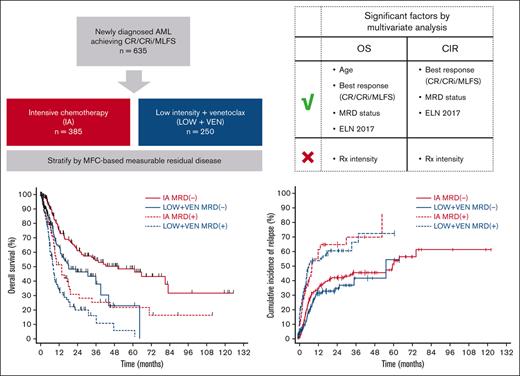
Key Points
Using multivariate analysis, treatment intensity was not significantly associated with OS or CIR in patients with AML responding to therapy.
Achievement of MRD− CR should be considered a key objective in AML therapy.
Abstract
Acute myeloid leukemia (AML) can be treated with either high- or low-intensity regimens. Highly sensitive assays for measurable residual disease (MRD) now allow for a more precise assessment of response quality. We hypothesized that treatment (Rx) intensity may not be a key predictor of outcomes, assuming that an optimal response to therapy is achieved. We performed a single-center retrospective study including 635 patients with newly diagnosed AML responding to either intensive cytarabine/anthracycline-based chemotherapy (IA; n = 385) or low-intensity venetoclax-based regimens (LOW + VEN; n = 250) and who had adequate flow cytometry–based MRD testing performed at the time of best response. The median overall survival (OS) was 50.2, 18.2, 13.6, and 8.1 months for the IA MRD−, LOW + VEN MRD−, IA MRD+, and LOW + VEN MRD+ cohorts, respectively. The 2-year cumulative incidence of relapse (CIR) was 41.1%, 33.5%, 64.2%, and 59.9% for the IA MRD−, LOW + VEN MRD−, IA MRD+, and LOW + VEN MRD+ cohorts, respectively. The CIR was similar between patients within MRD categories irrespective of the treatment regimen received. The IA cohort was enriched for younger patients and favorable AML cytogenetic/molecular categories. Using multivariate analysis, age, best response (complete remission [CR]/CR with incomplete hematologic recovery/morphologic leukemia-free state), MRD status, and European LeukemiaNet (ELN) 2017 risk remained significantly associated with OS, whereas best response, MRD status, and ELN 2017 risk were significantly associated with CIR. Treatment intensity was not significantly associated with either OS or CIR. Achievement of MRD− CR should be the key objective of AML therapy in both high- and low-intensity treatment regimens.
Introduction
Standard therapy for acute myeloid leukemia (AML) has long consisted of intensive chemotherapy based on a combination of cytarabine and an anthracycline. Since the original description of the 7 + 3 regimen1 (7 days of a continuous infusion of cytarabine plus 3 days of daunorubicin) in 1973, investigators have developed regimens incorporating upfront high-dose cytarabine, such as FLAG-Ida (fludarabine, high-dose cytarabine, granulocyte colony–stimulating factor, and idarubicin) and CLIA (cladribine, idarubicin, and high-dose cytarabine).2,3 However, because the median age at diagnosis in AML is 68 years,4 many patients are deemed ineligible for such intensive approaches because of age or comorbidities. This has prompted the development of low-intensity therapies for AML. Initial low-intensity regimens consisting of low-dose cytarabine (LDAC) or hypomethylating agent (HMA) monotherapy had limited efficacy.5-7 The addition of the oral BCL-2 inhibitor venetoclax (VEN) to low-intensity backbones has revolutionized the treatment of patients with AML who are older and/or unfit. In the phase 3 VIALE-A trial, VEN plus azacitidine was compared with azacitidine alone and showed an improvement in composite complete remission (CR) rates and overall survival (OS), defining a new standard of care for patients ineligible for intensive chemotherapy.8 VEN has also been combined with backbones based on LDAC and a triple nucleoside combination of cladribine, LDAC, and an HMA.9,10
Low-intensity VEN-based combinations are highly effective in inducing remissions, which can also be durable in many patients. However, they have not been directly compared with intensive chemotherapy regimens in prospective, randomized studies. In addition, in retrospective studies, the patient population treated with low-intensity therapy is generally older and enriched for AML with adverse genetic features, making comparisons of efficacy difficult between intensive and low-intensity regimens. Conceivably, many patients with AML are eligible for both intensive and low-intensity approaches (such as older patients aged <75 years without substantial comorbidities). In these cases, the optimal choice of therapy is controversial.11 Intensive approaches have been considered to be more effective but carry more toxicity, with the converse being applicable with low-intensity regimens. We hypothesized that treatment intensity may not be a determinant factor in patient outcome assuming achievement of an optimal response to therapy. In other words, in the context of AML therapy, the destination may matter more than the journey.
