Key Points
FOXP1 upregulates the cell stress sensor SIRT1 and supports AML cell resistance to therapeutic treatments.
FOXP1 limits superoxide anion levels and oxidative stress of myeloid leukemia cells.
Abstract
Transcription factor Forkhead box P1 (FOXP1) belongs to the same protein family as the FOXOs that are well-known regulators of murine hematopoietic stem progenitor cell (HSPC) maintenance via dampening oxidative stress. FOXP1 and FOXOs can play opposite, or similar, roles depending on cell context; they can crossregulate each other’s expression. In a previous study, we have shown that FOXP1 contributes to healthy human HSPC and acute myeloid leukemia (AML) cell growth. Here, we investigated the role of FOXP1 in HSPCs and AML cell oxidative stress defense in a human context. FOXP1 expression level was associated with an inferior survival outcome in patients with cytogenetically normal AML. FOXP1 knockdown enhanced superoxide anion levels of human-committed CD34+CD38+ cells but not stem cell–enriched CD34+CD38− HSPCs or AML cells in vitro. FOXP1 knockdown triggered enhanced NRF2 activity and increased cell oxidative stress. FOXP1 had no impact on FOXO1/3/4 expression in these cells; genetic and pharmacological inhibition of FOXOs did not change superoxide anion levels of human HSPCs or AML cells. Moreover, FOXP1 antioxidant activity was independent of changes in expression of superoxide dismutase 1 and 2 or catalase. Instead, FOXP1 upregulated expression of the stress sensor SIRT1 by stabilizing SIRT1 protein. FOXP1 loss sensitized AML cells to chemotherapy. Together, this study identified FOXP1 as a new safeguard against myeloid progenitor oxidative stress, which works independently of FOXOs but through SIRT1 and contributes to AML chemoresistance. It proposes FOXP1 expression/activity as a promising target to overcome drug resistance of AML HSPCs.
Introduction
Precise regulation of balance between self-renewal and differentiation of hematopoietic stem/progenitor cells (HSPCs) is critical to ensure the proper functioning of the blood-forming system. Subversion of molecular mechanisms regulating these processes is often causally linked to leukemia development. The ability to properly control cellular levels of reactive oxygen species (ROSs) is one of the best-known factors that regulate stem cell biology. Although excess amounts of ROS limit the function of hematopoietic stem cells (HSCs),1,2 some levels contribute to HSPC growth and sustain leukemic stem cell self-renewal.3 However, ROSs encompass an array of derivatives of molecular oxygen, including 2 key redox signaling agents, hydrogen peroxide and superoxide anion radical (O2.−) that occur as normal attributes of aerobic life and trigger oxidative stress. All these molecular oxygen derivatives individually exhibit vastly divergent chemical reactivity, which differentially contributes to cell pathophysiology, as revealed by recent methodological advances and development of selective tools targeting each of them.4 As an example, O2.− oxidizes Fe-S clusters at a high rate, thus releasing iron and impairing cell proliferation through respiratory chain complexes and RNA/DNA metabolism inhibition. It, however, is less apt for redox signaling to occur via thiols, as opposed to via hydrogen peroxide, whereas its uncharged protonated form, perhydroxyl radical, can diffuse in lipids and reacts with polyunsaturated lipids; it also reacts with other radicals, notably nitric oxide, which widens its activities.
