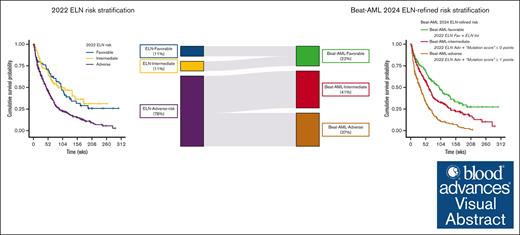
Key Points
Most (78%) patients aged ≥ 60 years with ND AML treated with LIT are classified as having 2022 ELN adverse risk.
The Beat-AML 2024 risk model incorporates IDH2, KRAS, MLL2, and TP53 to refine risk stratification among patients aged ≥ 60 years given LIT.
Visual Abstract
Although the 2022 European LeukemiaNet (ELN) acute myeloid leukemia (AML) risk classification reliably predicts outcomes in younger patients treated with intensive chemotherapy, it is unclear whether it applies to adults ≥60 years treated with lower-intensity treatment (LIT). We aimed to test the prognostic impact of ELN risk in patients with newly diagnosed (ND) AML aged ≥60 years given LIT and to further refine risk stratification for these patients. A total of 595 patients were included: 11% had favorable-, 11% intermediate-, and 78% had adverse-risk AML. ELN risk was prognostic for overall survival (OS) (P < .001) but did not stratify favorable- from intermediate-risk (P = .71). Within adverse-risk AML, the impact of additional molecular abnormalities was further evaluated. Multivariable analysis was performed on a training set (n = 316) and identified IDH2 mutation as an independent favorable prognostic factor, and KRAS, MLL2, and TP53 mutations as unfavorable (P < .05). A “mutation score” was calculated for each combination of these mutations, assigning adverse-risk patients to 2 risk groups: −1 to 0 points (“Beat-AML intermediate”) vs 1+ points (“Beat-AML adverse”). In the final refined risk classification, ELN favorable- and intermediate-risk were combined into a newly defined “Beat-AML favorable-risk” group, in addition to mutation scoring within the ELN adverse-risk group. This approach redefines risk for older patients with ND AML and proposes refined Beat-AML risk groups with improved discrimination for OS (2-year OS, 48% vs 33% vs 11%, respectively; P < .001), providing patients and providers additional information for treatment decision-making.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous and aggressive malignant clonal disorder of the myeloid progenitor cells that predominantly occurs in older adults, with a median age at diagnosis of 69 years.1,2 Outcomes of AML and response to therapy are associated with both patients’ individual clinical variables and cytogenetic and molecular features of the AML subtype. In 2022, the European LeukemiaNet (ELN) provided updated recommendations to risk-stratify patients with AML.3 Compared with the former 2017 ELN,4 certain mutations and cytogenetic abnormalities are no longer considered clinically prognostic in the 2022 ELN or now fall into a different risk category based on the presence of co-occurring genetic abnormalities.
With the approval of venetoclax (VEN)-azacitidine for treatment of patients with newly diagnosed (ND) AML who are ineligible for intensive chemotherapy (IC) based on results from the VIALE-A study, lower-intensity treatment (LIT) has become an important part of AML treatment.5-7 However, although the 2022 ELN risk classification has generally been shown to predict outcome in independent cohorts of patients homogeneously treated with IC and/or aged <60 years, it is unclear whether the ELN risk assignment schema applies to adults aged ≥60 years treated with LIT.8
Given the vital need to identify factors associated with poor outcomes that could support therapeutic decision-making in this large and difficult-to-treat patient cohort, we aimed to test the prognostic impact of the 2022 ELN risk classification in patients aged ≥60 years with ND AML treated with LIT, and to refine this risk stratification system using a large cohort of those treated in the Leukemia & Lymphoma Society–sponsored Beat-AML clinical trial.
Methods
Study population and molecular analysis
Patients aged ≥60 years with ND AML who met the screening criteria for enrollment in the Beat-AML trial (NCT03013998), provided consent before 10 May 2023, and received LIT were included in the study.9 Cytogenetic analysis from diagnostic assessment was centrally reviewed and reported in accordance with the International System for Human Cytogenomic Nomenclature.10 Complex karyotype was defined according to the presence of ≥3 unrelated chromosome abnormalities. Normal karyotype was defined according to the detection of 0 chromosome abnormalities. Next-generation sequencing was performed using FoundationOne Heme (Foundation Medicine).11 The presence of multiple hot spot mutations within 1 gene in an individual patient was counted as 1 gene mutation, and mutations were considered present at any detectable variant allele frequency (VAF). FLT3 internal tandem duplication (ITD) was detected using the LeukoStrat CDx FLT3 Mutation Assay (Invivoscribe). Informed consent was obtained according to the Declaration of Helsinki.