Traditional morphologic response assessment in AML lacks sensitivity. A patient in morphologic CR may still harbor up to a billion leukemic cells.12 A group of highly sensitive laboratory techniques capable of detecting the remaining submicroscopic disease, referred to as measurable residual disease (MRD), now allow for the detection of as little as ∼0.1% to 0.01% AML blasts, depending on the method, with some molecular techniques even being sensitive at the 10−6 level.13,14 Detectable MRD has been established as a strong and independent prognostic factor predicting worse OS and an increased risk of relapse.15-17 Although most of the evidence for the prognostic value of MRD is based on studies of intensive chemotherapy, a growing body of literature supports its value for patients treated with lower intensity regimens.18-20 The most widely applicable MRD assessment method in AML is multiparameter flow cytometry (MFC), relying on aberrant cell surface protein expression to identify and quantify AML blasts.21
In order to assess the relative contributions of treatment intensity and MRD response to outcomes, we conducted a retrospective, single-center study of patients with newly diagnosed AML responding to either intermediate to high-dose cytarabine-based intensive regimens or VEN-based low-intensity frontline regimens. We show that treatment intensity was not significantly associated with either OS or cumulative incidence (CI) of relapse (CIR) in a multivariate analysis considering factors such as response quality, MRD status, and cytogenetic/molecular risk categories. Assuming an optimal response is achieved, this suggests that outcomes are similar whether intensive or low-intensity approaches were used to achieve this state.
Methods
Study design and patients
This was a retrospective single-center study conducted at The University of Texas MD Anderson Cancer Center. We identified all patients with newly diagnosed AML who had achieved a response (CR, CR with incomplete hematologic recovery [CRi], or morphologic leukemia-free state [MLFS]) after either intensive chemotherapy regimens based on intermediate to high-dose cytarabine plus an anthracycline (IA cohort) or after low-intensity therapy based on a LDAC or HMA backbone plus VEN (LOW + VEN cohort). Patients who were nonresponding were excluded. Patients treated with additional investigational or targeted agents were included within the IA or LOW + VEN cohorts as per their backbone regimens, except for IA plus VEN, which was excluded, given the limited follow-up and experience with this specific combination. Patients with antecedent myelodysplastic syndrome were included only if they were not previously treated with an HMA.
Because MFC-based MRD was a key factor in our analysis, we excluded patients who lacked adequate MRD testing via MFC. Patients with a diagnosis of acute promyelocytic leukemia (APL) or core-binding factor (CBF) AML [AML with t(8;21)(q22;q22.1), RUNX1::RUNX1T1; or inv(16)(p13.1q22), CBFB::MYH11] were also excluded because MRD for these AML subtypes is tracked using quantitative polymerase chain reaction, as opposed to MFC, at our institution.
Baseline clinical, cytogenetic, and molecular characteristics were collected for all patients. Molecular profiling was performed using a next-generation sequencing panel interrogating hotspot or entire exonic regions of recurrently mutated genes in AML, and included either 28, 53, or 81 genes, depending on the year of testing. The patients were stratified into favorable-, intermediate-, or adverse-risk categories following the European LeukemiaNet (ELN) 2017 guidelines.22
Ethics approval for this study was obtained from The University of Texas MD Anderson Cancer Center Institutional Review Board, and the study was conducted in accordance with the Declaration of Helsinki.
Response and outcome definitions
Patients who were responding were defined as those who had achieved either CR, CRi, or MLFS, as defined by the ELN 2017 criteria.22 OS was defined as the time from best morphologic response to death from any cause, with censoring of patients who were alive at the time of last follow-up. Relapse was defined as reemergence of bone marrow blasts ≥5%, circulating blasts, or extramedullary disease.22 Treatment-related mortality (TRM) was defined as death from any cause without preceding AML relapse.
MRD assessment
MRD assessment was performed locally at the time of best morphologic response using 8 color MFC as previously described.21 In patients with multiple MRD assessments at the same level of best response, the first was retained. Briefly, MRD was analyzed by expert hematopathologists, using a combined leukemia-associated immunophenotype and different-from-normal (DfN) approach.16 A case was considered MRD+ if the sample contained an abnormal population consisting of at least 20 cells with an aberrant immunophenotype similar to the leukemia-associated immunophenotype at diagnosis or a population consisting of at least 20 cells with multiple distinctive aberrations compared to normal myeloid precursors/stem cells. The DfN approach carefully excluded the preleukemic phenotype.23 The sensitivity of this assay varied between 10−3 and 10−4 (0.1%-0.01%), depending on the cellularity of the specimen and the degree of immunophenotypic overlap between the residual leukemic blasts and the normal myeloid precursors. Specimens with negative results but suboptimal cell counts (<200 000 events and <200 CD34+ cells) were excluded.
