Key Points
Second generation tyrosine kinase inhibitors are more effective than imatinib for de novo CML-CP.
It is important to clarify which tyrosine kinase inhibitors is best to achieve DMR required for TFR.
Visual Abstract
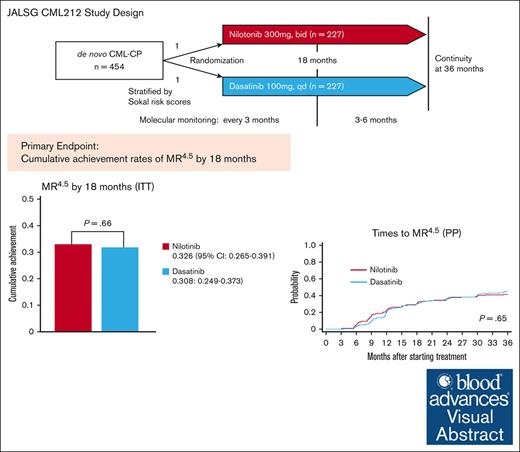
Deep molecular response (DMR) is a prerequite for treatment-free remission (TFR) in chronic myeloid leukemia in chronic phase (CML-CP). The JALSG (Japan Adult Leukemia Study Group) conducted a prospective randomized phase 3 CML212 study for de novo CML-CP to compare the cumulative achievement of molecular response 4.5 (MR4.5; international scale BCR::ABL1 ≤0.0032%) by 18 months between nilotinib and dasatinib treatment as a primary end point. A total of 454 patients were randomly assigned to the 300 mg nilotinib twice daily arm or to the 100 mg dasatinib daily arm (both n = 227). BCR::ABL1 messenger RNA levels were monitored every 3 months. Study treatment was stopped if the patients were judged as failure according to the European LekemiaNet 2009 criteria or showed intolerance. The cumulative achievement rates of MR4.5 by 18 months were 32.6% (95% confidence interval [CI], 26.5-39.1) in the nilotinib arm and 30.8% (95% CI, 24.9-37.3) in the dasatinib arm with no significant difference (P = .66). The cumulative achievement rates of early molecular response, complete cytogenetic response, and major molecular response by 12, 18, 24, and 36 months were almost the same between the 2 arms. There was no significant difference in progression-free survival (PFS) or overall survival (OS) between the 2 arms by log-rank tests (PFS, P = .58; OS, P = .64). These results suggest that nilotinib and dasatinib would be equally effective for patients with de novo CML-CP. This trial was registered in the University Hospital Medical Information Network Clinical Trials Registry as #UMIN000007909.
Introduction
The second generation (2G) tyrosine kinase inhibitors (TKIs) such as dasatinib, nilotinib, and bosutinib, were developed for patients with chronic myeloid leukemia (CML) with resistance or intolerance to 1G imatinib.1-7 Because 2G TKIs have more potent inhibitory activities on BCR::ABL1 than imatinib, they were compared with imatinib as the first-line TKI for de novo CML in chronic phase (CML-CP) in 3 prospective randomized trials (ENESTnd [Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients], DASISION [Dasatinib versus Imatinib Study in Treatment-Naive CML Patients], and BFORE [Bosutinib Trial in First-Line Chronic Myelogenous Leukemia Treatment]).8-12 All these 2G TKIs more efficiently achieved a major molecular response (MMR;BCR::ABL1 on the international scale [IS] ≤0.1%) than imatinib, which was the primary end point in these studies.
About 40% to 60% of patients with CML-CP with a durable deep molecular response (DMR; BCR::ABL1 IS ≤0.01%) can maintain molecular remission after TKI discontinuation.13-17 In addition, most patients do not experience disease progression, and the molecular remission was recovered by restarting the TKI, indicating that a TKI can be stopped safely in a substantial proportion of patients with CML-CP with a durable DMR. However, even if BCR::ABL1 messenger RNA (mRNA) was not detected by highly sensitive quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) after TKI discotinuation, BCR::ABL1 was still detected by DNA PCR.18,19 Therefore, this condition is not considered to be cured but is referred to as treatment-free remission (TFR).
TFR is now positioned as a new therapeutic goal for CML-CP in European LekemiaNet 2020 (ELN2020).20 In the ENESTnd and DASISION trials, both nilotinib and dasatinib were more effective at achieving DMR, which is a critical milestone for TFR, than imatinib.8-11 However, there is no direct comparison to determine which TKI is better to achieve DMR for patients with de novo CML-CP. Therefore, the Japan Adult Leukemia Study Group (JALSG) conducted a prospective randomized controlled phase 3 CML212 study to compare the cumulative achievement of less than or equal to molecular response 4.5 (MR4.5) measured by the IS (BCR::ABL1 IS ≤ 0.0032%) between nilotinib and dasatinib.
Methods
Patients
Diagnosis of CML was done by cytogenetic studies (G-banding or fluorescence in situ hybridization) and/or the detection of BCR::ABL1 mRNA by RT-PCR within 6 months before the study entry. The CP of CML was defined by <10% blasts, <20% basophils, and a platelet count of ≥100 ×109 per liter in the bone marrow (BM) or peripheral blood (PB). Eligible patients must have received no previous treatment for CML, except for hydroxyurea (within 1 month) or imatinib (within 2 weeks). In addition, patients had to have Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate hepatic, renal, pancreatic, cardiac, and lung functions. Patients with cardiac dysfunctions (such as QT prolongation >450 milliseconds, cardiomegaly on a chest X-ray, and left ventricular ejection fraction <45%), active another malignancy, a history of another invasive malignancy within 5 years, or active infection requiring treatment were excluded. In addition, pregnant patients, those with a possibility of being pregnant, and breastfeeding patients were excluded. Other inclusion and exclusion criteria are available in the supplemental Appendix.
