images showing the impact of VWF on memory B-cell differentiation and antibody secretion. (C) ASC counts in various culture conditions. The paired Student t test was used to compare data sets. These results demonstrate that VWF can mitigate FVIII-specific differentiation of memory B cells into ASCs and anti–FVIII antibody production.
Memory B-cell–mediated ELISPOT assay. Splenocytes were isolated from rhF8-immunized FVIIInull mice, and CD138+ plasma cells were depleted. The remaining cells (CD138−) were used as memory B-cell pools. CD138− memory B-cell pools were cultured with various doses of rhF8 with or without 1 U/mL rhVWF for 6 days to induce differentiation of memory B cells into ASCs. ASCs were determined by ELISPOT assay. Each spot represents a single ASC. (A) The rhF8 dose response on inducing differentiation of memory B cells into ASCs and anti–FVIII antibody production. (B) Representative ELISPOT images showing the impact of VWF on memory B-cell differentiation and antibody secretion. (C) ASC counts in various culture conditions. The paired Student t test was used to compare data sets. These results demonstrate that VWF can mitigate FVIII-specific differentiation of memory B cells into ASCs and anti–FVIII antibody production.
The impact of VWF on FVIII memory immune responses in NSGF8KO mice
We then used an in vivo model to further evaluate the impact of VWF on the memory immune response in HA mice. Since we are unable to mimic human ITI in FVIIInull mice, we used our recently established NSGF8KO mice that lack endogenous T and B cells, which was confirmed by flow cytometry analysis as shown in supplemental Figure 4A, and readily accept exogenous T- and B-cell transplant to investigate memory immune responses to a secondary FVIII immunization and the effect of the VWF/FVIII complex on this secondary immune response in vivo. We transferred CD138− splenic cell pools from rhF8-primed FVIIInull mice into NSGF8KO mice followed by rhF8 immunization in the presence or absence of rhVWF. Blood samples were collected 1 week after immunization for analysis. Twenty-four hours after receiving CD138− splenic cell transfer, both T and B cells were detected in peripheral blood, but no antibodies were detected in the recipients (data not shown), demonstrating that cell transfer was successful and CD138− cell pools transferred were free of ASCs.
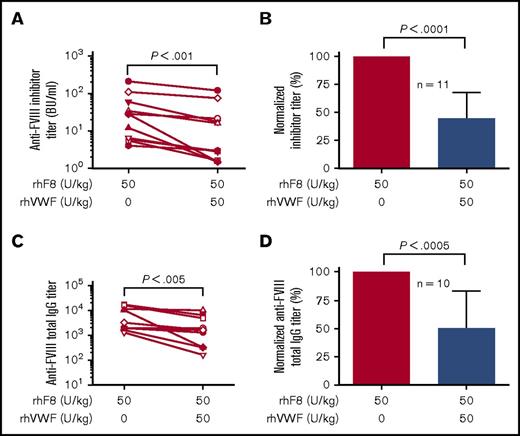
Flow cytometry analysis showed that CD4+, CD8+, and B cells were still detected in peripheral blood of all animals 1 week after rhF8 with or without rhVWF immunization. The percentages of CD4+, CD8+, and B cells were not significantly different among the rhF8 group and the rhF8 plus rhVWF group (supplemental Figure 5B-D). After 1 dose of rhF8 immunization, antibodies were detectable in animals that received FVIII-primed memory B-cell pool transfer with an average titer of ∼20% of the levels developed in their donors (data not shown). As shown in Figure 6A,C, the titers of inhibitors and anti-F8 total IgG antibodies in animals that received rhF8-primed CD138− splenic cell pools followed by rhF8 immunization were 45.9 ± 62.9 BU/mL and 5729 ± 6448, respectively. These titers were significantly higher than those obtained in animals that received the same pool of rhF8-primed CD138− splenic cell transfer and immunized with rhF8 together with rhVWF (23.9 ± 38.4 BU/mL and 2840 ± 3232, respectively).
The impact of VWF on FVIII memory immune responses in vivo. Splenocytes were isolated from rhF8-immunized FVIIInull mice, and CD138+ plasma cells were depleted. The remaining cells (CD138−) were used as memory B-cell pools. CD138− memory B-cell pools were infused into age- and sex-matched paired immunocompromised NSGF8KO littermates followed by rhF8 immunization with or without rhVWF. One week after immunization, plasma was collected for Bethesda (to determine inhibitor titers) and ELISA assays (to determine anti-FVIII total IgG titers). (A-B) Inhibitor titers. (C-D) Anti-FVIII total IgG titers. Data are normalized by defining the titer in the group immunized with rhF8 only as 100%. The paired Student t test was used to compare the actual titer data sets, and the Mann-Whitney U test was used to compare the normalized data sets. These data demonstrate that VWF can mitigate anti-FVIII memory immune responses in vivo.
