Key Points
The most commonly prescribed contraceptives in publicly insured patients with SCD are combination pills and depot medroxyprogesterone acetate.
No significant differences were found in TE rates in patients with SCD prescribed combined hormonal vs POCs.
Abstract
Patients with sickle cell disease (SCD) are at a risk of thromboembolism (TE), and use of hormonal contraception can further increase that risk. This study aims to assess patterns of hormonal contraceptive use and compare risk of contraception-related TE between combined hormonal contraceptives (CHCs) and progestin-only contraceptives (POCs). Patients with SCD aged between 12 and 44 years with a new prescription of a hormonal contraceptive in the Centers for Medicare and Medicaid Services Medicaid Analytic eXtract database (2006-2018) were followed up to 1 year. We identified 7173 new users: 44.6% initiated CHC and 55.4% initiated POC. Combined oral contraceptive pills (OCPs; 36.5%) and progestin-only depot medroxyprogesterone acetate (33.9%) were the most frequently prescribed agents. A total of 1.8% of contraception users had a new diagnosis of TE within 1 year of the first identified contraception prescription. There were no significant differences in TE event rates between CHC and POC users (17.2 and 24.7 events per 1000 person-years, respectively). In patients prescribed OCP, there were no differences in TE event rates based on estrogen dose or progestin generation. Transdermal patch had a 2.4-fold increased risk of TE as compared with that of OCP. Although limited by the retrospective study design and use of administrative claims data, this study found no significant differences in TE rates between new users of CHC and POC in patients with SCD. Careful evaluation of underlying TE risk factors should be considered for each patient with SCD before initiation of hormonal contraception.
Introduction
Sickle cell disease (SCD) is the most common, serious inherited blood disorder in the United States, affecting ∼100 000 individuals.1 With the advent of vaccinations, prophylactic penicillin, and disease remitting therapies such as hydroxyurea, most patients with SCD survive into adulthood. This improvement in survival creates the need to address many long-standing questions about the reproductive health of patients with SCD.2
Pregnancy in patients with SCD is associated with an increased frequency of adverse health events,3 including a several-fold increase in risk of severe maternal morbidity4,5 and mortality.4,6,7 Maternal and perinatal complications in patients with SCD are higher than in those without SCD, even when controlling for race.4,8,9 Patients with SCD also have high rates of unplanned pregnancy.10,11 Given the high unplanned pregnancy rates and increased maternal and fetal risks in pregnancy, patients with SCD require broad access to safe and highly effective contraceptive methods.
Although large-scale epidemiologic studies of contraception use in patients with SCD are lacking, several reports from different countries suggest variable use of hormonal contraception in individuals with SCD.11-15 Moreover, there is variability in provider preferences and practices related to contraception counseling for patients with SCD.16 The paucity of safety data for hormonal contraceptives in patients with SCD likely limits the providers’ ability to make informed recommendations and patients’ ability to make informed decisions.
The primary safety concern is that hormonal contraceptives carry a known risk of thromboembolism (TE) due to the prothrombotic effects of estrogen.17 One meta-analysis reported a pooled relative risk of 3.5 (95% confidence interval [CI], 2.9-4.3) for venous thromboembolism (VTE) with the use of combined oral contraceptives compared with nonuse in healthy patients.18 Use of these agents in patients with SCD, an inherently thrombophilic disease state,19 may further increase the TE overall risk. Progestin-only contraceptives (POCs) are generally considered safe for patients with SCD. A meta-analysis of contraceptive users among patients with SCD by Haddad et al20 reported no increased adverse events and a decreased risk of painful episodes and improved hematologic parameters among POC users. Limited data were available related to the safety of combined hormonal contraceptives (CHCs), including a lack of data on TE, leading to insufficient evidence to make definite recommendations.