Acute myeloid leukemia (AML) are genetically complex and highly heterogeneous fatal malignancies. Despite a high rate of complete remission after cytotoxic chemotherapies or the association of demethylating agents with BCL2 inhibitors, the 5-year overall survival remains very poor, with tumor regrowth initiated by chemoresistant leukemic cells. Even specific therapies have limited effects, as exemplified by Fms-like tyrosine kinase (FLT3) mutated AML subtypes, the most commonly observed mutations in AML, that showed modest clinical activity and rapid emergence of resistance upon treatment with various FLT3 kinase inhibitors.5 These observations indicate that understanding of the mechanisms of AML resistance is incomplete, and novel markers to identify new AML subcategories and to guide therapy are needed. Recently, chemotherapy-resistant human AML cells were shown to exhibit elevated oxidative phosphorylation with imbalanced redox homeostasis; these cells were more susceptible to changes in ROS content, which alters their sensitivity to chemotherapies.6,7 Among well-known ROS regulators are members of the FOXO subfamily of Forkhead box (FOX) transcription factors, as demonstrated in murine models.8,9 FOXOs favor cell-cycle arrest gene transcription, apoptosis, and ROS detoxification in a large variety of cell types,10 including murine HSCs.9,10 These activities made FOXOs primarily known as quiescence factors and tumor suppressors, depending on cellular contexts. Paradoxically, FOXOs also function as oncogenes whose upregulation promotes self-renewal and block differentiation of human CD34+ HSPCs.11 Their activity is associated with adverse prognosis in AML, which runs counter to established roles of AKT/FOXO tumor suppressor signaling in other human cancers.11,12 FOXP1 belongs to a distinct subtype of FOX transcription factors.13 As with other members of this family, FOXP1 has a diverse repertoire of functions, ranging from regulation of B-cell development and monocyte differentiation to facilitation of cardiac valve and lung development13; FOXP1 also appears to play a role in malignancy, functioning as a tumor suppressor or as an oncogene, depending on cell type, interacting partners, or subcellular localization.13,14 FOXP1 and the FOXOs have antagonistic functions: for example, FOXP1 antagonizes IL7RA induction by FOXO1 in naive T cells or drives a negative feedback loop to suppress FOXO-induced apoptosis, and modulates expression of a specific subset of FOXO-target genes in other cell types.15,16 These observations highlight complex cooperation between the 2 FOX factor subtypes.
We recently identified FOXP1 as an essential regulator of HSPC maintenance and AML cell growth and survival by its repression of p21CIP1 and p27KIP1 cell-cycle inhibitors.17 These inhibitors are multifunctional proteins that contribute to cell apoptosis or senescence and also to redox state, in addition to cell-cycle arrest.18 FOXP1 was further shown to regulate oxidative stress of a few cell models.19,20 Moreover, Seipel et al21 identified FOXP1 as an adverse prognostic factor in patients with AML who were intensively treated and received autologous stem cell transplant (auto-SCT) at remission; patients with AML with elevated FOXP1 gene expression at diagnosis had a significantly shorter progression-free and overall survival after intensive induction chemotherapy and auto-SCT than those without elevated FOXP1 gene expression. Knowing the role of redox homeostasis in chemotherapeutic response of malignant cells, we questioned the effect of regulatory activities of FOXP1 and FOXOs on the oxidative stress in healthy and malignant human myeloid progenitors. We observed that FOXP1, but not FOXOs, dampens O2.– levels in these cells, which limits their oxidative stress; we identified FOXP1 as a new risk factor in AML that enhances expression of the stress sensor SIRT1, and contributes to chemotherapy sensitivity.
Materials and methods
Reagents and supplemental Materials and Methods can be found in supplemental File.
Cell purification and culture
AML bone marrow samples and human cord blood (CB) units were collected per institutional guidelines (CPP IdF2 N°2015-08-11 MS3 DC), with a written informed consent from donors in accordance with the Declaration of Helsinki. Handling, characterization, and storage of patient samples were performed by Cochin Hospital’s Cell Biobank. For samples from patients with AML, the selection criterion was high levels of FLT3-internal tandem duplication (ITD):wild type (WT) ratio (ITD:WT > 0.5, supplemental Table 3). After thawing, AML bone marrow mononuclear cells were kept in culture in Iscove modified Dulbecco medium supplemented with bovine serum albumin, insulin, and transferrin (Stem Cell Technologies), thrombopoietin peptide (20 nM), human interleukin-3 (10 ng/mL), human stem cell factor (50 ng/mL), and human FLT3-L (50 ng/mL) as previously described.17 CB CD34+ cells were purified per the manufacturer’s recommendations (Miltenyi), and cultured in Iscove modified Dulbecco medium, as previously described.22
Lentiviral constructions and transduction
Short hairpin RNA (shRNA) sequences (supplemental Table 4) were inserted down to the H1 promoter in a pSuper.neovector (OligoEngine); H1-shRNA expression cassettes were introduced into the pTRIPΔU3-GFP lentiviral vector, as previously described.22 shRNA directed against luciferase sequence (shCtl) or empty lentiviral vector were used as control. Human FOXP1 and SIRT1 lentiviral constructs were engineered per Gateway Technology (Life Technologies), using the pINDUCER21 vector (Addgene, 19068). Lentiviral particles were produced and titrated as previously described17 and added on cells once, at adjusted minimal multiplicities of infection from 3 to 8 for AML cell lines to get ∼90% transduction, and 20 for primary cells. Primary CD34+ HSPC transduction occurred 24 hours after purification, whereas AML cells were transduced 5 or 7 days after thawing, when cell viability of >80% had been reached. All cells were collected at day 3 after transduction, at a time point when cell viability exceeded 70%, as assessed by flow cytometry using proper forward- and side-scatter gating. The percentage of transduced cells was estimated via green fluorescent protein (GFP) fluorescence-activated cell sorting analysis; it reached ∼90% for every AML cell line and primary HSPC subtype (CD34+, CD34+CD38+, and CD34+CD38−) and 25% or 40% for patient cells; the latter samples were sorted based on GFP expression 2 or 3 days after transduction. When indicated, transgene expression was induced by doxycycline treatment (0.1 μg/mL) for 15 hours.