Study design and treatment
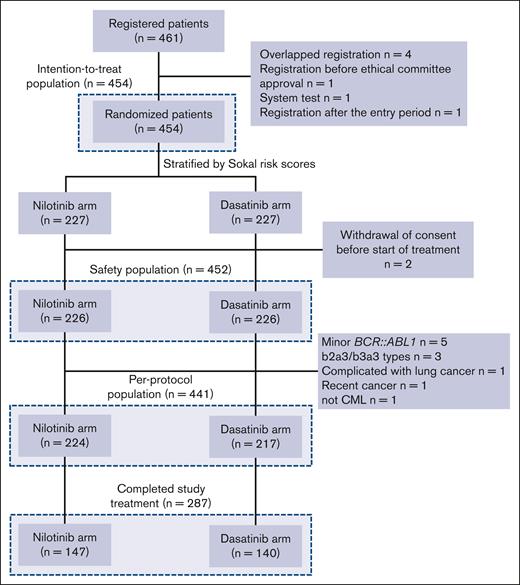
The JALSG CML212 study is a multicenter, open-labeled, prospective randomized controlled phase 3 study for de novo CML-CP. This study was approved by the institutional review board of each institute and registered in the University Hospital Medical Information Network Clinical Trials Registry (identifier UMIN000007909). All patients provided written informed consent before enrollment. A total of 461 patients were enrolled from 82 institutes from May 2012 to January 2016. Of 461 patients who registered, 7 patients were excluded because they were duplicate registrations (n = 4), they registered before a center’s ethical committee approval (n = 1), it was a test registration after system error (n = 1), or registered after the enrollment period (n = 1). From the remaining 454 patients (intention-to-treat [ITT] population), 2 patients were excluded because of withdrawal of agreement before the drug administration (safety population, n = 452). Then, 11 patients were further excluded because they had variant or minor BCR::ABL1 mRNA (n = 8), lung cancer (n = 1), a recent history of the cancer (n = 1), and not CML (n = 1). Therefore, 441 patients were treated as the per-protocol (PP) population (nilotinib arm, n = 224; dasatinib arm, n = 217; Figure 1).
Flow diagram of the patients and analyzed population. ITT population consisted of all patients who were randomized. Safety population consisted of individuals who received at least 1 dose of the protocol treatment. PP population consisted of subjects who received at least 1 dose of the protocol treatment and excluded those who were determined to be ineligible for the study.
Flow diagram of the patients and analyzed population. ITT population consisted of all patients who were randomized. Safety population consisted of individuals who received at least 1 dose of the protocol treatment. PP population consisted of subjects who received at least 1 dose of the protocol treatment and excluded those who were determined to be ineligible for the study.
The 454 patients were randomly assigned in a 1:1 ratio to receive either nilotinib (300 mg twice daily) or dasatinib (100 mg once daily) (both, n = 227) with Sokal risk scores21 as a stratification factor (Figure 1) by the JALSG Kanazawa data center. Treatment responses were evaluated according to the ELN2009 criteria.22 According to these criteria, failure was defined as no complete hematologic response (CHR) at 3 months, no cytogenetic response (Ph+ cells >95%) at 6 months, no partial cytogenetic response (Ph+ cells >35%) at 12 months, and no complete cytogenetic response (CCyR) at 18 months. Adverse events were managed by treatment interruptions and/or dose reductions of the allocated TKI according to the criteria detailed in the supplemental Appendix. Dose escalation of dasatinib to 140 mgdaily was permitted for suboptimal responses or failure, but such cases were treated as stopping the study treatment. Dose escalation of nilotinib was not allowed. These dose modifications were based on the package leaflet of these drugs in Japan. Study treatment was discontinued if the patients were judged to have failed according to the ELN2009 criteria or if they showed intolerance (repetitive ≥grade 3 or continuous grade 2 side effects) to the allocated TKI.
Definition of AP and BP
Accelelated phase (AP) was defined as follows: ≥10% (but <30%) blasts in PB or BM, ≥30% blasts and promyelocytes in PB and BM, ≥20% basophiles in PB, or <109/L platelets unrelated to the treatment. Blastic phase (BP) was defined as follows: ≥30% blasts in PB or BM or extramedullary lesion except hepatosplenomegaly, which was confirmed in the biopsy specimen (eg, chloroma).
End points
The primary end point was the cumulative achievement rate of MR4.5 by 18 months. Major secondary endpoints were as follows: the safety and continuity of nilotinib and dasatinib; clinical outcomes, including progression-free survival (PFS), event-free survival (EFS), and overall survival (OS); the rates of early molecular response (EMR; BCR::ABL1 IS ≤ 10%) at 3 months; the rates of CCyR, MMR, MR4.0, and MR4.5 by 12, 18, 24, 36 months; time to CCyR, MMR, MR4.0, and MR4.5; and the efficacy of nilotinib and dasatinib by Sokal and European Treatment and Outcome Study (EUTOS) risk groups.21,23
Evaluation of efficacy
Cytogenetic responses were evaluated at baseline, 3, 6, 12, 18, 24, and 36 months until achieving CCyR as measured by the G-banding method using BM samples. If the evaluation by G-banding was not sufficient, cytogenetic responses were evaluated using PB neutrophils fluorescence in situ hybridization. BCR::ABL1 IS levels in PB were monitored as the ratio of BCR::ABL1 transcripts to ABL1 transcripts every 3 months until 18 months and then every 3 to 6 months until 36 months using a highly sentitive qRT-PCR kit with sensitivities that were equal to or greater than MR4.5 (supplemental Appendix).
OS was defined as the interval from the date of randomization to the date of death from any causes. PFS was defined as the interval from the date of randomization to the date of progression to AP or BP or death from any causes. EFS was defined as the interval from the date of randomization until the date of the earliest of following defined events: loss of the CHR, reduction in cytogenetic responses (loss of CCyR or partial cytogenetic response [PCyR]), increase in white blood cell count in patients without CHR, progression to AP or BP, discontinuation of the allocated TKI, or death.
Detection of point mutaions in resistant patients
We conduced mutational analyses if the BCR::ABL1 IS level increased fivefold during the monitoring. For these analyses, we used the commercial direct sequence method at BML Inc (Tokyo, Japan), which can detect point mutations in the BCR::ABL gene at codon 225 to 505 (supplemental Appendix).
Evaluation of safety
Adverse events were evaluated by the Common Terminology Criteria for Adverse Events, version 4.0, of the National Cancer Institute.