Statistical analysis
The differences in patient baseline characteristics between the IA and LOW + VEN cohorts were compared using the χ2 test for categorical variables and the Wilcoxon Mann-Whitney test for continuous variables. The Kaplan-Meier product-limit method was used to estimate OS. The methods of Gooley et al were used to estimate the CIR and CI of TRM using the time of best morphologic response as the start date.24 We used the methods of Fine and Gray to model potential risk factors for the CIR, considering death as a competing event.25 For the primary analysis, OS and CIR were censored without event at the time of allogeneic stem cell transplantation (SCT). A secondary analysis without any censoring for SCT was also performed. A univariate Cox proportional hazards regression was used to identify any association between variables and OS, whereas the Fine and Gray methods were used to identify associations with CIR. Factors that were significantly associated with OS at P < .05, using univariate analysis, were retained to perform the multivariate analysis using a Cox proportional hazards model for OS or the Fine and Gray methods for CIR. All statistical analyses and figures were generated using Stata/SE version 16.1 (Stata Corp LP, College Station, TX) and GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA).
Results
Patient and response characteristics
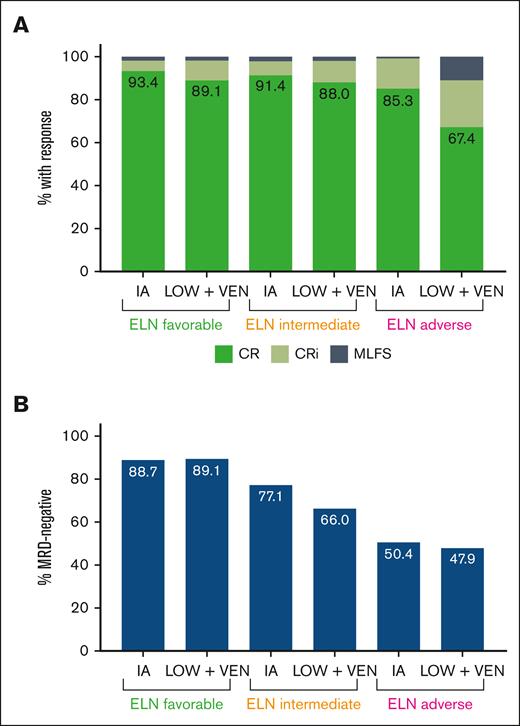
Between 4 February 2010 and 5 October 2021, we identified 635 patients with newly diagnosed, non-CBF, non-APL AML who achieved a first response (CR, CRi, or MLFS) and had adequate MRD testing results. In total, 385 patients were treated with IA regimens and 250 with LOW + VEN regimens. Of the patients treated with IA or LOW + VEN, 95 of 385 (24.7%) and 59 of 250 (23.6%), respectively, had an additional investigational agent added to the regimen. Almost all the younger patients (age, <60 years) received IA, with only 11 patients aged <60 years treated with LOW + VEN. Patient baseline characteristics are shown in Table 1. The patients treated with LOW + VEN regimens were significantly older. This group was also enriched for ELN adverse-risk disease and high-risk cytogenetic features, such as −5/5q–, −7/7q–, −17/17p–, and complex karyotype. The median time from the start of therapy to best morphologic response was 32 days (interquartile range, 27-38 days) in the IA cohort and 39 days (interquartile range, 30-63 days) in the LOW + VEN cohort. The LOW + VEN cohort had a lower proportion of patients who achieved CR (as opposed to CRi/MLFS) compared with the IA cohort (76.0% CR with LOW + VEN vs 89.4% CR with IA). This was driven by an increased incidence of ELN adverse-risk disease in the LOW + VEN cohort, which had a lower CR rate. Response distribution by treatment intensity and ELN category are shown in Figure 1A. The rate of MRD negativity was also lower in the LOW + VEN cohort compared with the IA cohort (60.4% with LOW + VEN vs 71.4% with IA). MRD negativity was similar between treatment cohorts within ELN categories (Figure 1B). Of the responding patients, MRD− CR was achieved in 66.2% (255 of 385) in the IA cohort and in 55.2% (138 of 250) in the LOW + VEN cohort. The rate of allogeneic SCT was approximately double in the IA cohort (50.1%, 193 of 385 patients) compared with the LOW + VEN cohort (24.8%, 62 of 250 patients).
Responses and MRD negativity rates based on the treatment cohort and ELN risk category. (A) Distribution of responses based on the ELN risk category and treatment cohort. (B) MRD negativity rates based on the ELN risk category and treatment cohort. These figures include responding patients only.