The impact of VWF on FVIII memory immune responses in vivo. Splenocytes were isolated from rhF8-immunized FVIIInull mice, and CD138+ plasma cells were depleted. The remaining cells (CD138−) were used as memory B-cell pools. CD138− memory B-cell pools were infused into age- and sex-matched paired immunocompromised NSGF8KO littermates followed by rhF8 immunization with or without rhVWF. One week after immunization, plasma was collected for Bethesda (to determine inhibitor titers) and ELISA assays (to determine anti-FVIII total IgG titers). (A-B) Inhibitor titers. (C-D) Anti-FVIII total IgG titers. Data are normalized by defining the titer in the group immunized with rhF8 only as 100%. The paired Student t test was used to compare the actual titer data sets, and the Mann-Whitney U test was used to compare the normalized data sets. These data demonstrate that VWF can mitigate anti-FVIII memory immune responses in vivo.
For comparison, the titers in the rhF8-immunized groups were normalized to 100%. The titers in the groups immunized with rhF8 plus rhVWF were 44.8 ± 23.2% in inhibitors and 49.1 ± 33.9% in anti-F8 total IgG antibodies. The average titer of inhibitors and total anti-F8 IgG antibodies in the rhF8 groups were 2.23- and 2.04-fold higher than those obtained in the rhF8 plus rhVWF group (Figure 6C-D). Taken together, these data demonstrate that infusion of rhVWF together with rhF8 can mitigate anti-F8 memory responses in vivo.
Discussion
It has become increasingly clear that the interaction between VWF and FVIII is not only important for hemostasis but also critical to HA therapy.15 Since it is still unclear whether a product containing VWF influences the efficacy of ITI, we investigated whether VWF affects FVIII-primed T cells and memory B cells in a secondary response to FVIII. We found that VWF can modulate FVIII memory immune responses in HA mice in a setting of preexisting anti-FVIII immunity. In our studies, FVIII-primed CD4+ T cell proliferation and cytokine IFN-γ production were attenuated in response to rhF8 restimulation when VWF was present. VWF mitigated FVIII-specific memory B-cell differentiation and anti–FVIII antibody production both ex vivo and in vivo. Together, our data indicate that VWF modulates the immunogenicity of FVIII in memory immune responses in HA with preexisting anti-FVIII immunity.
Multiple lines of evidence have demonstrated that generation of anti–FVIII antibodies from both primary and secondary immune responses is a CD4+ T-cell–dependent process.38-40 While the exact mechanisms of the aggressive FVIII infusion–mediated ITI are still uncertain, the potential mechanisms may include inhibition of memory B-cell response and induction of T-cell anergy, anti-idiotypic antibodies, or suppressor T cells.8,31,40,41 In our studies, 1 U/mL rhVWF significantly attenuated FVIII-sensitized CD4+ T-cell proliferation in response to rhF8 restimulation at both low (1 U/mL) and high (10 U/mL) concentrations in vitro. The suppressive function in the 1 U/mL treatment group is not significantly different from in the 10 U/mL group, indicating that a ratio of 1:10 (IU/IU) between VWF and FVIII is sufficient to suppress the FVIII immune response in primed CD4+ T cells. VWF is a set of multimers with an average molecular weight (MW) of ∼20 MDa. In contrast, the MW of FVIII is ∼250 kDa. The monomeric subunit of VWF from which multimers are derived is ∼260 kDa, which is approximately the MW of FVIII. Using the monomeric MW of VWF at 10 μg/mL and the MW of FVIII at 200 ng/mL produces their respective molar concentrations, revealing the VWF monomers are in molar excess (50-fold) of FVIII. Our previous studies have also demonstrated that a lower ratio of VWF to FVIII (1:10 or 1:5) already has a dramatic effect on the stability of FVIII in the inhibition reaction preceding the activity assay and only a moderate further impact when the ratio increases to 1:1.26 These data indicate that even a small amount of VWF can significantly impact the biophysiological properties of FVIII.
Previous studies by Wu and colleagues42 have demonstrated that after recombinant human full-length FVIII (fFVIII) infusion, immune responses in a mouse model are of IgG subclasses, including both Th1- and Th2-induced IgG, which is similar to those in human patients. They showed that CD4+ splenocytes from fFVIII-sensitized FVIIInull mice produced both Th1 and Th2 representative cytokines (eg, IFN-γ, IL-4, and IL-10) after fFVIII restimulation in vitro. Studies by Qadura and colleagues22 have demonstrated that infusion of pdFVIII induces a distinct splenic cytokine microenvironment in FVIIInull mice compared with rhF8 infusion. They showed that rhF8 predominately induced Th1 cytokine, while pdFVIII induced Th2 cytokine production. In our model, when rhF8-primed splenocytes were restimulated with rhF8, both Th1 and Th2 cytokines were produced. In our studies, IFN-γ production was significantly attenuated in primed splenocytes when restimulated with rhF8 in the presence of rhVWF. IFN-γ production reduced an average of 40% and 22%, respectively, when splenocytes from rhF8-sensitized FVIIInull mice were restimulated with either a low (1 U/mL) or high (10 U/mL) dose of rhF8 together with 1 U/mL of rhVWF compared with the groups stimulated with rhF8 only. IFN-γ is the primary cytokine that defines Th1 cells. It is produced with a positive feedback loop, promoting more undifferentiated CD4+ T cells to Th1 cells, resulting in augmentation of antigen processing and presentation.43 Upon rhF8 restimulation, IL-10, which is produced by Th2 cells, was also downregulated in the presence of rhVWF, but only in the high-dose groups. Thus, our data indicate that VWF inhibits CD4+ T cell proliferation predominately via the Th1 pathway.