According to the US Medical Eligibility Criteria (MEC) 2016,21 there are no restrictions on the use of POCs for patients with SCD. CHCs, which contain both estrogen and progestin, are also considered safe for use because the advantages generally outweigh the theoretical or proven risks. These recommendations were based on limited safety data such as the lack of adverse effects of POCs on hematologic parameters and their benefit in alleviating clinical symptoms associated with SCD. The National Heart Lung and Blood Institute of the United States consensus–adapted recommendations support these MEC recommendations.22 However, a recent meta-analysis showed that not all POCs may be completely free of TE risk.23 In the general population, injectable POC users had a pooled relative VTE risk of 2.62 (95% CI, 1.74-3.94) as compared with nonusers of contraception based on the 3 case-control studies included in the analysis. Furthermore, there is little information available regarding the safety of POC use in patients with medical complexity, such as those with SCD who have an inherent and coexisting TE risk factor from their disease. The possible risk of TE with injectable POC depot medroxyprogesterone acetate (DMPA) is particularly important to study because this agent is commonly prescribed to individuals with SCD.11,15,24,25
This study used population-level administrative claims data for publicly insured patients to assess patterns of hormonal contraception use among patients with SCD and evaluate the risk of TE during the first year after initial contraception prescription. Our primary hypothesis was that rates of TE would be higher in patients with SCD who were prescribed CHC than in those who were prescribed POC.
Study design and methods
Data source
We obtained data using the Centers for Medicare and Medicaid Services Medicaid Analytic eXtract (CMS MAX) and Transformed Analytic Files (TAF) data systems from 2006 to 2018. CMS MAX and TAF contain data from Medicaid enrollees in each state program. Data include inpatient and outpatient diagnoses, pharmacy claims (National Drug Codes), coverage dates, and demographic information of Medicaid enrollees. Each patient was assigned a unique eligible identification number, which allowed for the linkage of all inpatient, outpatient, and pharmacy records over the duration of the study period. The protocol was reviewed by the institutional review board at Nationwide Children’s Hospital and was deemed to meet the federal criteria for research exempt from full institutional review board review. It was conducted according to the Declaration of Helsinki. Data were analyzed by statisticians (J.R.S. and S.K.V.), and J.R.S. had access to the primary data source. Information regarding diagnoses and/or procedures was obtained using International Classification of Diseases (ICD) 9 and 10; Current Procedural Terminology and Healthcare Common Procedure Coding System codes are available as supplemental Table 1.
Study population
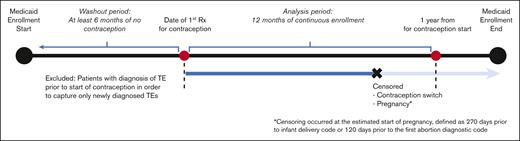
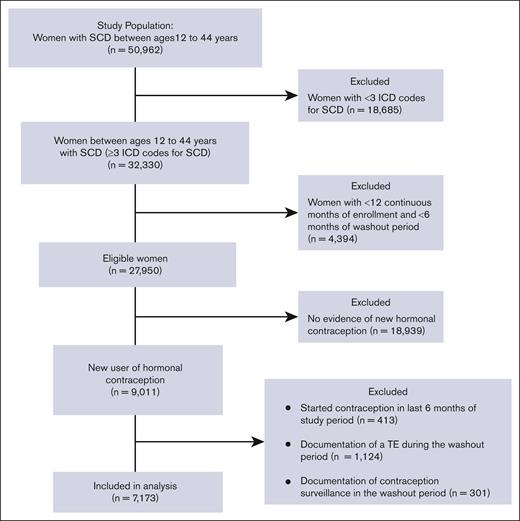
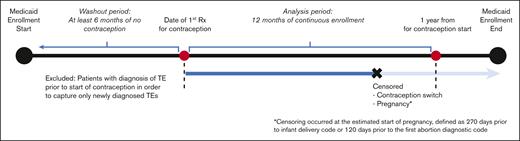
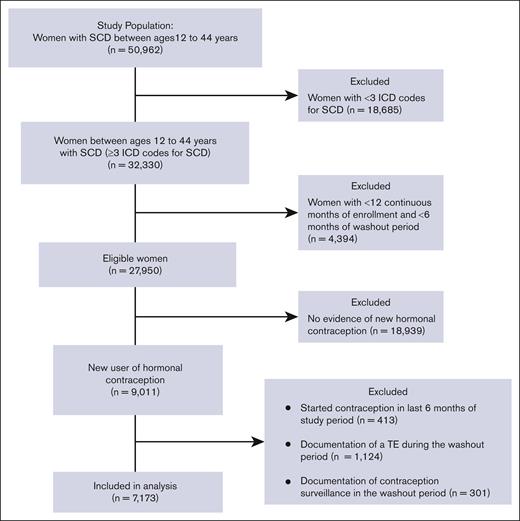
The study included patients with SCD of reproductive age between 12 and 44 years. SCD was defined as the presence of ≥3 ICD-9 or ICD-10 codes consistent with SCD, an algorithm that has been shown to be highly sensitive for identifying individuals with SCD.26 SCD genotype was identified with ICD codes (an individual’s genotype was assigned as the most commonly used genotype for each patient). If a patient had 2 different genotypes used equally often, they were categorized as having nonspecific SCD. To meet the eligibility criteria of a new contraception user, patients should have had (i) a prescription for a contraceptive agent, (ii) 12 continuous months of Medicaid enrollment (analysis period), and (iii) ≥6 months of a washout period before the initiation of the contraceptive (Figure 1). During the washout period, patients with any prescription of a hormonal contraceptive agent, any diagnosis code for surveillance of contraception, or procedure code for removal of intrauterine device (IUD) or implant were excluded from the analysis in order to capture only new users of hormonal contraception. Furthermore, patients with any diagnostic codes for TE during the washout period were also excluded from the analysis.