Statistical analysis
The cumulative rates of MR4.5 by 18 months was assessed from the results of the ENESTnd (21% in the nilotinib 300mg, twice daily arm24) and the DASISION (13% in the dasatinib 100mg once daily arm25) studies and from 2 phase 2 studies conducted at the MD Anderson Cancer Center (21% in the nilotinib study and 6% in the dasatinib study, respectively).26,27 From these results, we assumed that the cumulative rates of MR4.5 by 18 months were 21% among patients with de novo CML-CP who were treated with nilotinib 300 mg twice daily and 9.5% among those treated with dasatinib 100 mg once daily. A total sample size of 450 was required to verify the hypothesis that nilotinib is superior to dasatinib in the cumulative rates of MR4.5 by 18 months when using 1:1 randomization with a statistical power of 90% and a .05 2-sided significance level with a stratified Cochran-Mantel-Haenszel test and allowing 10% loss to follow-up. All efficacy analyses were performed on the ITT population in principle. A safety analysis was performed on the safety population.
Results
Patients
The baseline characteristics of the patients are shown in Table 1. The median age of the patients was 53 years in both arms. The proportions of Sokal low-, intermediate-, and high-risk groups were 44.1%, 37.0%, and 18.9%, respectively, in the nilotinib arm and 44.5%, 37.0%, and 18.5%, respectively, in the dasatinib arm without a significant difference (P = 1.00). In addition, there was no significant difference in the Eastern Cooperative Oncology Group performance status, EUTOS risk groups, blood cell counts, complications, or frequencies of additional chromosomal abnormalities between the arms, indicating that the baseline demographic and disease characteristics were well balanced between the 2 arms.
Baseline demographic and disease characteristics in the ITT population
| Characteristics . | Nilotinib (n = 227) . | Dasatinib (n = 227) . | P value∗ . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 53 (19-85) | 53 (17-90) | .70 |
| Sex, n (%) | |||
| Male | 142 (62.6) | 149 (65.6) | .56 |
| Female | 85 (37.4) | 78 (34.4) | |
| ECOG performance status, n (%) | |||
| 0 | 204 (89.9) | 199 (87.7) | .66 |
| 1 | 22 (9.70) | 26 (11.5) | |
| 2 | 1 (0.40) | 2 (0.90) | |
| Sokal risk group, n (%) | |||
| Low risk | 100 (44.1) | 101 (44.5) | 1.00 |
| Intermediate risk | 84 (37.0) | 84 (37.0) | |
| High risk | 43 (18.9) | 42 (18.5) | |
| EUTOS risk group, n (%) | |||
| Low risk | 196 (86.3) | 196 (86.3) | 1.00 |
| High risk | 31 (13.7) | 31 (13.7) | |
| Complications, n (%) | |||
| Yes | 67 (29.5) | 53 (23.3) | .17 |
| No | 158 (69.6) | 172 (75.8) | |
| Missing | 2 (0.90) | 2 (0.90) | |
| Medical history, n (%) | |||
| Yes | 73 (32.2) | 66 (29.1) | .67 |
| No | 147 (64.8) | 155 (68.3) | |
| Unknown | 3 (1.30) | 2 (0.90) | |
| Missing | 4 (1.80) | 4 (1.80) | |
| Additional chromosomal changes | |||
| Yes | 33 (14.5) | 29 (12.8) | .59 |
| No | 191 (84.1) | 196 (86.3) | |
| Missing | 3 (1.30) | 2 (0.90) | |
| Blood cell counts at diagnosis | |||
| WBC (per μL), median (range) | 35 230 (102.5-557 300) | 39 400 (49.8-586 500) | .38 |
| Hb (g/dL), median (range) | 12.9 (6.7-18.2) | 13.1 (6.6-19) | .67 |
| PLT (×104/μL), median (range) | 48 (10.2-282) | 48.1 (10.4-338) | .32 |
| Characteristics . | Nilotinib (n = 227) . | Dasatinib (n = 227) . | P value∗ . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 53 (19-85) | 53 (17-90) | .70 |
| Sex, n (%) | |||
| Male | 142 (62.6) | 149 (65.6) | .56 |
| Female | 85 (37.4) | 78 (34.4) | |
| ECOG performance status, n (%) | |||
| 0 | 204 (89.9) | 199 (87.7) | .66 |
| 1 | 22 (9.70) | 26 (11.5) | |
| 2 | 1 (0.40) | 2 (0.90) | |
| Sokal risk group, n (%) | |||
| Low risk | 100 (44.1) | 101 (44.5) | 1.00 |
| Intermediate risk | 84 (37.0) | 84 (37.0) | |
| High risk | 43 (18.9) | 42 (18.5) | |
| EUTOS risk group, n (%) | |||
| Low risk | 196 (86.3) | 196 (86.3) | 1.00 |
| High risk | 31 (13.7) | 31 (13.7) | |
| Complications, n (%) | |||
| Yes | 67 (29.5) | 53 (23.3) | .17 |
| No | 158 (69.6) | 172 (75.8) | |
| Missing | 2 (0.90) | 2 (0.90) | |
| Medical history, n (%) | |||
| Yes | 73 (32.2) | 66 (29.1) | .67 |
| No | 147 (64.8) | 155 (68.3) | |
| Unknown | 3 (1.30) | 2 (0.90) | |
| Missing | 4 (1.80) | 4 (1.80) | |
| Additional chromosomal changes | |||
| Yes | 33 (14.5) | 29 (12.8) | .59 |
| No | 191 (84.1) | 196 (86.3) | |
| Missing | 3 (1.30) | 2 (0.90) | |
| Blood cell counts at diagnosis | |||
| WBC (per μL), median (range) | 35 230 (102.5-557 300) | 39 400 (49.8-586 500) | .38 |
| Hb (g/dL), median (range) | 12.9 (6.7-18.2) | 13.1 (6.6-19) | .67 |
| PLT (×104/μL), median (range) | 48 (10.2-282) | 48.1 (10.4-338) | .32 |
ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; PLT, platelet; WBC, white blood cell.
Fisher exact test
Continuity and patient status
At 36 months, 149 of 224 (66.5%) (95% confidence interval [CI], 59.9-72.7) and 141 of 217 (65.0%; 95% CI, 58.2-71.3) patients continued nilotinib and dasatinib until 36 months, respectively (P = .76; supplemental Table 1). Before that time, 75 (33.4%) and 76 (35.0%) patients discontinued the allocated TKI treatment in the nilotinib arm and dasatinib arm, respectively. The major reasons for discontinuation of the protocol treatment were as follows: 4 (5.3%) and 5 (6.6%) patients were determined to have failed treatment according to the ELN2009 criteria; 48 (64.0%) and 48 (63.2%) patients discontinued because of intolerance in the nilotinib and dasatinib arms, respectively (supplemental Table 1); and 15 patients (nilotinib arm, n = 10; dasatinib arm, n = 5) discontinued the allocated TKI with the category “judge by doctor.” Among them, 4 patients in the nilotinib arm and 3 patients in the dasatinib arm discontinued the allocated TKI because of the insufficient efficacies that did not satisfy the criteria of failure according to the ELN2009 criteria, such as suboptimal response (eg, CCyR but not MMR at and after 18 months).