Statistical analysis
Continuous and categorical variables were summarized by reporting medians and ranges or frequencies and percentages, respectively. Group comparisons of dichotomous variables and continuous variables were made using Fisher exact and Mann-Whitney tests, respectively. Overall survival (OS) was estimated using the Kaplan-Meier method from the date of trial inclusion until death or last follow-up. Group differences for censored outcomes were calculated using the log-rank test.
To evaluate the impact of additional molecular abnormalities among patients with ELN-defined adverse-risk AML, these patients were randomly divided into a training (70%) and test (30%) set while maintaining a balanced distribution of mutations (TP53, RUNX1, ASXL1, TET2, SRSF2, IDH1, IDH2, FLT3-ITD, KRAS, and NRAS) in each set.12 These mutations were selected because of their high mutation frequency among adverse-risk patients, the existence of clinical targeted therapies against these gene mutations (ie, FLT3-ITD, IDH1/2), and their known prognostic implications in AML from the literature. Cox proportional hazard models were developed using the training set (n = 316) to assess the relative risk of each variable for mortality over time. First, univariable analysis was conducted, then multivariable analysis used the backward elimination approach starting with all variables that were significant on the univariable analysis and removing the variables with the highest P values from the model until all variables in the final model had a P value below the threshold of <.05. The proportional hazard assumption was tested using a score test of the multivariable model. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were summarized for each variable. A “mutation score” was obtained by calculating the total sum of the gene mutations present in each individual patient that was associated with an HR >1 (ie, +1 point for each gene mutation with a >1 HR, ie, detected within an individual patient) minus the number of gene mutations associated with an HR <1 (−1 point) as identified by the final multivariable Cox proportional hazard models. Then, the mutation score was validated in the test set. The refined Beat-AML favorable-risk group was defined by combining the 2022 ELN favorable- and intermediate-risk categories. The Beat-AML intermediate-risk group was defined by ELN adverse risk plus a mutation score of −1 to 0, and the refined Beat-AML adverse-risk group was defined by ELN adverse risk plus a mutation score of ≥1. The Harrell C-index was used to assess the model’s discrimination ability. The nonparametric C-estimator was used to compare 2 correlated C-indices.13 All statistical tests were 2-sided. Figures were generated in Microsoft Excel, version 16.80 and RStudio, version 4.3.2 (Posit).
Results
Patient characteristics
We identified a total of 595 patients with ND AML who were aged ≥60 years at the time of diagnosis, received LIT, and had sufficient data available to assign risk stratification based on the 2022 ELN criteria. The median age at diagnosis was 73 years (range, 60-92 years), and 42% of patients were female. Mutation analysis was available for all patients (N = 595), and cytogenetic data were available for 588 of 595 (99%) patients; 198 (34%) patients had a normal karyotype, 199 (34%) had a complex karyotype, and 10 (2%) had core-binding factor AML (Table 1). The treatment regimens are summarized in supplemental Table 1.
ELN risk assignment
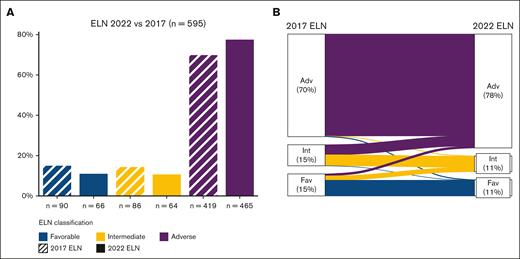
Risk stratification was performed according to the 2022 ELN,3,4 with 66 (11%) patients classified as favorable, 64 (11%) as intermediate, and 465 (78%) as adverse risk. Among these, 7 patients were classified as having adverse-risk AML on the basis of the detection of TP53 mutation with a VAF ≥10% in the absence of available cytogenetic data. Compared with the prior 2017 ELN classification, 88% (n = 525) of the patients were reclassified into the same risk category, whereas 12% (n = 67) were reclassified into the higher-risk and 0.5% (n = 3) into the lower-risk category according to the presence of in-frame bZIP-mutated CEBPA (Figure 1). The frequency of patients with adverse-risk AML increased from 70% by ELN 2017 to 78% by 2022 ELN.