It has been shown that infusion of pdFVIII, but not rhF8, results in an increase of Treg cells in spleens of FVIIInull mice.22 While the major feature of pdFVIII is that the product contains its carrier protein VWF, pdFVIII may contain other proteins that might also modulate anti-FVIII immune responses upon pdFVIII infusion. In the current studies, we used rhVWF to explore how VWF affects subsets of FVIII-primed CD4+ T cells, limiting the potential influences by other plasma-derived proteins on anti-FVIII immune responses. We found that both Treg cell percentage and proliferation were not affected by VWF when whole splenocytes from rhF8-immunized FVIIInull mice were restimulated with rhF8 in vitro. However, rhVWF significantly attenuated the proliferation of both effector (CD4+CD44+CD62L−) and central memory (CD4+CD44+CD62L+) CD4+ T cells when FVIII-primed splenocytes were restimulated with rhF8 in the presence of VWF compared with without VWF, suggesting that reduction of CD4+ T cell proliferation when VWF is present is not due to the suppression of Treg cells.
Since FVIII-specific differentiation of memory B cells into ASCs requires the help of activated CD4+ T cells, reducing effector CD4+ T-cell proliferation will impede the differentiation of memory B cells into ASCs and mitigate anti–FVIII antibody production. Indeed, when memory B-cell pools from rhF8-sensitized FVIIInull mice were restimulated with rhF8 in the presence of rhVWF, ASCs and anti–FVIII antibody production as determined by in vitro ELISPOT assay were dramatically reduced in both low- and high-dose rhF8-treated groups. Some ASCs were still detected in the rhF8 plus rhVWF group, indicating that the effect of VWF on FVIII-specific memory B cells varies among clones. The underlying mechanisms by which FVIII-specific B-cell maturation and antibody production are mitigated by VWF are still unclear and warrant further investigation. Importantly, both inhibitors and total anti–FVIII antibodies were significantly mitigated by rhVWF in vivo in NSGF8KO mice after memory B-cell pool transfer followed by rhF8 immunization. The reduction in inhibitor titers appeared to be greater than in anti-FVIII total IgG, but there was no statistical difference. There was concern about antigenic competition when comparing the FVIII immune response in FVIIInull mice that repeatedly received a human FVIII infusion with or without human VWF.22,23 In our in vivo model, immunocompromised animals received rhF8-primed CD138− splenic cell transfer, which did not contain VWF-primed T and B cells, followed by rhF8 infusion in the presence or absence of rhVWF; anti–FVIII antibody titers were measured 1 week after infusion. Thus, in our system, no VWF-specific memory B cells were transferred, which may circumvent the potential influence of antigenic competition by rhVWF while we studied FVIII-specific memory immune responses.
Our data from current studies also support our previous findings that inhibitor titers declined with time after targeting FVIII expression to platelets in the inhibitor models24,33,44 and that infusion of platelets containing FVIII does not elicit a secondary immune response in FVIIInull mice with preexisting anti-FVIII immunity.45 In these scenarios, FVIII is expressed and stored together with VWF in platelet α-granules, resulting in an intracellular preformed VWF/FVIII complex within platelets. A preformed VWF/FVIII complex plus abundant platelet-derived transforming growth factor 1,46 which is an important immune modulator that can induce Treg production and suppress immune responses, could be the critical elements making platelet FVIII less immunogenic, even in a setting of preexisting anti-FVIII immunity.
In summary, our ex vivo and in vivo data demonstrate that VWF attenuates FVIII-primed CD4+ T cells and memory B cells in response to rhF8 restimulation. Our results indicate that for ITI treatment, infusion of FVIII products containing VWF may reduce anti-FVIII memory responses and be beneficial in HA patients with inhibitors.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant HL-102035 (Q.S.), a Biotest Inc. Research grant (Q.S.), a Bayer Hemophilia Award (Q.S.), and generous gifts from the Children’s Hospital Foundation (Wisconsin) and the Midwest Athletes Against Childhood Cancer Fund (Q.S.).
Authorship
Contribution: J.C. designed the study, performed experiments, and analyzed data; X.L. performed experiments; J.A.S. performed experiments and made comments on the manuscript; and Q.S. designed and conducted research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qizhen Shi, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: qizhen.shi@bcw.edu.