We also analyzed the responses of both treatment arms according to the ELN2020 criteria.20 Optimal responses were 94.3%, 92.0%, and 74.1% in the nilotinib arm and 90.0%, 90.1%, and 78.4% in the dasatinib arm at 3, 6, and 12 months, respectively, without an apparent difference. In addition, there was no obvious difference in the warning and failure (supplemental Table 2).
These results suggest that nilotinib and dasatinib have similar continuity in terms of the efficacy and durability.
Efficacy
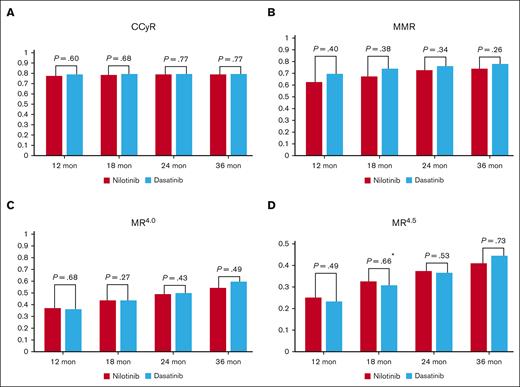
In the ITT population, the cumulative achievement rates of MR4.5 by 18 months were 32.6% (74/227) (95% CI, 26.5-39.1) in the nilotinib arm and 30.8% (70/227) (95% CI, 24.9-37.3) in the dasatinib arm with no significant difference between the arms (P = .66) (the primary end point; Figure 2A; supplemental Table 3). In addition, the cumulative achievement rates of MR4.5 by 12, 24, and 36 months were 25.1%, 37.4%, and 41.0%, respectively, in the nilotinib arm and 23.3%, 36.6%, and 44.5%, respectively, in the dasatinib arm with no significant difference (Figure 3D; supplemental Table 3). This finding was confirmed in the PP population, namely 33.0% (95% CI, 26.9-39.6) in the nilotinib arm and 31.8% (95% CI, 25.7-38.4) in the dasatinib arm (P = .82; supplemental Table 4). We also found that the rates of cumulative achievement rates of MR4.5 by 18 months did not differ between the nilotinib and dasatinib arms, regardless of the Sokal and EUTOS risk groups in both the ITT and PP populations (supplemental Tables 5 and 6).
Comparison of MR4.5 and EMR between the nilotinib arm and the dasatinib arm. Cumulative achievement rates of MR4.5 (A) and achievement rates of EMR (B). The results were calculated on the ITT population by means of the Cochran-Mantel-Haenszel (CMH) test.
Comparison of MR4.5 and EMR between the nilotinib arm and the dasatinib arm. Cumulative achievement rates of MR4.5 (A) and achievement rates of EMR (B). The results were calculated on the ITT population by means of the Cochran-Mantel-Haenszel (CMH) test.
Comparison of cytogenetic and molecular responses between the nilotinib arm and the dasatinib arm. Cumulative achievement rates of CCyR (A), MMR (B), MR4.0 (C), and MR4.5 (D) by 12, 18, 24, and 36 months. The results were calculated on the ITT population by means of the CMH test. ∗Primary end point and same as Figure 2B.
Comparison of cytogenetic and molecular responses between the nilotinib arm and the dasatinib arm. Cumulative achievement rates of CCyR (A), MMR (B), MR4.0 (C), and MR4.5 (D) by 12, 18, 24, and 36 months. The results were calculated on the ITT population by means of the CMH test. ∗Primary end point and same as Figure 2B.
Stable MR4.5 is important for TFR. Therefore, we analyzed loss of MR4.5, which was defined by 2 consequent losses of MR4.5 but with MMR and loss of MMR occurring once only. Among 93 patients who achieved MR4.5 by 36 months in the nilotinib arm, 10 patients lost MR4.5, but 7 patients subsequently achieved MR4.5 again. Also, among 101 patients who achieved MR4.5 by 36 months in the dasatinib arm, 6 patients lost MR4.5 but 3 patients regained MR4.5. Combining both arms, 96.9% (188/194) of the patients maintained MR4.5 continuously.
The rates of EMR at 3 months were 74.5% (169/227; 95% CI, 68.3-80.0) in the nilotinib arm and 73.1% (166/227; 95% CI, 66.9-78.8) in the dasatinib arm with no significant difference (P = .26; Figure 2B).
The cumulative CCyR rates by 12, 18, 24, and 36 months were 77.5%, 78.4%, 78.9%, and 78.9%, respectively, in the nilotinib arm and 78.9%, 79.3%, 79.3%, and 79.3%, respectively, in the dasatinib arm without a significant difference (Figure 3A; supplemental Table 3). In addition, there was no significant difference between the arms in the cumulative achievement rates of MMR or MR4.0 in the ITT population (Figure 3B-C; supplemental Table 3). These results were also confirmed in the PP population (supplemental Table 4).
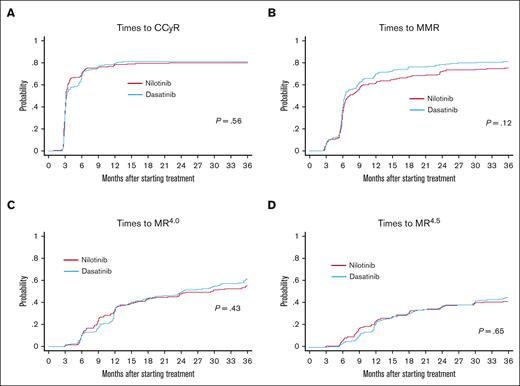
We also examined the times to cytogenetic and molecular responses in the PP population by the cumulative incidence method. As shown in Figure 4, there was no significant difference in the times to the first achievement of CCyR, MMR, MR4.0, or MR4.5 between the nilotinib and dasatinib arms, indicating that both TKIs are equivalently effective at achieving these responses in terms of rapidity. Similar results were obtained when using the Kaplan-Meier method in the PP population (supplemental Figure 1).