ELN risk classification. (A) Proportion of patients (n = 595) with ELN favorable risk (blue), intermediate risk (yellow), and adverse risk (purple). The dashed fill shows the 2017 ELN classifications, and solid fill shows the 2022 ELN classification. (B) Alluvial plot comparing 2017 ELN (left) with 2022 ELN (right).
ELN risk classification. (A) Proportion of patients (n = 595) with ELN favorable risk (blue), intermediate risk (yellow), and adverse risk (purple). The dashed fill shows the 2017 ELN classifications, and solid fill shows the 2022 ELN classification. (B) Alluvial plot comparing 2017 ELN (left) with 2022 ELN (right).
2022 ELN risk and gene mutations
There were marked differences in the mutation spectrum between the ELN risk groups. Genetic mutations that were strongly associated with ELN adverse risk were TP53, RUNX1, and ASXL1, each accounting for 37%, 31%, and 28% of the group. As expected, NPM1 was most strongly associated with favorable risk (82% vs 15% overall; P < .001), followed by DNMT3A and IDH2, which were both also frequently found among intermediate-risk patients. FLT3-ITD was detected in 48% of the intermediate-risk patients, as opposed to 12% of the overall patient population. Gene mutations that were most strongly associated with ELN risk are shown in Figure 2. When considering all genes tested in the next-generation sequencing panel, the total number of mutations was not different between the 2022 ELN risk groups, with a median of 11 mutations in each group (P = .485).
Distribution of the 10 gene mutations most significantly associated with 2022 ELN risk. Colors indicate the 2022 ELN risk groups: favorable risk (blue), intermediate risk (yellow), and adverse risk (purple).
Distribution of the 10 gene mutations most significantly associated with 2022 ELN risk. Colors indicate the 2022 ELN risk groups: favorable risk (blue), intermediate risk (yellow), and adverse risk (purple).
Survival analysis
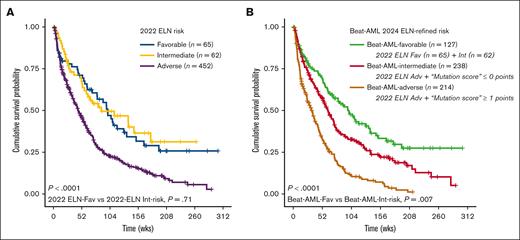
OS data were available for 579 of 595 (97%) patients. The 2022 ELN risk stratification reliably assigned those with poor OS to adverse risk (P < .001; C-index, 0.55) but did not differentiate favorable from intermediate risk (P = .71; 2-year OS, 47% vs 50% vs 23% for ELN favorable, intermediate, and adverse risk, respectively; Figure 3A). We reconsidered the impact of molecular abnormalities to refine our ability to risk-stratify patients with ND AML aged ≥60 years receiving LIT, focusing first on those categorized as having adverse risk by 2022 ELN (n = 452).
Survival analysis by ELN risk. Survival analysis in patients with ND AML aged ≥60 years treated with LIT by (A) 2022 ELN and by (B) the Beat-AML 2024 ELN-refined risk.
Survival analysis by ELN risk. Survival analysis in patients with ND AML aged ≥60 years treated with LIT by (A) 2022 ELN and by (B) the Beat-AML 2024 ELN-refined risk.
Univariable (supplemental Table 2) and multivariable analyses were conducted on the training set (n = 316). In the final multivariable model, IDH2 mutation (HR, 0.65; 95% CI, 0.46-0.94) was identified as an independent favorable prognostic variable, although treatment with an IDH inhibitor was not associated with a significant difference in outcome for those with an IDH2 mutation (HR, 0.62; 95% CI, 0.31-1.23), KRAS (HR, 1.75; 95% CI, 1.09-2.76), MLL2 (HR, 1.43; 95% CI, 1.03-1.99), and TP53 (HR, 1.90; 95% CI, 1.47-2.47) mutations were identified as independent unfavorable prognostic variables (P < .05; Table 2). The outcomes of FLT3-ITD mutated patients treated with an FLT3 inhibitor (n = 27) did not significantly differ from those of patients not treated with an FLT3 inhibitor (P = .62). No violations of proportional hazard assumptions were observed.