Responses and MRD negativity rates based on the treatment cohort and ELN risk category. (A) Distribution of responses based on the ELN risk category and treatment cohort. (B) MRD negativity rates based on the ELN risk category and treatment cohort. These figures include responding patients only.
OS and CIR stratified based on treatment intensity and MRD status
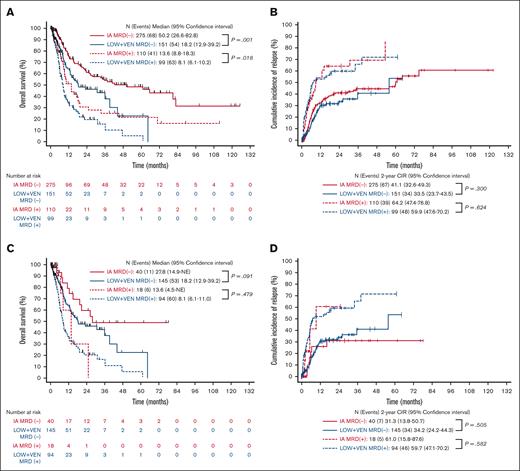
We then stratified the full cohort into 4 groups based on treatment intensity (IA vs LOW + VEN) and MRD status (positive vs negative). Two hundred seventy-five patients were stratified into the IA MRD− group, 151 into the LOW + VEN MRD−, 110 into the IA MRD+, and 99 into the LOW + VEN MRD+ group. With a median follow-up of 38.9 months, the median OSs for the IA MRD−, LOW + VEN MRD−, IA MRD+, and LOW + VEN MRD+ groups were 50.2, 18.2, 13.6, and 8.1 months, respectively (Figure 2A). The 2-year CIRs for the IA MRD−, LOW + VEN MRD−, IA MRD+, and LOW + VEN MRD+ groups were 41.1%, 33.5%, 64.2%, and 59.9%, respectively (Figure 2B). The 2-year CIs of TRM for the IA MRD−, LOW + VEN MRD−, IA MRD+, and LOW + VEN MRD+ groups were 7.8%, 29.5%, 9.4%, and 30.4%, respectively (supplemental Figure 1A).
OS and CIR stratified based on treatment intensity and MRD status (censored for SCT). (A,B) Complete cohort. (C,D) Only those aged ≥60 years. (A,C) OS stratified based on treatment intensity and MRD status. (B,D) CIR stratified based on treatment intensity and MRD status. NE, not estimable.
OS and CIR stratified based on treatment intensity and MRD status (censored for SCT). (A,B) Complete cohort. (C,D) Only those aged ≥60 years. (A,C) OS stratified based on treatment intensity and MRD status. (B,D) CIR stratified based on treatment intensity and MRD status. NE, not estimable.
Patients who were MRD− had significantly longer OS when treated with IA (median OS, 50.2 months with IA vs 18.2 months with LOW + VEN; P = .001) but similar CIR (2-year CIR, 41.1% with IA vs 33.5% with LOW + VEN; P = .300; Figure 2A-B). A similar finding was observed with the patients who were MRD+ (median OS, 13.6 months with IA vs 8.1 months with LOW + VEN; P = .018; 2-year CIR, 64.2% with IA vs 59.9% with LOW + VEN; P = .624; Figure 2A-B). The CI of TRM curves grouped together based on treatment received, as opposed to MRD status, with the lower TRM observed within the patients treated with IA likely reflecting the younger age and better fitness of this group (supplemental Figure 1A).
Because older patients may derive less benefit from IA, we repeated this analysis by including only the patients aged ≥60 years (n = 297). There was no significant difference in OS or CIR based on the treatment intensity within MRD categories in the patients aged ≥60 years (Figure 2C-D). The corresponding figure for CI of TRM in the older patients is shown in supplemental Figure 1B. The limited number of patients aged <60 years who received LOW + VEN precluded analyses comparing the impact of treatment intensity in this population.
Univariate analysis for OS and CIR
Given the imbalances in patient characteristics between patients treated with IA and those treated with LOW + VEN, we sought to explore the variables that were associated with OS and CIR. We first performed a univariate analysis to identify associations between individual factors and OS. Age, sex, best response (CR, CRi, or MLFS), treatment intensity (IA vs LOW + VEN), MRD status, ELN 2017, and specific cytogenetic findings were included in this analysis. Using univariate analysis, younger age, intensive therapy (IA), achievement of CR (as opposed to CRi/MLFS), MRD negativity, lower ELN 2017 risk, and nonadverse karyotype [eg, patients without 11q23 rearrangements, inv(3), −5/5q–, −7/7q–, −17/17p–, and/or complex cytogenetics] were significantly associated with improved OS (Table 2). For CIR, the significant favorable factors were achievement of CR, MRD negativity, lower ELN 2017 risk, and the absence of the same cytogenetic findings (Table 3). Notably, treatment intensity was not significantly associated with CIR in the univariate analysis.