Comparison of times to cytogenetic and molecular responses between the nilotinib arm and the dasatinib arm. Times to the first CCyR (A), MMR (B), MR4.0 (C), and MR4.5 (D). Times to cytogenetic and molecular responses were defined as the intervals from the date of first dose to the response events and analyzed on the PP population by the cumulative incidence method with a Gray test. If the patients died or were lost to follow-up without achieving the events, these patients were treated as censored cases at the date of death or the last follow-up.
Comparison of times to cytogenetic and molecular responses between the nilotinib arm and the dasatinib arm. Times to the first CCyR (A), MMR (B), MR4.0 (C), and MR4.5 (D). Times to cytogenetic and molecular responses were defined as the intervals from the date of first dose to the response events and analyzed on the PP population by the cumulative incidence method with a Gray test. If the patients died or were lost to follow-up without achieving the events, these patients were treated as censored cases at the date of death or the last follow-up.
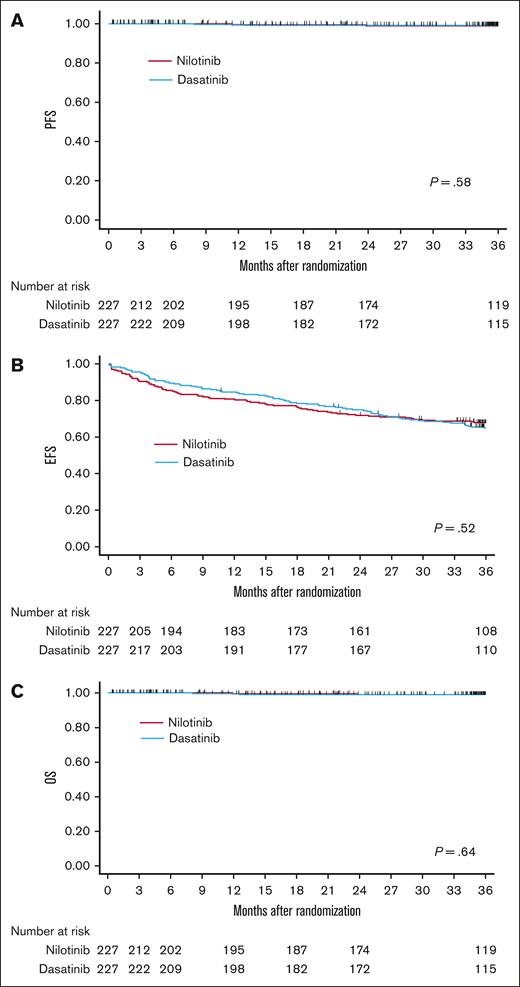
We next compared the clinical outcomes between the nilotinib and dasatinib arms in the ITT population by using the Kaplan-Meier method. There was no significant difference in PFS, EFS, or OS between the 2 arms when using log-rank tests (the estimated rates at 36 months: 98.9%, 67.7%, and 98.9% in the nilotinib arm; 99.0%, 64.8%, and 99.0% in the dasatinib arm, respectively; Figure 5). In addition, PFS, EFS and OS did not differ between the 2 arms regardless of the Sokal and EUTOS risk groups (supplemental Figures 2 and 3). In the ITT population (each n = 227), 1 patient (0.44%) in the nilotinib arm progressed to AP/BP at 12 months and 2 patients (0.88%) in the dasatinib arm progressed at 8 and 51 months, respectively.
Comparison of PFS, EFS, and OS between the nilotinib arm and the dasatinib arm. PFS (A), EFS (B), and OS (C). PFS, EFS, and OS were defined as the interval from the date of randomization until the date of progression or death from any causes, whichever came first (PFS); until the date of the earliest of defined events (EFS); and until the date of death from any causes (OS), respectively. PFS, EFS, and OS were analysis on the ITT population using the Kaplan-Meier method with a log-rank test.
Comparison of PFS, EFS, and OS between the nilotinib arm and the dasatinib arm. PFS (A), EFS (B), and OS (C). PFS, EFS, and OS were defined as the interval from the date of randomization until the date of progression or death from any causes, whichever came first (PFS); until the date of the earliest of defined events (EFS); and until the date of death from any causes (OS), respectively. PFS, EFS, and OS were analysis on the ITT population using the Kaplan-Meier method with a log-rank test.
According to the protocol, we conduced muatational analyses if the BCR::ABL1 IS level increased fivefold during the monitoring. Accordingly, a total of 30 tests (10 tests in 10 nilotinib-allocated patients; 20 tests in 14 dasatinib-allocated patients) were conducted. Although no mutation was detected in these tests, we detected a 35 bp insertion (not mutation but splicing variant) in exon 8/9 in 2 dasatinib-allocated patients. We speculate that these negative results might be because of the sensitivity of our method.
Safety
Because both TKIs are commonly used and their safety profiles are well-known, we collected the data of AEs greater than or equal to grade 3. In the safety population, the AEs classified as grade 3 or greater that were observed at a frequency of ≥10% were lipase elevation (11.5%) in the nilotinib arm and neutropenia (12.8%) and thrombocytopenia (16.8%) in the dasatinib arm (Table 2).