Next, a negative point (−1) was assigned to IDH2, and a positive point (+1) to each of KRAS, MLL2, and TP53. A “mutation score” was calculated for each combination of mutations, assigning patients into 2 risk groups: ≤0 points (“Beat-AML intermediate”) vs ≥1 points (“Beat-AML adverse”) (supplemental Table 3). As an example, the co-occurrence of IDH2, KRAS, and MLL2 in 1 individual patient resulted in a score of +1. The robustness of the 2 risk groups based on the mutation score was validated in the test set (P = .03; supplemental Figure 1). Considering the entire cohort of patients who received LIT, we further reclassified those who were assigned 2022 ELN favorable risk (n = 65) and intermediate risk (n = 62) into a combined “Beat-AML favorable-risk” category (n = 127), resulting in a refined risk model (2-year OS, Beat-AML favorable risk 48% [n = 127] vs Beat-AML intermediate risk 33% [n = 238] vs Beat-AML adverse risk 11% [n = 214]; P < .001; C-index, 0.60; Figure 3B). Comparing the C-index obtained from the 2022 ELN risk group with the one obtained from our refined risk model, the refined model showed stronger discrimination power in predicting OS (P < .001).
Baseline characteristics are summarized in Table 1. Age, race, and ethnicity were similar across the 3 refined risk groups. Female sex was proportionally overrepresented among Beat-AML favorable-risk patients (P = .007). The incidence of normal karyotype was higher in the Beat-AML favorable-risk group (69% vs 33% overall), and the incidences of complex karyotype (71% vs 33% overall) and chromosome 5/5q (52% vs 23% overall) and chromosome 7 (41% vs 22% overall) abnormalities were higher in the Beat-AML adverse-risk than in the Beat-AML favorable- and Beat-AML intermediate-risk groups. On the basis of the VAF cutoff of ≥10% for TP53 as an ELN adverse-risk criterion, 2 TP53-mutated patients with a VAF <10% were included in the Beat-AML favorable-risk group. A third patient with a TP53 mutation was classified as having Beat-AML favorable risk based on the presence of a co-occurring in-frame bZIP-mutated CEBPA. Furthermore, because the ELN specifically indicates that myelodysplastic syndrome (MDS)–related gene mutations should not be used as an adverse prognostic marker if they co-occur with the favorable-risk AML subtypes, several patients classified as having Beat-AML favorable risk had 1 or more MDS-related gene mutations.
Given the large variety in LIT regimens (supplemental Table 1), we subsequently applied the model to evaluable patients exclusively treated with a combination regimen of hypomethylating agents (HMA) plus VEN (n = 202) to test the applicability of the model on a uniformly treated patient cohort. The refined Beat-AML 2024 model continued to demonstrate statistical significance (P = .0001) in patients treated with HMA plus VEN and outperformed the 2022 ELN model with a C-index of 0.597 compared with 0.551 (P = .004) (supplemental Figure 2).
Prognostic value of myelodysplasia-related gene mutations in patients given LIT
An important change in the 2022 ELN compared with the 2017 ELN is the removal of clinical history (de novo, secondary after an antecedent MDS or MDS/myeloproliferative neoplasm, or therapy-related) and the incorporation of 9 myelodysplasia-related gene mutations. Although these mutations are highly associated with AML following MDS or MDS/myeloproliferative neoplasm, they confer adverse-risk prognosis even when they occur in de novo AML.3,14-16 Given that the prognostic significance of the ELN risk model is based on patients who were treated with IC and aged <60 years, univariable analysis was conducted on patients aged ≥60 years given LIT (n = 579). Except for STAG2 (HR, 0.67; 95% CI, 0.48-0.93), none of the myelodysplasia-related genes were independently prognostic of OS (supplemental Table 4) among older patients treated with LIT.
Discussion
In this study, we tested the utility of the 2022 ELN risk classification in patients aged ≥60 years with ND AML given LIT using a large cohort of patients enrolled in the Beat-AML clinical trial. The vast majority of patients (78%) receiving LIT were classified as having ELN adverse risk. Further, the other 22% were equally distributed between the favorable-risk (11%) and intermediate-risk (11%) groups, with no survival difference between the 2 groups. We redefined risk for older patients with ND AML and propose refined Beat-AML favorable- (22% of patients), Beat-AML intermediate- (41%), and Beat-AML adverse-risk (37%) groups with improved discrimination for OS (2-year OS, 48% vs 33% vs 11%, respectively; P < .001).