Measurement of ROS levels
Cells were incubated with dihydroethidium (DHE; 5 μM) or MitoSOX Red (5 μM) for 30 or 10 minutes, respectively, before analyzing fluorescence intensity of viable GFP+ cells via flow cytometry; data were expressed as the mean fluorescence intensity in cytometer arbitrary units.
Statistical analysis
GraphPad7 software (Mann-Whitney and one-way analysis of variance tests) was used for all analyses. All experiments were performed at least 3 times independently. Data are expressed as mean ± standard error of the mean. Significance scores were nonsignificant; ∗P ≤ .05; ∗∗P ≤ .01; and ∗∗∗ P ≤ .001.
Results
FOXP1 limits oxidative stress of AML cells
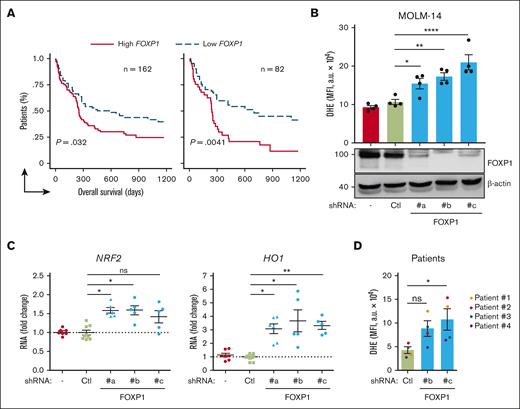
Data mining analyses of publicly accessible transcriptomic databases from cohorts of patients with primary AML at diagnosis were first performed to evaluate FOXP1 contribution to chemoresistance among patients with AML. From a large cohort (n = 163) of those with cytogenetically normal (CN) AML who received intensive chemotherapy (cytarabine and anthracycline), followed by consolidation chemotherapy (GSE12417),23 patients could be divided in 2 equal groups, considering the median expression value of the FOXP1 gene (1 sample at group boundary was discarded). Patients with lower FOXP1 gene expression scores had longer overall survival than those with higher scores (P = .032; log-rank [Mantel-Cox] tests; Figure 1A, left panel). When taking first and third quartiles into account, the overall survival probability was even more significantly different between high and low FOXP1–expressing samples (P = .0041; Figure 1A, right panel). These data suggested that FOXP1 expression level somehow contributed to CN-AML cell resistance to therapeutic treatments, in agreement with Seipel et al.21 The FOXP1 main targets p21CIP1 and p27KIP1 we identified in AML cells, regulate not only cell-cycle arrest but also contribute, among other roles, to the redox cycle.18 We questioned whether FOXP1 has a direct impact on CN-AML cell oxidative stress. We made use of well-validated lentiviral vectors encoding 1 of 3 distinct shRNAs against FOXP1, with shFOXP1#b targeting the second coding exon, sh#c targeting the last coding exon, and sh#a targeting the sequence within the 3’ untranslated region (supplemental Figure 1A) in addition to a GFP marker; these shRNAs limit FOXP1 expression to variable extends, as we previously showed.17 Consequences of FOXP1 knockdown (KD) on cell oxidative stress were then analyzed and compared with those in controls. Among CN-AML, FLT3 mutations are the most frequent events, detected in ∼25% to 30% of patients with AML. Upon lentiviral transduction of MOLM-14 FLT3-ITD AML cell line, FOXP1 KD was more induced, as compared with control untransduced or shLuciferase-expressing cells. Consequently, enhanced O2.– level was detected in shFOXP1-expressing cells, as assessed via a dihydroxyethidium probe, in which the fluorescence essentially relies on the presence of O2.− (Figure 1B). To assess global changes of oxidative stress upon FOXP1 KD, we focused on nuclear factor erythroid–derived 2-like 2 (encoded by NFE2l2 gene; NRF2), which is commonly induced upon oxidative stress; it is currently recognized as 1 of the main regulators of cellular defense mechanisms against oxidative stress. NRF2 controls environmental stress response by changing the expression of genes encoding cytoprotective enzymes/proteins critical to antioxidative response and ROS detoxification.24 FOXP1 KD enhanced NRF2 expression