Study population, including eligibility criteria for enrollment and follow-up.
The patients were followed up for a period of 1 year after contraceptive initiation because risk of contraception-related TE is highest in the first year of use, in particular, the first 3 to 6 months.27 Patients were censored before the 1-year follow-up period in the event of contraceptive switch (described below). Because pregnancy and postpartum states are risk factors for TE, data of patients were censored from analysis during those timeframes to avoid confounding with TE risk of hormonal contraception (Figure 1).28 Patients in the CMS MAX data set could have had multiple eligible periods for observation because of changes or lapses in health insurance coverage. For those with multiple eligible periods, only the first timeframe meeting the aforementioned criteria was included in the analysis.
Hormonal contraception
Hormonal contraception was identified using pharmacy claims and procedure codes. Patients were stratified into 2 categories based on type of hormonal contraception prescribed. CHC included combined oral contraceptive pill (OCP), transdermal patch, and vaginal ring. POC included progestin-only pill (POP), injectable (DMPA), subdermal implant, or IUD. POP-containing oral medroxyprogesterone was not included. Each CHC user was further classified based on the type of progestin (new generation progestins such as dienogest, drospirenone, desogestrel, and norgestimate vs other progestins such as levonorgestrel, norethindrone, ethynodiol, and norethynodrel) and amount of estrogen present (ultralow dose, <30 μg; low dose, 30 to <50 μg; and high dose, ≥50 μg) because these have been noted to affect the risk of TE.29
Data of patients were censored in the event of contraceptive switch during the study period, that is, if a different prescription for either oral contraceptives, patch, vaginal ring, or DMPA occurred during the enrollment period. For methods that are primarily coded as procedures (IUD and subdermal implants), we calculated the duration of use through placement, surveillance, and removal procedure codes. If no removal codes were detected after IUD or implant placement, then we assumed that the contraceptive remained in place throughout the 12-month follow-up timeframe (Figure 1).
TE events
TE events were identified using ICD-9 and ICD-10 diagnostic codes for VTE, which included deep vein thrombosis (DVT) and pulmonary embolism (PE), and arterial events, such as stroke and myocardial infarction (MI). The primary outcome of TE event rates was measured from the time of initiation of hormonal contraception to the first TE event identified during the study period. To qualify as a contraception-related TE, the TE should have been diagnosed between the time of initial CHC and POC prescription to 1-year follow-up or censoring. Patients with evidence of TE before starting the use of contraception (during the washout period) were excluded from the study because of inability to differentiate a new contraception-related TE event from past history of TE. To strengthen the confidence that the TEs were newly diagnosed, venous events were required to have ≥2 inpatient or outpatient claims with an ICD diagnostic code for VTE. Arterial events were identified as the presence of ≥1 inpatient claim with an ICD code for MI or stroke.
Statistical analysis
Descriptive statistics were calculated based on exposure, with individual patients contributing varying observation time (person-years) to each exposure. As required for CMS MAX/TAF data, if the number of observations was ≤10 in any category, we did not report exact frequencies to protect patient confidentiality.
Univariable comparisons of demographic and clinical characteristics between the CHC and POC groups were completed using nonparametric methods, such as χ2 or Wilcoxon rank-sum tests. For the primary analysis of estimating risk of TE by exposure to a contraceptive, we used Cox regression analyses and presented results as hazard ratios (HRs) and corresponding 95% CIs. With time-to-first TE as the dependent variable, TE risk analyses included covariates for SCD-specific and general thrombotic risk factors. SCD-specific factors included SCD severity and SCD genotype. Severe SCD was defined as per prior clinical trials as ≥3 inpatient admissions during a 1-year time period,30,31 which we designated as 6 months before and after the start of contraception. General TE risk factors included the age at initiation of contraception, obesity, smoking, diabetes, hypertension, and malignancies, excluding dermal cancer, which were identified using ICD-9 or 10 diagnostic codes.