Survival analysis demonstrated that ELN risk was prognostic for OS but did not differentiate favorable from intermediate risk. This observation is consistent with a recent study that evaluated a cohort of 148 patients aged ≥60 years treated with HMA or HMA plus VEN and reported no significant difference in OS according to the 2022 ELN risk (P = .926 and P = .498, respectively).17 Others conducted an exploratory analysis evaluating 2017 ELN in 392 patients who were enrolled in a nonrandomized phase 1b trial (NCT02203773) and the confirmatory phase 3 VIALE-A randomized trial (NCT02993523).8 The median OS was similar for ELN favorable and intermediate risk, with a significantly shorter median OS in adverse-risk patients (P value not reported). A third study, however, did show a distinction in OS based on 2022 ELN risk among 179 patients with ND AML treated with HMA and VEN (P < .001). Yet, most (71%) were classified as having adverse risk, consistent with our study.18
Treatment decision-making is heavily influenced by prognostic risk stratification and genomics, particularly in this older patient population given that most will not proceed to hematopoietic stem cell transplantation. Because 78% of the older patients given LIT are classified as adverse risk by the 2022 ELN, we investigated the impact of molecular abnormalities to improve the ability to risk-stratify patients with ND AML aged ≥60 years with adverse-risk AML receiving LIT. We developed a “mutation score” that incorporated independently significant gene mutations (IDH2, MLL2, KRAS, and TP53) among ELN adverse-risk patients based on multivariable Cox regression analysis, resulting in our refined Beat-AML ELN risk model. Döhner et al also recently proposed a 4-gene approach incorporating FLT3-ITD, KRAS, NRAS, and TP53, which performed better in predicting OS and event-free survival than the 2022 ELN system, and was validated in a larger cohort of 159 patients with AML treated with HMA plus VEN.8,18 Patients with a mutation in TP53 were allocated to the low-benefit group; patients with a mutation in FLT3-ITD, NRAS, or KRAS were allocated to the intermediate-benefit group; and the remaining patients were allocated to the higher-benefit group. Although their model incorporated KRAS and TP53, the significance of FLT3-ITD is uncertain given that only a minority of patients (3.9%) in the validation cohort had this mutation.
We defined IDH2 as a favorable prognostic gene mutation among ELN adverse-risk patients. The incidence of IDH mutations increases with age19 and the prognostic utility of IDH mutations remains controversial, although a somewhat favorable prognosis may be seen with IDH2 mutations.20 Notably, in our cohort, outcomes did not differ between patients with ELN adverse risk and IDH2 gene mutation who received azacitidine with an IDH inhibitor and those who did not. TP53 mutation was most strongly associated with inferior OS, and 88% had a complex karyotype (n = 147/167). With high reported resistance rates in the literature against both IC and LIT and uniformly poor outcomes, our observation again emphasizes the need for improved treatment strategies for this difficult-to-treat population.21,22
Finally, we assessed the prognostic impact of myelodysplasia-related gene mutations, which are considered to indicate adverse-risk prognosis, among patients given LIT. Except for STAG2, none of the myelodysplasia-related gene mutations were prognostic of OS in our cohort of patients aged ≥60 years who received LIT, suggesting that they may not be helpful in further delineating adverse risk in this patient population that already does have poor outcomes due to other high-risk disease and patient characteristics. This warrants further investigation to determine whether this new category should remain separate for patients aged ≥60 years receiving LIT.
The major limitations of the study include its retrospective design, the exclusion of patients with AML requiring urgent therapy (which precluded their enrollment in the Beat-AML clinical trial), and the heterogeneity among the LIT regimens, including treatment with various molecularly directed therapies. However, we demonstrated that the model also performed significantly better in predicting OS compared with the ELN when applied to a uniformly treated subpopulation of patients receiving a combination of HMA and VEN. Further validation using external data, or a prospective study cohort is needed to validate the Beat-AML 2024 ELN-refined risk model.
In conclusion, this large multi-institutional study highlights the limited applicability of the ELN model in patients aged ≥60 years with ND AML given LIT. We propose a refined Beat-AML classification using a genomically derived “mutation score” incorporating IDH2, KRAS, MLL2, and TP53 mutations for patients previously classified as having 2022 ELN adverse risk while also redefining Beat-AML favorable risk to improve risk prediction for most older patients with AML treated with LIT.