of FLT3-ITD AML cells; it further increased expression of the NRF2 main gene target, heme-oxidase-1 (HO-1), indicative of a global increase of oxidative stress upon FOXP1 loss (Figure 1C). Analysis of antioxidant activity of FOXP1 was further extended to a larger panel of AML cell lines (supplemental Table 2). It it worth noting that, depending on cells, shFOXP1-expressing vectors induced variable FOXP1 KD, with some cells being refractory to FOXP1 KD, irrespective of the shFOXP1 tested (THP-1; data not shown), whereas others, such as KASUMI-1 cells, showed FOXP1 KD with only 1 of the 3 shRNA used. Such variability could be related to the differences in FOXP1 messenger RNA sequence accessibility, depending on cell types. Alternatively, AML cells might express variable FOXP1 isoforms from alternative translation start and/or end exons, similar to what was identified in malignant and nonmalignant B-lymphoid cells,25,26 making our shRNA inactive. However, every time FOXP1 KD was efficiently induced, increased O2.− levels were observed; when FOXP1 KD was low, upper levels of O2.− were still detected but were statistically not significant (supplemental Figure 1B). FOXP1 KD further induced increased O2.− levels in primary FLT3-ITD AML cells (Figure 1D; supplemental Figure1C). Altogether, these data indicate that FOXP1 limits anion superoxide levels and dampens oxidative stress of human AML cells.
FOXP1 limits oxidative stress of AML cells. (A) Kaplan-Meier survival curves of patients with CN-AML (GSE12417; n = 163) based on FOXP1 gene expression levels, using median expression value cutoff (left) or first and third quartile statistical analysis (right). Log-rank (Mantel-Cox) test was used for statistical significance between groups. (B-C) MOLM-14 or (D) primary human FLT3-ITD AML cells (supplemental Table 3) were transduced with the indicated GFP- and shRNA-encoding lentiviral vector. After 3 days, oxidative stress was assessed in viable GFP+ cells using a DHE probe and flow cytometry (B,D) (n = 4). In parallel, indicated protein (B) (bottom) and RNA transcript (C) (n = 5 or 6) expression levels were analyzed. (BD) Data are expressed as MFI arbitrary unit (a.u); (C) normalized RNA transcript levels were expressed relative to mean level of control shRNA-expressing cells (n = 9); 1 spot represents 1 independent sample from independent experiments. − indicates no vector. Ctl, control shRNA directed against luciferase; MFI, mean fluorescence intensity.
FOXP1 limits oxidative stress of AML cells. (A) Kaplan-Meier survival curves of patients with CN-AML (GSE12417; n = 163) based on FOXP1 gene expression levels, using median expression value cutoff (left) or first and third quartile statistical analysis (right). Log-rank (Mantel-Cox) test was used for statistical significance between groups. (B-C) MOLM-14 or (D) primary human FLT3-ITD AML cells (supplemental Table 3) were transduced with the indicated GFP- and shRNA-encoding lentiviral vector. After 3 days, oxidative stress was assessed in viable GFP+ cells using a DHE probe and flow cytometry (B,D) (n = 4). In parallel, indicated protein (B) (bottom) and RNA transcript (C) (n = 5 or 6) expression levels were analyzed. (BD) Data are expressed as MFI arbitrary unit (a.u); (C) normalized RNA transcript levels were expressed relative to mean level of control shRNA-expressing cells (n = 9); 1 spot represents 1 independent sample from independent experiments. − indicates no vector. Ctl, control shRNA directed against luciferase; MFI, mean fluorescence intensity.