Multivariate analysis for OS and CIR
We then considered all factors that were significantly associated with OS by univariate analysis to perform a multivariate analysis. Individual cytogenetic features were not used to build the model because these were already accounted for within the ELN 2017 classification. By multivariate analysis, age, best response, MRD status, and ELN 2017 risk remained significantly associated with OS (Table 4). For CIR, the significant factors were best response, MRD status, and ELN 2017 risk (Table 4). Notably, treatment intensity (IA or LOW + VEN) was not significantly associated with either OS or CIR in the multivariate model. When the model was restricted to the older patients (age ≥60 years), only best response and ELN 2017 risk remained significantly associated with OS, whereas best response, MRD status, and ELN 2017 risk were significantly associated with CIR (Table 4). Treatment intensity was not significantly associated with OS or CIR in the older cohort. To assess for potential collinearity between best response and MRD status, we repeated the multivariate analysis with sequential exclusion of best response or MRD status, which resulted in similar findings as the primary analysis (supplemental Tables 1 and 2).
OS and CIR based on the ELN 2017 risk category
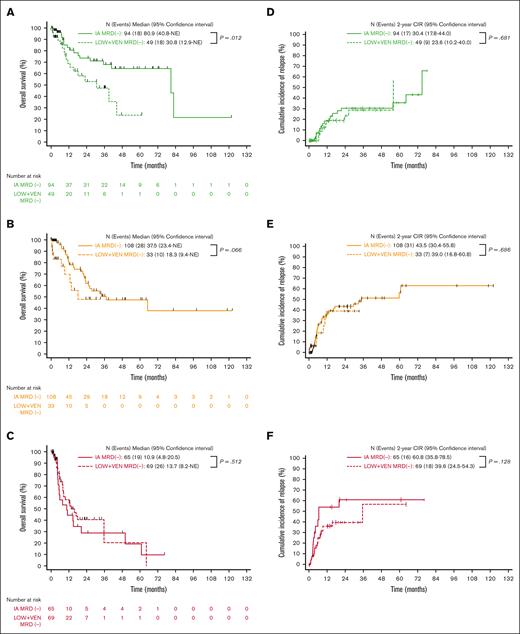
We then stratified all patients who had achieved MRD negativity (n = 426) based on the ELN 2017 risk category. Within the ELN 2017 favorable category, OS was significantly longer in those treated with IA vs those treated with LOW + VEN (median OS 80.9 vs 30.8 months; P = .012; Figure 3A). This was driven by an increased incidence of death in remission among the patients within the LOW + VEN cohort who did not receive transplantation (11 events [22.4%] with LOW + VEN vs 6 events [6.4%] with IA); this group was composed of an older patient population with a greater comorbidity burden. OS was not significantly different between the 2 treatment regimens in ELN intermediate and adverse-risk groups (Figure 3B-C). In the patients who were MRD−, the CIR was similar between the different treatment regimens within all ELN risk categories (Figure 3D-F).
OS and CIR stratified based on treatment intensity for patients achieving MRD negativity within ELN categories (censored for SCT). (A) ELN favorable, MRD−. (B) ELN intermediate, MRD−. (C) ELN adverse, MRD−. (A-C) OS stratified based on the treatment. (D) ELN favorable, MRD−. (E) ELN intermediate, MRD−. (F) ELN adverse, MRD−. (D-F) CIR stratified based on the treatment.
OS and CIR stratified based on treatment intensity for patients achieving MRD negativity within ELN categories (censored for SCT). (A) ELN favorable, MRD−. (B) ELN intermediate, MRD−. (C) ELN adverse, MRD−. (A-C) OS stratified based on the treatment. (D) ELN favorable, MRD−. (E) ELN intermediate, MRD−. (F) ELN adverse, MRD−. (D-F) CIR stratified based on the treatment.
Impact of allogeneic SCT on findings
We then repeated the analysis without any censoring for SCT. In the uncensored analysis, the patients treated with IA had significantly improved OS within MRD categories in the full cohort as well as the older patients (age, ≥60 years; supplemental Figure 2A,C), likely reflecting the survival benefit of SCT, which was performed much more frequently in the IA cohort. The CIR was similar within MRD categories between the treatment regimens for the full cohort (supplemental Figure 2B). In the older patients, the IA MRD+ group had improved CIR compared with the LOW + VEN MRD+ group, likely reflecting a beneficial effect of SCT on relapse risk in the IA MRD+ group, although the number of patients was small (n = 18) (supplemental Figure 2D). Treatment intensity was significantly associated with OS but not CIR, using univariate analysis without censoring for SCT (supplemental Tables 3 and 4). Treatment intensity was not a significant factor for either OS or CIR in the uncensored multivariate analysis (supplemental Table 5). OS and CIR in the patients who were MRD−stratified based on the treatment and ELN 2017 criteria are shown in supplemental Figure 3.