Acknowledgments
The authors thank the pharmaceutical sponsors who paid the cost of performing the specific substudies with their investigational drugs.
This study was sponsored by the Leukemia & Lymphoma Society. Funding for the trial was made possible by the Harry T. Mangurian Jr. Foundation, many other donors, and the sites that enabled resources for rapid turnaround for cytogenetics and other monitoring requirements of patients. Substudies in this trial were supported by the pharmaceutical sponsor.
Authorship
Contribution: F.W.H. and Y.F.M. designed and supervised the research; F.W.H, Y.H., and K.J.A. analyzed the data; W.G.B., Y.H., R.L.W., R.T.S., E.T., E.M.S., T.L.L., P.A.P., R.H.C., M.R.B., V.H.D., M.L.A., W.S., O.O., R.L.R., T.K., M.W.D., J.F.Z., R.L.O., C.C.S., J.M.F., G.J.S., E.K.C., K.L.K., N.A.H., T.C., M.M., M.S., S.G.M., L.R., B.J.D., R.L.L., A.B., A.O.Y., U.M.B., A.S.M., J.C.B., and Y.F.M. collected and assembled data, and were involved in patient care; all authors reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: W.G.B. has served on advisory boards of AbbVie and Syndax; and has received research funding from Immune-Onc Therapuetics, Meryx, and Nkarta. E.T. has participated in advisory boards and/or consulting for AbbVie, Astellas, Daiichi Sankyo, Servier, and Rigel; and has received research funding from Prelude Therapeutics, Schrodinger, Incyte, and AstraZeneca. E.M.S. has served on the advisory boards of Astellas Pharma, AbbVie, Genentech, Daiichi Sankyo, Novartis, Amgen, Seattle Genetics, Syros Pharmaceuticals, Syndax Pharmaceuticals, Agios Pharmaceuticals, and Celgene; and is an equity holder in Auron Therapeutics. T.L.L. has received research funding from BioPath Holdings, Astellas Pharma, Celyad Oncology, Aptevo Therapeutics, Cleave Biosciences, CicloMed, Jazz Pharmaceuticals, and Kura Oncology; and serves on the advisory board of Servier. P.A.P. has served on the advisory board of Agios Pharmaceuticals. M.R.B. has received institutional funding from AbbVie, Ascentage Pharma, Astellas, Gilead, Kura, and Takeda Pharmaceuticals. O.O. has served on the advisory boards of Servier, Rigel, AbbVie, Incyte; and the data safety monitoring board for Treadwell Therapeutics. T.K. has served on advisory boards of Astellas, Rigel, and Takeda; has received honoraria/consulting fees from Servier; and has received institutional research funds from AbbVie, Gilead, Novartis, and Syndax Pharmaceuticals. M.W.D. is a paid consultant for Novartis, Takeda Pharmaceuticals, Blueprint, Incyte, Dava Oncology, CTI BioPharma, Syneos, Cogent, Pfizer, and Dispersol. J.F.Z. has received honoraria from advisory boards from AbbVie, Bristol Myers Squibb (BMS), Daiichi Sankyo, Gilead, Immunogen, Servier, and Shattuck Labs; has received consulting fees from AbbVie, Foghorn, Novartis, Gilead, Sellas, Servier, and Sumitomo Dainippon Pharma; and has received research funding from AbbVie, Arog, Astex, Jazz, Gilead, Merck, Newave, Shattuck Labs, Stemline Therapeutics, Sumitomo Dainippon Pharma, and Takeda Pharmaceuticals. R.L.O. has received research funds from Cellectis; and consulted for Actinium, Astellas, AbbVie, Rigel, and Servier. C.C.S. has received research funds from AbbVie, BMS, Erasca, Revolution Medicines, and Zentalis Pharmaceuticals; and served on advisory boards for AbbVie, Genentech, and Astellas. G.J.S. has commercial interests in BMS, Amgen, and Johnson & Johnson; has received fees from AbbVie, Agios, Amgen, Astellas, BMS, Incyte, Janssen, Jazz, Karyopharm, Kite, Pharmacyclics, Sanofi/Genzyme, and Stemline Therapeutics; and has received research funds from AbbVie, Actinium, Actuate, Arog, Astellas, AltruBio, AVM Biotechnology, BMS/Celgene, Celator, Constellation, Daiichi Sankyo, Deciphera, Delta-Fly, Forma, Fujifilm, Gamida, Genentech-Roche, Glycomimetics, Geron, Incyte, Karyopharm, Kiadis, Kite/Gilead, Kura, Marker, Mateon, Onconova