Adverse events during the study treatment in the safety population
| . | Nilotinib (n = 226) . | Dasatinib (n = 226) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade . | 3 . | 4 . | 5 . | ≥3 . | 3 . | 4 . | 5 . | ≥3 . |
| Hematologic toxicities, n (%) | ||||||||
| Leukopenia | 5 (2.2) | 0 (0) | 0 (0) | 5 (2.2) | 6 (2.7) | 1 (0.4) | 0 (0) | 7 (3.1) |
| Neutropenia | 6 (2.7) | 3 (1.3) | 0 (0) | 9 (4.0) | 22 (9.7) | 7 (3.1) | 0 (0) | 29 (12.8) |
| Lymphopenia | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 2 (0.9) | 2 (0.9) | 0 (0) | 4 (1.8) |
| Decreased Hb | 3 (1.3) | 0 (0) | 0 (0) | 3 (1.3) | 14 (6.2) | 2 (0.9) | 0 (0) | 16 (7.1) |
| Thrombocytopenia | 6 (2.7) | 3 (1.3) | 0 (0) | 9 (4.0) | 32 (14.2) | 6 (2.7) | 0 (0) | 38 (16.8) |
| Nonhematologic toxicities, n (%) | ||||||||
| Rash | 5 (2.2) | 0 (0) | 0 (0) | 5 (2.2) | 4 (1.8) | 0 (0) | 0 (0) | 4 (1.8) |
| Edema | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 6 (2.7) | 0 (0) | 0 (0) | 6 (2.7) |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Nausea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Myalgia | 4 (1.8) | 0 (0) | 0 (0) | 4 (1.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pleural effusion | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (4.4) | 1 (0.4) | 0 (0) | 11 (4.9) |
| QTc prolongation | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Interstitial pneumonia | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) |
| Gastrointestinal bleeding | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) |
| Other bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 3 (1.3) |
| Increased glucose | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Increased amylase | 3 (1.3) | 2 (0.9) | 0 (0) | 5 (2.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Increased lipase | 23 (10.2) | 3 (1.3) | 0 (0) | 26 (11.5) | 4 (1.8) | 0 (0) | 0 (0) | 4 (1.8) |
| Increased bilirubin | 14 (6.2) | 0 (0) | 0 (0) | 14 (6.2) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Increased AST | 10 (4.4) | 0 (0) | 0 (0) | 10 (4.4) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Increased ALT | 19 (8.4) | 1 (0.4) | 0 (0) | 20 (8.8) | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) |
| Increased creatinine | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| . | Nilotinib (n = 226) . | Dasatinib (n = 226) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade . | 3 . | 4 . | 5 . | ≥3 . | 3 . | 4 . | 5 . | ≥3 . |
| Hematologic toxicities, n (%) | ||||||||
| Leukopenia | 5 (2.2) | 0 (0) | 0 (0) | 5 (2.2) | 6 (2.7) | 1 (0.4) | 0 (0) | 7 (3.1) |
| Neutropenia | 6 (2.7) | 3 (1.3) | 0 (0) | 9 (4.0) | 22 (9.7) | 7 (3.1) | 0 (0) | 29 (12.8) |
| Lymphopenia | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 2 (0.9) | 2 (0.9) | 0 (0) | 4 (1.8) |
| Decreased Hb | 3 (1.3) | 0 (0) | 0 (0) | 3 (1.3) | 14 (6.2) | 2 (0.9) | 0 (0) | 16 (7.1) |
| Thrombocytopenia | 6 (2.7) | 3 (1.3) | 0 (0) | 9 (4.0) | 32 (14.2) | 6 (2.7) | 0 (0) | 38 (16.8) |
| Nonhematologic toxicities, n (%) | ||||||||
| Rash | 5 (2.2) | 0 (0) | 0 (0) | 5 (2.2) | 4 (1.8) | 0 (0) | 0 (0) | 4 (1.8) |
| Edema | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 6 (2.7) | 0 (0) | 0 (0) | 6 (2.7) |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Nausea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Myalgia | 4 (1.8) | 0 (0) | 0 (0) | 4 (1.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pleural effusion | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (4.4) | 1 (0.4) | 0 (0) | 11 (4.9) |
| QTc prolongation | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Interstitial pneumonia | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) |
| Gastrointestinal bleeding | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) |
| Other bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 3 (1.3) |
| Increased glucose | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Increased amylase | 3 (1.3) | 2 (0.9) | 0 (0) | 5 (2.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Increased lipase | 23 (10.2) | 3 (1.3) | 0 (0) | 26 (11.5) | 4 (1.8) | 0 (0) | 0 (0) | 4 (1.8) |
| Increased bilirubin | 14 (6.2) | 0 (0) | 0 (0) | 14 (6.2) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Increased AST | 10 (4.4) | 0 (0) | 0 (0) | 10 (4.4) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
| Increased ALT | 19 (8.4) | 1 (0.4) | 0 (0) | 20 (8.8) | 2 (0.9) | 0 (0) | 0 (0) | 2 (0.9) |
| Increased creatinine | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) |
ALT, Alanine transaminase; AST, Aspartate aminotransferase; QTc, Corrected QT interval.
Angina was observed in 5 patients in the nilotinib arm (grade 3 or greater, n = 4; grade 2, n = 1) and in 1 patient (grade 3) in the dasatinib arm. Myocardial infarction, cerebrovascular events, and peripheral arterial events were not observed in this study. However, in the nilotinib arm, cerebral stenosis was detected by magnetic resonance angiography in 1 patient. Pulmonary hypertension equal to or greater than grade 3 was observed in 1 patient in each of the nilotinib and dasatinib arms. Pleural effusion at was observed in 11 patients (4.9%) in the dasatinib arm, which was largely consistent with the frequency in the DASAION Japanese cohort.28 Any unknown or unexpected side effect was not observed in either of the arms during the study treatment.
Discussion
Nilotinib is a more selective BCR::ABL1 inhibitor than imatinib, whereas dasatinib is a multikinase inhibitor, which inhibits Src family kinases and serine/threonine kinases. Therefore, nilotinib and dasatinib have different profiles in terms of side effects. However, both TKIs showed similar superiority to imatinib in achieving CCyR, MMR, and DMR in the ENESTnd and DASISION trials.8-11 Based on these data, these 2G TKIs were approved for de novo CML-CP in many countries. However, there was no significant difference in the OS between the 2G TKIs and imatinib in these trials. In addition, 2G TKIs increased the risk for cardiovascular events. Therefore, ELN2020 recommends imatinib, nilotinib, and dasatinib equally as the first-line TKI for de novo CML-CP.20 However, in the recent National Comprehensive Cancer Network guideline (http://www.nccn.org/professionals/physician_gls/default.aspx#cml), 2G TKIs are preferred over imatinib for intermediate- and high-risk CML-CP to prevent disease progression.