Together, these analyses suggest that, assuming response is achieved, disease-related factors (ELN 2017–defined risks) and response quality (CR/CRi/MLFS and MRD status) appear to be more strongly associated with outcomes (OS and CIR) than treatment intensity, irrespective of censoring for SCT.
Discussion
The addition of VEN to low-intensity treatment regimens has led to both improved response rates and better quality responses among patients with AML who are unfit for intensive chemotherapy.8,9 In practice, the determination of a patient’s fitness for intensive chemotherapy is often left to the judgment of the treating physician. Many predictive tools, including the hematopoietic cell transplantation comorbidity index, the geriatric assessment, the AML composite model, and the early (4-week) mortality score, among others, have been developed to make this decision more objective.26-32 These tools help identify patients in whom intensive chemotherapy should be avoided but do not answer the question whether intensive chemotherapy is better than low-intensity therapy for patients potentially eligible for both (such as older patients who otherwise have minimal comorbidities), and this question has not yet been answered in prospective randomized trials. We hypothesized that treatment intensity may not be the key determinant of outcome, assuming an optimal response to therapy can be achieved (ie, elimination of MRD).
In our study, we demonstrated that treatment intensity was not significantly associated with OS or CIR in responding patients with AML using multivariate analyses considering other factors such as age, response quality (CR/CRi/MLFS), MRD status, and ELN risk categories. Our results are in line with those of 2 recent retrospective studies that compared the outcomes of patients with AML treated with low-intensity therapy (HMA + VEN) with those of a propensity score–matched control group treated with intensive chemotherapy. In the first study,33 Maiti et al compared outcomes in 85 patients with AML treated with 10-day decitabine plus VEN (DEC10-VEN) with a comparator cohort treated with intensive chemotherapy and matched for baseline characteristics (n = 85). In this analysis, compared with patients treated with intensive chemotherapy, the patients treated with DEC10-VEN had higher rates of CR/CRi (81% vs 52%; P < .001), lower 30-day mortality (1% vs 24%; P < .01), lower rates of relapse (34% vs 56%; P = .01), and longer OS (median, 12.4 vs 5 months; P < .01).33 In another study, a propensity-matched analysis (controlling for age, ELN risk, and receipt of SCT) was performed to compare patients treated with azacitidine plus VEN (n = 48) with patients treated with intensive chemotherapy (n = 48). There were no significant differences in OS or progression-free survival between the 2 treatment regimens, although a trend toward longer OS was observed in the azacitidine plus VEN cohort compared with the intensive chemotherapy cohort (median OS, not reached vs 705 days; P = .0667).34 Therefore, a growing amount of literature (albeit limited by its retrospective nature) lends support to the notion that there is no clear benefit of intensive therapy over lower intensity, VEN-based regimens in older individuals with AML after patient and disease-related risk factors are adjusted for.
Another important finding in our study was that traditional response assessment criteria (CR, CRi, and MLFS) retained their significance in the multivariate model even when MRD status was considered. These findings are consistent with those of another retrospective study that included 245 patients with AML and showed that both MRD status and response (CR vs CRi vs CR with incomplete platelet recovery [CRp]) were predictive of relapse in a multivariate model.35 This is somewhat counterintuitive because MRD status is considered a much more sensitive assessment of response than morphological criteria and blood count recovery. This finding is likely explained by the imperfect nature of MFC-based MRD in AML. Up to 30% of patients who are tested MRD− by MFC still eventually relapse,36 suggesting the method occasionally fails to detect leukemic blasts either because they are immunophenotypically indistinguishable from normal myeloblasts or are present at a level below the limit of detection. Lack of full count recovery (ie, achievement of CRi/MLFS as opposed to CR) may reflect residual disease undetected using MFC-based MRD methods with negative implications. Indeed, responses other that CR (such as CRp or CRi) have been associated with more frequent detection and higher levels of MRD.35 A recent meta-analysis demonstrated the prognostic impact of MRD in patients achieving responses other than CR (eg, CRi and MLFS).17 Therefore, it appears that both MRD and quality of morphologic/hematologic response contribute independently to outcomes in AML.