Interaction between contraceptive exposure and severity of SCD was evaluated in each model. A significance level of 0.05 for the interaction term and biologic rationale determined whether models stratified according to severity were calculated. All HRs were adjusted for the aforementioned covariates of interest; however, if a covariate was rare and had frequencies that were at or near zero on a given subanalysis, the covariate was not included in the multivariable model. TE event rates were presented per 1000 person-years, with corresponding 95% CIs calculated using Poisson regression models. All P values were 2-sided, and those <.05 were considered statistically significant. We used SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
The data set provided by CMS MAX/TAF included 32 330 patients who had ≥3 ICD codes for SCD, with 7173 of these patients having a new prescription for hormonal contraception and meeting the study eligibility criteria (Figure 2). Demographic and clinical characteristics of the 2 groups of hormonal contraceptive users are summarized in Table 1. The majority of patients in both groups were Black and non-Hispanic and aged <35 years at the time of contraception initiation. Nearly two-thirds of the eligible patients in both groups were categorized as having hemoglobin (Hb) SS genotype, and the majority had ≥3 years of continuous enrollment and ≥ 2 years of washout period in the CMS MAX/TAF database. POC users had significantly longer duration of enrollment than CHC users (P < .0001). POC users were more likely to be older, have severe SCD, and have a higher number of SCD claims than CHC users. Smoking and hypertension were also more prevalent in POC users than in CHC users (Table 1).
Patterns of hormonal contraceptive use in patients with SCD
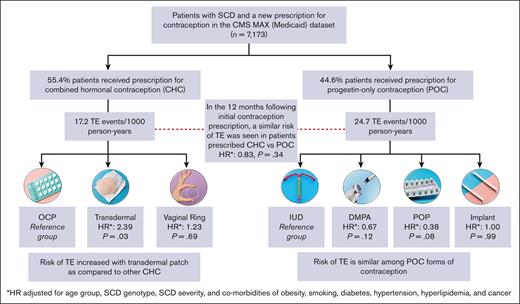
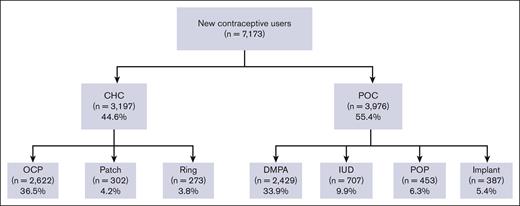
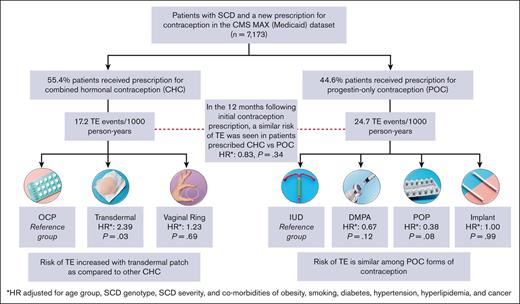
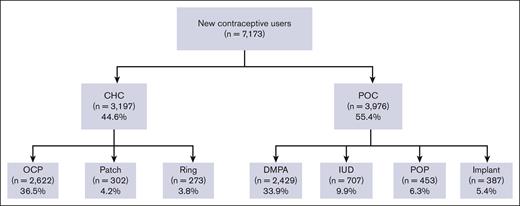
Among the patients who were prescribed a new hormonal contraceptive agent (n = 7173), 44.6% were prescribed CHC and 55.4% were prescribed POC (Figure 3). The most frequently prescribed contraceptive was combined OCP (36.6%; n = 2622), followed by DMPA (33.9%; n = 2429). Long-acting reversible contraceptives (LARCs), such as IUD and implants were prescribed to 9.9% and 5.4% of the patients, respectively.