In a previous retrospective study, Iriyama et al reported that DMR (BCR::ABL1 IS ≤ 0.0032%) rates by 6, 12, 18, and 24 months did not differ between nilotinib and dasatinib in Japanese patients with CML-CP (7%, 17%, 24%, and 28% with nilotinib; 3%, 16%, 25%, and 29% with dasatinib).29 However, it has not been determined which TKI is better in achieving DMR among patients with de novo CML-CP in a prospective randomized study. This study failed to conclude that nilotinib was superior to dasatinib in achieving DMR by 18 months. At this point, this was a negative study. However, we believe that the results of multiple secondary endpoints are noteworthy. The secondary end point results demonstrated that nilotinib and dasatinib are equally effective in achieving MR4.5 and CCyR, EMR, MMR, and MR4.0. In addition, the times to these responses were almost the same in both arms. The dose intensities are important to compare the efficacies and safeties between nilotinib and dasatinib. Regrettably, these data were not collected in this study. However, dose interruption and dose reduction was conducted according to the package leaflets of both drugs in this study. Therefore, we considered that our results would reflect the efficacies of both drugs in daily practice. Therefore, despite the limit of this study design, we suppose that nilotinib and dasatinib would be equally effective for patients with de novo CML-CP in terms of the efficacy. Nilotinib and dasatinib showed similar treatment efficacies in this study, although their off-target and AE profiles were different. These results suggest that if TKIs are selected with more careful consideration of the AEs and patient background, treatment efficacies may be better than achieved in this study, not to mention in the ENESTnd and DASISION studies.
In addition to ATP-competitive BCR::ABL1 inhibitors, an allosteric BCR::ABL1 inhibitor, asciminib, was shown to be superior to bosutinib as the third-line treatment for CML-CP.30 Furthermore, the efficacies of asciminib alone or in combination with other BCR::ABL1 inhibitors are now examined for patients with de novo CML-CP. Therefore, our study would provide useful information to select the first-line BCR::ABL1 inhibitor(s) as a single agent or in combination with asciminib.
The continuity of nilotinib (300 mg twice daily) until 5 years was 59.9% in the ENESTnd trial and that of dasatinib (100 mg once daily) was 61% in the DASISION trial. In this study, the continuity of nilotinib by 36 months was 65.6% and that of dasatinib was 63.9%, indicating that the continuities of both TKIs were almost equivalent. The most frequent reasons to discontinue TKI were intolerance but not resistance in both arms. Pleural effusion was a major AE that led to discontinuation of dasatinib and biochemical abnormalities were a major cause of intolerance to nilotinib. As for cardiovascular events, grade 3 angina was observed in 2 patients in the nilotinib arm and grade 3 pulmonary artery hypertension was observed in 1 patient in the dasatinib arm. These rates were lower than those observed in the other studies.8-11 This may be owing to the strict inclusion and exclusion criteria as related to the cardiovascular risks in this study and/or characteristics of Japanese patients who develop less cardiovascular occlusive diseases than European and American populations. However, our protocol was actually compiled in 2010 to 2011. At that time, only corrected QT interval (QTc) prolongation and peripheral arterial occlusive disease were known as major cardiovascular events related to nilotinib. In addition, pulmonary hypertension was not considered to be related to dasatinib. Although most of the doctors routinely monitored electrocardiogram, ultrasound cardiography (UCG), and ankle brachial index every 6 to 12 months according to the Japanese guideline for the treatment of CML-CP after recognizing their risks, these tests were not specified in our protocol. Because these tests were conducted only through the complaints of the patients, the frequencies of cardiovascular and pulmonary toxicities might be underestimated in our study.
Because nilotinib and dasatinib have divergent characteristics, their effects on anti-CML immunity, which is totally orchestrated by cytotoxic T lymphocytes, natural killer cells, regulatory T cells, and other factors, are different. Therefore, it remains to be determined which TKI is better to stop treatment for the patients with durable DMR. This study also aimed to accumulate patients with CML-CP who can be candidates for the following TKI stop studies. We are now conducting 2 stop studies, namely JALSG N-STOP216 and D-STOP216, which include patients with CML-CP with durable MR4.5 registered in this trial. These results, together with the results of this paper, will answer a clinical question about which TKI is better to achieve TFR for patients with de novo CML-CP.
Acknowledgment
The authors thank S. Amano at the Japan Adult Leukemia Study Group (JALSG) office for the assistance in conducting the clinical trial.
This study was supported by a grant from the Japan Agency for Medical Research and Development (AMED)(grants 15ck0106089h001,16ck0106189h0002 and 17ck0106189h0003).
This study was supported by the NEW TARGET study conducted by the Japanese Society of Hematology (JSH), a general incorporated association in Japan. This study was also supported by the JALSG and JALSG receives donations from a variety of pharmaceutical companies.
JSH and JALSG had no involvement with specific companies, especially those related to chronic myeloid leukemia.
Authorship