A major limitation of our study was its retrospective single-center design. Our results may also not apply to AML subtypes in which transcript-based MRD is performed (CBF-AML) because these were excluded from this study. In addition, retrospective comparisons between patients treated with intensive chemotherapy vs low-intensity regimens are inherently biased because patients who are intensively treated are younger, have better performance status, have lower comorbidity burdens, and are enriched for more favorable cytogenetic/molecular disease features. Therefore, multivariate analyses are critical when attempting such comparisons. Unmeasured potential confounders are another possible limitation of this study.
Furthermore, our study included only responding patients and therefore could not evaluate whether the overall response rates were different between the IA- and LOW + VEN–treated cohorts. However, we did note that CR with MRD negativity was achieved in a higher proportion of patients treated with IA than in those treated with LOW + VEN, apparently driven by an increased incidence of ELN adverse-risk disease in the LOW + VEN cohort. It is, therefore, possible that the better quality of responses in the IA cohort was because of more favorable disease features as opposed to greater efficacy of the therapy. Time to best morphologic response may also be longer in low-intensity therapies when compared with IA regimens, as was observed in our study, potentially introducing an immortal time bias. To mitigate this, we used the best response date as time 0 for both OS and CIR calculations. Finally, we emphasize that patients receiving low-intensity therapy in our study as well as in the 2 other retrospective studies discussed were almost exclusively older patients (age, ≥60 years).33,34 Therefore, at the present time, the applicability of our findings are limited to older patients with AML.
Overall, our findings suggest that, assuming an optimal response to therapy is achieved, disease control may be similar irrespective of the treatment used to achieve this state, although this needs to be confirmed in prospective studies. This supports the notion that the primary goal of therapy in AML should be to achieve MRD– CR, with more effective novel low-intensity regimens that can achieve deep responses being potentially equivalent to traditional cytotoxic regimens. This is reflected by the recognition of MRD− responses in the 2017 ELN recommendations and their most recent update.22,37 In younger patients (age, <60 years), intensive chemotherapy–based approaches remain the standard of care and are potentially curative. The use of low-intensity therapy in younger patients is investigational, and it is unknown whether such approaches can cure AML in the absence of SCT. A clinical trial to evaluate frontline lower intensity regimens among younger patients (#NCT03573024) is ongoing.38 In older patients eligible for both intensive and low-intensity regimens, the optimal approach remains to be determined. We expect that an ongoing randomized phase 2 study (#NCT04801797) will be instrumental in answering this question.39
Acknowledgments
The authors thank all members of the Leukemia Department at The University of Texas MD Anderson Cancer Center who provided clinical care to our patients. The authors thank the patients whose data made this study possible.
This research was funded in part by a Cancer Center Support grant from the National Cancer Institute, National Institutes of Health (P30 CA016672) and the Leukemia SPORE.
Authorship
Contribution: F.R., N.J.S., and A.B. designed the study; S.P. provided the patient database; G.N.G. and X.H. provided the statistical analysis; A.B. analyzed the data and wrote the manuscript; S.A.W., W. Wang, S.L., J.J., and K.P. performed the flow cytometry MRD analysis and provided this data; T.K., N.J.S., G.B., C.D., N.D., Y.A., F.G.H., A.M., K.S., M.Y., P.T., W. Wierda, G.G.-M., M.A., E.J., M.K., H.K., and F.R. provided clinical care to the patients; and all authors contributed intellectually to the study and critically reviewed and edited the manuscript.
Conflict-of-interest disclosure: T.K. reports consultancy from AbbVie, Agios, Bristol Myers Squibb (BMS), Genentech, Jazz Pharmaceuticals, Novartis, Servier, and PinotBio; research funding from AbbVie, BMS, Genentech, Jazz Pharmaceuticals, Pfizer, Cellenkos, Ascentage, GenFleet, Astellas, AstraZeneca, Amgen, Cyclacel Pharmaceuticals, Delta-Fly Pharma, Iterion, GlycoMimetics, and Regeneron; and honoraria from Astex. N.J.S. reports consultancy from Takeda Oncology, AstraZeneca, Amgen, Novartis, and Pfizer; research funding from Takeda Oncology, Astellas, and Stemline Therapeutics; and honoraria from Amgen. G.B. reports research funding from Astex, Ryvu Therapeutics, and PTC Therapeutics; membership on an entity’s board of directors or advisory