TE events
A total of 126 patients (1.8%) who had a new prescription for a hormonal contraceptive developed a TE event within the first year of use of the agent. The most common TE event was PE (49.2%; n = 62), followed by DVT (40.5%; n = 51). Arterial events were found in <10 patients, and <10 patients had multiple TE types. The TE event rate was 21 events per 1000 person-years, and the median time to TE from the initiation of contraception was 170 days (interquartile range [IQR], 70-257 days). The median (IQR) age of patients who developed a new TE event was 25.4 years (IQR, 22.0-29.8 years). Table 2 compares new users of contraception between patients with and without TE. Those with TE were older, had more SCD claims, and were more likely to have severe SCD, obesity, diabetes, and hypertension. In a subanalysis of patients with severe SCD, we found that the rates of TE among patients with severe SCD were much higher than the rates of TE among those without severe SCD in both contraception groups. However, the HR for CHC vs POC showed no significant differences in TE rates for either patients with or without severe SCD (supplemental Tables 2-4).
The TE event rate was 17.2 events per 1000 person-years for CHC users and 24.7 events per 1000 person-years for POC users (unadjusted HR, 0.70; 95% CI, 0.48-1.00; P = .051). After adjustment of covariates, no statistically significant differences were observed in the TE event rate between CHC and POC users (HR, 0.83; 95% CI, 0.58-1.21; P = .336). In the 2 most commonly prescribed hormonal contraceptives, we found no significant differences in TE event rates between OCP and DMPA users (HR, 0.84; 95% CI, 0.53-1.32; P = .446). Interaction of the contraception type with disease severity was considered for multivariable Cox models, but the interaction term was not significant and, ultimately, not included in the final analysis.
TE event rates were then compared across different formulations of CHC and POC (Table 3). Users of high-dose estrogen (n < 10) were excluded from this subanalysis. In the CHC formulation group, transdermal patch was associated with a significantly higher risk of TE than the combined OCP (adjusted HR, 2.39; 95% CI, 1.09-5.24; P = .031), whereas there were no significant differences in TE rates between vaginal ring and combined OCP (adjusted HR, 1.23; 95% CI, 0.43-3.51; P = .694). In the POC formulation group, IUD was used a reference group because it has no increased risk of TE as compared with nonusers of hormonal contraception in the general population.32,33 There were no significant differences between adjusted rates of TE among DMPA, POP, and implant users as compared with those among IUD users (Table 3). Even when compared to POP, DMPA use was not significantly associated with an increased risk of TE in our study cohort (adjusted HR, 1.74; 95% CI, 0.62-4.88; P = .295). Models of risk factors for TE among different contraception groups are available in supplemental Tables 5-7. HRs are estimates of risk of TE as compared with those without TE.
Finally, rates of TE were compared based on estrogen dose and generation of progestin in users of combined OCP (Table 4). After excluding patients who had prescriptions for >1 dose level of estrogen during the analysis period (n = 105) and those with prescription for high-dose estrogen (n < 10), there were no significant differences in TE event rates between low-dose and ultra-low-dose OCP (adjusted HR, 0.76; 95% CI, 0.36-1.60; P = .472). Similarly, after excluding patients who had prescriptions for both new and other generation progestins during the analysis period (n = 69), the subanalysis in the combined OCP users demonstrated no significant differences in TE event rates based on progestin generation (adjusted HR, 1.05; 95% CI, 0.52-2.13; P = .895).
Discussion
In this national cohort of publicly insured patients with SCD, we identified that although POCs were more frequently prescribed than CHCs overall, among new users, the most frequently used hormonal contraceptive agent was combined OCP (36.6%), closely followed by DMPA (33.9%). LARCs such as IUD and implant were less frequently prescribed (15.3%). Nearly 2% of new users of hormonal contraception had a new diagnostic code of TE in the first year of use, primarily venous events such as PE and DVT. No statistically significant differences were found between TE event rates of CHC and POC users even after adjustment for covariates of interest. Use of transdermal contraceptive patch was associated with a 2.4-fold increased risk of TE as compared with combined OCP. DMPA was not associated with a significant increased risk of TE as compared with IUD.
The use of combined oral contraceptives has been associated with a threefold to sixfold increased risk of VTE as compared with that in nonusers.27,29 This translates into a low absolute risk of 0.1 to 0.3 events per 1000 person-years34,35 in the general population. In this study, the TE event rate including both arterial and venous TE events in patients with SCD during the first year of use of hormonal contraception was 21 events per 1000 person-years. Although the above rates cannot be directly compared with those of our study population because of differences in methodology and patient selection, it appears that the rates of TE in new users of hormonal contraception in patients with SCD are higher than the published rates of TE in the general population of hormonal contraception users.