Therapeutics, Pfizer, Precog, REGiMMUNE, Samus, Sangamo Therapuetics, SELLAS, Stemline Therapeutics, Syros, Takeda Pharamceuticals, Tolero, Trovagene, Agios, Amgen, Jazz, Orca, ONO PHARMA -UK, and Novartis. M.S. has been a consultant for Eilean Therapeutics. B.J.D. has served on the scientific advisory boards for Adela Bio, Aileron Therapeutics (inactive), Therapy Architects/ALLCRON (inactive), Cepheid, Labcorp, Nemucore Medical Innovations, Novartis, and the RUNX1 Research Program; has served on the scientific advisory boards and holds stock in Aptose Biosciences, Blueprint Medicines, Enliven Therapeutics, Iterion Therapeutics, GRAIL, and Recludix Pharma; has served on boards of directors and holds stock in Amgen and Vincerx Pharma; has served on boards of directors for Burroughs Wellcome Fund and CureOne (inactive); has participated in the joint steering committee of Leukemia & Lymphoma Society’s Beat acute myeloid leukemia trial; has served on advisory committee for Multicancer Early Detection Consortium; is the founder of VB Therapeutics; has sponsored research agreements in AstraZeneca, DELiver Therapeutics, ImmunoForge, Terns, Enliven Therapeutics (inactive), and Recludix Pharma (inactive); has received clinical trial funding from Novartis and AstraZeneca; has received royalties from patent 6958335 (Novartis exclusive license) and Oregon Health & Science University and the Dana-Farber Cancer Institute (1 Merck exclusive license, 1 CytoImage, Inc exclusive license, 1 DELiver Therapeutics nonexclusive license, and 1 Sun Pharma Advanced Research Company nonexclusive license); and holds US Patents 4326534, 6958335, 7416873, 7592142, 10473667, 10664967, and 11049247. R.L.L. is on the supervisory board of Qiagen; is a scientific adviser to Imago, Mission Bio, and Syndax; receives equity support from Zentalis, Ajax, Bakx, Auron, Prelude, C4 Therapeutics, and IsoPlexis; receives research support from Ajax and AbbVie; has consulted for Incyte, Janssen, MorphoSys, and Novartis; and has received honoraria from AstraZeneca and Kura for invited lectures and from Gilead for grant reviews. U.M.B. has been a consultant for Genentech, Daiichi Sankyo, Takeda, Pfizer, AbbVie/Genentech, and Novartis. A.S.M. has served on the advisory boards of AbbVie/Genentech, Novartis, Ryvu Therapeutics, Rigel Therapeutics, Treadwell Therapeutics, and Foghorn Therapeutics. J.C.B. is a current equity holder in Vincerx Pharma Inc (a publicly traded company), Eilean Therapeutics, and Kurome Therapeutics; holds membership on the boards of directors or advisory committees of Vincerx, Newave, Eilean, Kartos, and Orange Grove Bio; and has been a consultant for and received honoraria from Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, and Syndax. Y.F.M. has received honoraria/consulting fees from BMS, Kura Oncology, Blueprint Medicines, Geron, OncLive, MD Education, VJHemOnc, and Medscape Live; has participated in advisory boards and received honoraria from Sierra Oncology, Stemline Therapeutics, Blueprint Medicines, MorphoSys, Taiho Oncology, Sobi, Rigel Pharmaceuticals, Geron, Cogent Biosciences, and Novartis; and has received travel reimbursement from Blueprint Medicines, MD Education, and MorphoSys. None of these relationships were related to this work. The remaining authors declare no competing financial interests.
The current affiliation for P.A.P. is Servier Pharmaceuticals, Boston, MA.
Correspondence: Yazan F. Madanat, Harold C. Simmons Comprehensive Cancer Center, The University of Texas Southwestern Medical Center, 2201 Inwood Rd, NC8.106, Dallas, TX 75390-8565; email: yazan.madanat@utsouthwestern.edu.
References
Author notes
Data are available on request from the corresponding author, Yazan F. Madanat (yazan.madanat@utsouthwestern.edu).
The full-text version of this article contains a data supplement.