Contribution: I.M., S.O., Y.A., Y.M., N.T., C.N., N.I., K.F., K.K., Y.O., T.O., M.O., T.T., T.M., K.O., N.A., T.N., H.K., Y.Miyazaki. conceived and design the study; I.M., S.O., Y.A., M.K., Y.M., N.T., C.N., N.I., K.F., K.K., Y.O., T.O., M.O., T.T., T.M., K.O., E.S., S.F., Y.K., N.A collected and assembled the data; and all authors analyzed and interpreted the data, contributed to manuscript writing, approved the final manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: I.M. reports honoraria from the Japan Agency for Medical Research and Development (during the conduct of study), Sumitomo Pharma Co, Ltd, and Shionogi & Co, Ltd, Taiho Pharmaceutical Co Ltd, Nippon Shinyaku Co Ltd, Japan Blood Products Organization, Teijin Pharma Ltd, Nihon Pharmaceutical Co Ltd, Mundipharma Kabushiki Kaisha, Boehringer Ingelheim, AYUMI Pharmaceutical Corporation, Eli Lilly Japan Kabushiki Kaisha, Mitsubishi Tanabe Pharma Corporation, Nippon Kayaku Co Ltd, Bayer Yakuhin, Ltd, CSL Behring, and Nippon Boehringer Ingelheim Co Ltd; serves on the speakers bureau of Bristol Myers Squibb and Novartis Pharmaceuticals during the conduct of the study; reports honoraria from, and serves on the speakers bureau of Chugai Pharmaceutical Co Ltd, Kyowa Kirin Co Ltd, Takeda Pharmaceutical Company Limited, Asahi Kasei Pharma Corporation, Eisai Co Ltd, ONO PHARMACEUTICAL Co Ltd, AbbVie GK, Daiichi Sankyo, Ltd, SymBio Pharmaceuticals Limited, and Otsuka Pharmaceutical Co, Ltd; and serves on the speakers bureau of Astellas Pharma Inc and Janssen Pharmaceutical Kabushiki Kaisha, Pfizer Japan Inc, AstraZeneca, Sanofi Kabushiki Kaisha, Meiji Seika Pharma Co, Ltd, and Alexion Pharmaceuticals, Inc. Y.A. reports receiving consulting fees from JCR Pharmaceuticals Co Ltd and Kyowa Kirin Co Ltd; lecture fees from Otsuka Pharmaceutical Co Ltd, Chugai Pharmaceutical Co Ltd, Novartis Pharma Kabushiki Kaisha, and AbbVie GK; and honoraria from Meiji Seika Pharma Co Ltd, not related to the content of the manuscript. Y.M. reports receiving consulting fees from Takeda Pharmaceutical Co Ltd, BostonGene, and CIMIC; and research fundings from CIMIC, Ono, PREMIA, Bristol Myers Squibb, Novartis, Pfizer, Daiichi Sankyo, Otsuka Pharmaceutical, and Astellas. N.T. reports receiving research funding and honoraria from Novartis Pharma and Otsuka Pharmaceutical; research funding from Asahi Kasei Pharma Corporation; and honoraria from Pfizer. C.N. reports receiving honoraria from Pfizer, Fujimoto Pharmaceutical, Janssen Pharmaceutical, and Takeda Pharmaceutical. N.I. reports receiving honoraria from and serving on the speakers bureau of Bristol Myers Squibb and Novartis Pharma Kabushiki Kaisha K.F. reports receiving honoraria from Bristol Myers Squibb, Otsuka Pharmaceuticals, Novartis Pharma Kabushiki Kaisha, AbbVie, Pfizer, Janssen Pharmaceutical Kabushiki Kaisha, Nippon Shinyaku, Chugai Pharmaceutical Co Ltd, Meiji Seika Pharma Co Ltd, Takeda Pharmaceutical Co Ltd, and CSL Behring Kabushiki Kaisha T.O. reports receiving honoraria and research funding from Kyowa Kirin Co Ltd, Chugai Pharmaceutical Co Ltd, and ONO PHARMACEUTICAL Co Ltd; research funding from Taiho Pharmaceutical Co Ltd and Japan Blood Products Organization; honoraria from, and serving on the speakers bureau of Novartis Pharma Kabushiki Kaisha, Bristol Myers Squibb, Pfizer Japan lnc, and Otsuka Pharmaceutical Co Ltd; and honoraria from Takeda Pharmaceutical, Astellas Pharma lnc, Eisai Co, Ltd, Janssen Pharmaceutical Kabushiki Kaisha, Daiichi Sankyo, and Mundipharma Kabushiki Kaisha T.M. reports receiving honoraria and research funding from Kyowa Kirin Co, Ltd; research funding from Chugai Pharmaceutical Co Ltd; and honoraria from Takeda Pharmaceutical, Otsuka Pharmaceutical Co, Ltd, MSD Co, Ltd, Astellas Pharma lnc, Janssen Pharmaceutical Kabushiki Kaisha, and AbbVie Inc. E.S. reports serving on the speakers bureau of, and receiving consultancy fees and research funding from Bristol Myers Squibb; serving on the speakers bureau of and receiving consultancy fees from Novartis Pharma Kabushiki Kaisha and Pfizer Japan lnc; receiving consultancy fees from Otsuka Pharmaceutical Co Ltd; and serving on the speakers bureau of Takeda Pharmaceutical, Janssen Pharmaceutical Kabushiki Kaisha, and Sanofi Kabushiki Kaisha S.F. reports serving on the speakers bureaus of and receiving research funding from Otsuka Pharmaceutical, Chugai Pharmaceutical, and Asahi Kasei; receiving research funding from Shionogi and Kyowa Kirin, and Daiichi Sankyo, Amgen Kabushiki Kaisha; and serving on the speakers bureaus of Novartis, Pfizer, Bristol Myers Squibb, Nipppon Shinyaku, MSD, Takeda, Sanofi, Janssen, AstraZeneca, AbbVie Inc, Meiji Seika Pharma, and CSL Behring Kabushiki Kaisha Y.K. reports receiving consultancy fees from Special Reference Laboratory. N.A. reports receiving consultancy fees from Nippon Shinyaku Co Ltd and Novartis Pharmaceuticals Co Ltd. T.N. reports receiving consultancy fees from Nippon Shinyaku and Astellas Pharma; and honoraria from Bristol Myers Squibb, Pfizer, and Sysmex. H.K. reports receiving research funding from Fujifilm, Kyowa Kirin, Bristol Myers Squibb, Otsuka, Perseus Proteomics, Daiichi Sankyo, CURED; honoraria and research funding from AbbVie, Astellas Pharma, Chugai, research funding from Zenyaku Kogyo, Nippon Shinyaku, Eisai, Takeda, Sumitomo Pharma, and Sanofi; and honoraria from Novartis. Y.Miyazaki. reports receiving research funding from Chugai Pharma; and honoraria from Nippon Shinyaku, Bristol Myers Squib, Novartis, Sumitomo Pharma, Kyowa Kirin, AbbVie, Daiichi Sankyo, Takeda, Janssen Pharmaceutical, Astellas, Pfizer, Chugai, and Otsuka Pharmaceutical. The remaining authors declare no competing financial interests.
The current affiliation for Y.K. is Nadogaya Hospital, Chiba, Japan.
A complete list of the members of the Japan Adult Leukemia Study Group appears in the supplemental Appendix
Correspondence: Itaru Matsumura, Department of Hematology and Rheumatology, Kindai University Faculty of Medicine, 377-2, Ohno-Higashi, Osaka-Sayama, Osaka 589-8511, Japan; email: imatsumura@med.kindai.ac.jp.
References
Author notes
Presented orally at the 62th American Society of Hematology Annual Meeting and Exposition, San Diego, CA, 5 to 8 December 2020 (available at https://doi.org/10.1182/blood-2020-134168).
The data that support the findings of this study are available upon reasonable request. Individual participant data consisting of the results in this article will be shared after deidentification, if proposals are approved by the investigators in the Japan Adult Leukemia Study Group . The request should be sent to the corresponding author, Itaru Matsumura (imatsumura@med.kindai.ac.jp).
The full-text version of this article contains a data supplement.