Although, overall, the study found no increased risk of TE associated with the use of CHC when compared with that associated with the use of POC, patients using POC had increased TE risk factors, which could bias the results of not being able to find a difference in TE rates. In addition, only 15% of our cohort had severe SCD, defined as ≥3 admissions in 1 year, which is less than reported rates in the literature.36 Although limitations in our data source prohibit us from comparing CHCs with POCs in cohorts with balanced baseline TE risk, we attempted to address this limitation by performing a subanalysis of those with severe SCD. Rates of TE in patients with severe SCD using CHC vs POC showed no significant differences. Because of the limitations in the definition of disease severity and other potentially unmeasured differences in baseline risk between the CHC and POC groups, we still advise clinical judgment when considering CHC for patients with multiple risk factors for TE. Moreover, the US MEC cautions against the use of CHC in patients with nephropathy, retinopathy, or neuropathy secondary to endocrine disorders, such as diabetes.21 Although these recommendations are not specific to patients with SCD, the aforementioned complications are likely to occur in patients with SCD secondary to end-organ damage, and the use of CHC should be assessed based on the severity of the condition.
The use of estrogen-containing transdermal patch as a contraceptive has been associated with a twofold increased risk of VTE as compared with that among combined OCP users in the general population37,38 and among women with diabetes.39 The increased risk of thrombosis is attributed to the higher total estrogen exposure in transdermal as compared to oral delivery.40 Our study found transdermal patch users to have a 2.4-fold increased risk of TE as compared with combined OCP users. There was no statistically significant increased risk of TE in DMPA users as compared with that in IUD users, which is different from previous studies in the general population demonstrating an increased risk of VTE in users of injectable POC as compared with that in nonusers, which were limited by a small sample size or retrospective design.33,41,42 Furthermore, we also did not find any significant differences in TE event rates between DMPA and POP users in our study cohort. Although the use of DMPA in patients with SCD is reassuring with respect to the risk of TE, its exposure has been associated with the loss of bone mineral density,43,44 which may be concerning in patients with SCD who are already at risk of low bone mineral density.45,46 This warrants further investigation of the risk-benefit profile of DMPA in patients with SCD, given its impact on bone health, and must be weighed against potential benefits on frequency of vaso-occlusive pain crises.
Contraception care is sought in patients with SCD not only for the purpose of prevention of pregnancy but also for menstrual suppression to reduce acute pain crises triggered by menses.2 Hormonal contraceptive uptake has been reported to be <50% in patients with SCD across several single-center cross-sectional studies.12,14,15 Although DMPA has been reported to be the most frequently used hormonal contraceptive agent in patients with SCD,11,15,25 our study, although limited to new prescriptions, found a slightly greater percentage of patients who were prescribed combined OCP than of those who were prescribed DMPA. This is similar to the results of the cross-sectional, survey-based single-center study conducted by Pecker et al,14 in which OCP and DMPA ever being used were reported in 46% and 44% of patients, respectively. The rate of LARC use was lower than the rates demonstrated in the study by Pecker et al14 (15.3% vs 22%, respectively) but similar to the rates of LARC used by the general population in the United States, ranging between 10.4% and 14%.47,48 Because our study only captured new users of contraception, as evidenced by the first prescription of a particular agent, and often patients do not choose upfront use of LARC, the use of these agents might have been underestimated.
Limitations
The use of administrative claims data relies on coding and documentation by providers, which is variable. The use of ICD codes for the identification of SCD genotype has been shown to have low accuracy,49,50 and information related to SCD genotype needs further validation. Our methodology might have led some patients with less severe genotypes to be misclassified as having HbSS. Despite this bias, we still identified an increased risk of TE in patients labeled as having HbSS. Our analysis focused on chronic risk factors of TE, such as patient age, genotype, SCD severity, and comorbidities rather than acute events, such as hospitalizations, trauma, immobility, or recent surgery. We did find that 55% of the patients with TE had at least 1 inpatient claim for hospitalization within 90 days of the event, demonstrating the importance of prospective observational studies that can more accurately capture details of acute TE risk factors in the setting of hormonal contraceptive use. In addition, risk factors such as smoking and obesity are typically underreported in administrative claims studies.51,52 Presence of central venous lines and its risk with TE could not be assessed because of limitations of ICD coding system for identification of central lines.