committees from Pacylex, Novartis, CytomX, and Bio Ascend; and consultancy from Catamaran Bio, AbbVie, PPD Development, Protagonist Therapeutics, and Janssen. S.L. reports consultancy from AbbVie, GLG, and QualWorld; equity ownership in publicly traded company from AbbVie; honoraria from PeerView; and research funding from Astellas and Amgen. C.D. reports membership on an entity’s board of directors or advisory committees from Genmab, GlaxoSmithKline, Kura, and Notable Labs; honoraria from Kura, Astellas, bluebird bio, BMS, Foghorn, Immune-Onc, Novartis, Takeda, Gilead, and Jazz Pharmaceuticals; is a current holder of stock options in a privately held company from Notable Labs; consultancy from AbbVie and Servier; and research funding from Servier, BMS, Foghorn, Immune-Onc, LOXO, Astex, Cleave, and Forma. N.D. reports research funding from Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, ImmunoGen, Pfizer, BMS, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, GlycoMimetics, and Trillium; advisory role with Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, BMS, Kite, Actinium, Arog, ImmunoGen, Arcellx, and Shattuck; data monitoring committee member from Kartos and Jazz Pharmaceuticals; consultancy and membership on an entity’s board of directors or advisory committees from Agios, Celgene, Sobi, and Star Therapeutics; and research funding from Karyopham Therapeutics and Newave Pharmaceutical. Y.A. reports research funding from Jazz Pharmaceuticals, FibroGen, Sun Pharma, BerGenBio, Daiichi-Sankyo/Lilly, and Astex. K.S. reports consultancy and research funding from Novartis; honoraria from Otsuka Pharmaceuticals; and membership on an entity’s board of directors or advisory committees of Novartis, Pfizer, and Daiichi-Sankyo. M.Y. reports research funding from Daiichi-Sankyo and Pfizer. G.G.-M. reports research funding from Astex Pharmaceuticals, Novartis, AbbVie, BMS, Genentech, Aprea Therapeutics, Curis, and Gilead Sciences; consultancy from Astex Pharmaceuticals, Acceleron Pharma, and BMS; and honoraria from Astex Pharmaceuticals, Acceleron Pharma, AbbVie, Novartis, Gilead Sciences, Curis, Genentech, and BMS. M.A. reports research funding from Daiichi Sankyo, Breast Cancer Research Foundation, AstraZeneca, Oxford Biomedical UK, Brooklyn ITX, Senti Bio, PinotBio, and Syndax; membership on an entity’s board of directors or advisory committees of Cancer UK, Leukemia & Lymphoma Society, Aptose, German Research Council, National Cancer Institute of National Institutes of Health, CLL Foundation, and Brooklyn ITX; and is a current holder of stock options in a privately held company from Reata, Aptose, Eutropics, Senti Bio, and Chimerix. E.J. reports research funding from Amgen, Pfizer, AbbVie, Adaptive Biotechnologies, Astex, and Ascentage and consultancy from Amgen, Pfizer, AbbVie, Takeda, Adaptive Biotechnologies, Astex, Ascentage, Genentech, Novartis, BMS, Jazz Pharmaceuticals, Hikma Pharmaceuticals, and Incyte. M.K. declares research funding from AbbVie, Genentech, F. Hoffman-La Roche, Stemline Therapeutics, Forty-Seven, Eli Lilly, Cellectis, Calithera, Ablynx, Agios, Ascentage, AstraZeneca, Rafael Pharmaceuticals, Sanofi, and Novartis; consultancy from AbbVie, Genentech, F. Hoffman-La Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji, Janssen, Eli Lilly, and Cellectis; honoraria from F. Hoffman-La Roche, Forty-Seven, and Kisoji; membership on an entity’s board of directors or advisory committees for F. Hoffman-La Roche, Stemline Therapeutics, and Janssen; current equity holder in private company from Reata Pharmaceuticals; and patents and royalties from Reata Pharmaceuticals, Eli Lilly, and Novartis. H.K. reports research funding from AbbVie, Amgen, Ascentage, BMS, Daiichi Sankyo, ImmunoGen, Jazz Pharmaceuticals, and Novartis and honoraria from AbbVie, Amgen, Amphista, Ascentage, Astellas, Biologix, Curis, Ipsen Biopharmaceuticals, KAHR Medical, Novartis, Pfizer, Precision BioSciences, Shenzhen Target Rx, and Takeda. F.R. reports research funding from Amgen, Astex/Taiho, BMS/Celgene, Syos, AbbVie, Prelude, Xencor, Astellas, and Biomea Fusion, Inc.; honoraria from Amgen, BMS/Celgene, Syos, AbbVie, and Astellas; membership on an entity’s board of directors or advisory committees for Astex/Taiho; and consultancy from BMS/Celgene, Syos, Novartis, AbbVie, AstraZeneca, and Astellas. The remaining authors declare no competing financial interests.
Correspondence: Farhad Ravandi, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030-4009; e-mail: fravandi@mdanderson.org.
References
Author notes
The data set presented in this study is not publicly available to protect patient confidentiality.
Reasonable requests for deidentified data are available on request from the corresponding author, Farhad Ravandi (fravandi@mdanderson.org).
The full-text version of this article contains a data supplement.