Because of the high turnover rate of Medicaid enrollees and our eligibility criteria requiring 12 continuous months of enrollment, we could potentially underestimate rates of new TE events. In contrast, given the limitations of our data source, we did not require pharmacy claims for anticoagulant use in our definition of a new TE, which might have led to overidentification of TE events. Those who had previous history of TE before Medicaid enrollment, which could influence a provider’s choice of contraception prescription, could not be excluded, although we did require a minimum of 6-month washout period without TE or contraception use before initial contraceptive prescription. Moreover, adherence to agents based on prescription fills would not be known. Because pharmacy data are applicable to the short-acting agents as opposed to the LARCs, this limitation could bias our results toward not finding an increased risk of TE with estrogen compared with that with progestin.
There is large statewide variability in medication reporting, including missing data in “days supplied” and “units dispensed” fields in pharmacy claim data in CMS MAX. State-by-state variations in reporting these data further reduces the quality of prescription related data. Medications from homecare pharmacies, Title X clinics, such as Planned Parenthood, or prescriptions paid by cash, at no cost, or those under $4 could not be captured. Given these limitations, we did not require pharmacy claims for anticoagulant use in our definition of a new TE event, which might have led to the overidentification of TE events using ICD diagnostic codes without additional anticoagulation prescription used in other administrative data set studies. To mitigate this bias, we used 2 ICD codes for VTE instead of just 1 code, and for arterial TE events, such as stroke and MI, which unanimously will require an inpatient admission, the use of 1 ICD code along with evidence of hospital admission was considered sufficient for identification. Duration of exposure to hormonal contraception, such as oral pills, transdermal patch, and vaginal ring, were likely overestimated because we presumed the exposure for all patients to be 1 year from initial prescription of a new hormonal contraceptive agent, unless a documented switch in contraception type or pregnancy occurred. Moreover, distinction between hormonal and nonhormonal IUD could not be made based on procedure codes used to identify IUD use. Lastly, although the majority of patients with SCD are covered by public insurance,53,54 the results of this study are limited to those under Medicaid insurance and may not be applicable to those with private insurance.
Conclusions
Despite these limitations, the study helps elucidate patterns of hormonal contraception prescriptions and the risks of contraception-related TE in publicly insured patients with SCD in the US. The use of transdermal patch was associated with a 2.4-fold increased risk of TE as compared with that of OCP; however, overall, no significant differences were found in TE event rates on comparing all CHC users with all POC users. These data empower patients with SCD to make the appropriate choices with evidence-based information and help physicians guide prescription practices. Appropriate knowledge, access, and use of contraceptive care in patients with SCD can not only prevent unintended pregnancies but also give opportunities for preconception counseling and safety planning for pregnancies in patients with SCD. Future directions include exploration of additional factors informing the risks and benefits of contraceptive choices in patients with SCD, specifically better understanding of the impact of CHC and POC hormonal contraception on bone density and menses-related vaso-occlusive crises.
Acknowledgment
This study was supported by research funding from the National Heart, Lung, and Blood Institute at the National Institutes of Health (R21 HL 150487; S.H.O.).
Authorship
Contribution: N.S.B. participated in study design and data interpretation, and wrote the manuscript; S.H.O. conceptualized and designed the study, interpreted data, and wrote the manuscript; J.R.S. had full access to the data set and analyzed data in addition to editing and reviewing the manuscript; S.K.V. assisted in study design, analyzed the data, and critically reviewed the manuscript; and S.E.C., R.M.C., A.H.R., and W.X. assisted in study design and critically reviewed the manuscript.
Conflict-of-interest disclosure: S.H.O. serves as a consultant for Pharmacosmos, AstraZeneca, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Sarah H. O’Brien, Division of Pediatric Hematology/Oncology/BMT, Nationwide Children's Hospital and The Ohio State University, College of Medicine, 700 Children’s Dr, Columbus, OH 43205; e-mail: sarah.obrien@nationwidechildrens.org.
References
Author notes
Presented in abstract form at the 64th American Society of Hematology Annual Meeting and Exposition, New Orleans, LA, 12 December 2022.
The data source for this proposal was the Centers for Medicare and Medicaid Services Medicaid Analytic eXtract data system. This data source is proprietary. The investigative team only had access to the data during their National Institutes of Health R21 award period and will not have permission to share raw data with others. Investigators interested in using CMS data are encouraged to contact the CMS Research Data Assistance Center at https://resdac.org/.
The full-text version of this article contains a data supplement.