FOXP1 regulates O2.− levels of primary healthy human myeloid progenitors
We wondered whether such FOXP1 antioxidant function was malignancy dependent or, instead, reflected FOXP1 general activity in human myeloid progenitors. Human CB CD34+ HSPCs were transduced with the same shFOXP1- or control shRNA–encoding lentiviral vectors as described earlier. FOXP1 KD triggered enhanced O2.− levels, as assessed by DHE probe (Figure 2A). It further enhanced expression of NRF2, indicative of a global increase in oxidative stress upon FOXP1 KD in human HSPCs (Figure 2B). Interestingly, FOXO antioxidant activities had been reported in murine HSPCs; these activities were repeatedly observed in murine HSC–enriched lineage–negative Lin−Sca+cKit+ HSPCs, as opposed to immature Lin−cKit+ myeloid progenitors.1,27 We thus questioned whether FOXP1 antioxidant activity was similarly differential between human HSC–enriched primitive CD34+CD38− and more committed CD34+CD38+ progenitors. FOXP1 KD did not affect O2.− levels of the HSC-enriched subpopulation; however, it enhanced O2.− levels in less immature CD34+CD38+ progenitors (Figure 2C). Intracellular O2.− production takes place within and outside mitochondria. To determine which O2.− pool was regulated by FOXP1, a mitoSOX probe, which accumulates in mitochondria, was used to assess mitochondrial O2.− levels. FOXP1 KD did not change mitochondrial O2.− levels of human CD34+ HSPCs, as opposed to treatment with rotenone drug used as positive control (supplemental Figure 2). These data indicated that FOXP1 limited O2.− levels of primary human myeloid progenitors, acting largely on the cytosolic O2.− pool. Altogether, these data provide evidence that FOXP1 exhibit antioxidant activities in both normal progenitors and malignant myeloid cells in humans.
FOXP1 loss enhances HSPC oxidative stress. CD34+ HSPCs were isolated from healthy human CB samples and transduced with the indicated GFP- and shRNA-encoding lentiviral vectors directed against FOXP1 (#a, #b, and #c) or luciferase as a control (Ctl). After 3 days, FOXP1 (A; bottom) and NRF2 (B) protein expression was assessed using western blot; β-actin was used as a loading control; size markers (kDa) are indicated on the left (1 representative experiment; n ≥ 3); (A [top],C) O2.− levels of total (−, untreated) or GFP+ cells were assessed using a DHE probe and flow cytometry in total CD34+ (A) (n = 7), and CD34+CD38+ and CD34+CD38− (C) (n = 3) cell populations. Data are expressed as MFI (a.u.); 1 spot represents 1 independent sample from independent experiments.
FOXP1 loss enhances HSPC oxidative stress. CD34+ HSPCs were isolated from healthy human CB samples and transduced with the indicated GFP- and shRNA-encoding lentiviral vectors directed against FOXP1 (#a, #b, and #c) or luciferase as a control (Ctl). After 3 days, FOXP1 (A; bottom) and NRF2 (B) protein expression was assessed using western blot; β-actin was used as a loading control; size markers (kDa) are indicated on the left (1 representative experiment; n ≥ 3); (A [top],C) O2.− levels of total (−, untreated) or GFP+ cells were assessed using a DHE probe and flow cytometry in total CD34+ (A) (n = 7), and CD34+CD38+ and CD34+CD38− (C) (n = 3) cell populations. Data are expressed as MFI (a.u.); 1 spot represents 1 independent sample from independent experiments.
FOXP1 antioxidant activity is independent of FOXOs factors
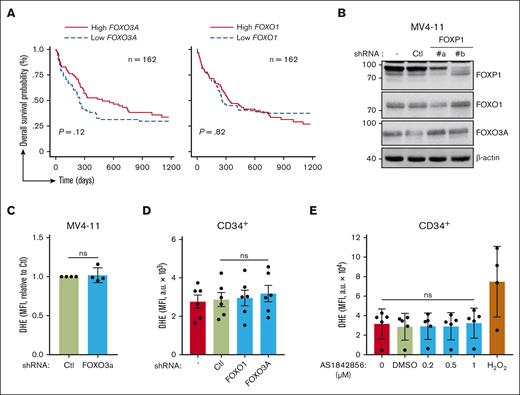
We explored FOXP1 antioxidant activity in the context of its crossregulation with FOXOs.13,16,27 Data mining analyses of the same publicly accessible transcriptomic databases at diagnosis of patients with primary CN-AML were performed to evaluate FOXO1 and FOXO3 contribution to chemoresistance. Interestingly, as opposed to FOXP1 data, patients with higher FOXO1 or FOXO3A gene expression scores had no, or even longer, overall survival (Figure 3A). These data suggested that FOXP1 contribution to therapeutic resistance of CN-AML cells was independent of FOXOs. The impact of FOXP1 on FOXOs activities in AML cells was further assessed upon FOXP1 KD. FOXO1, FOXO3A, and FOXO4 (at a much lower level) transcripts were expressed; FOXO6 expression levels were below the limits of detection. FOXP1 KD did not change FOXO1, FOXO3A, or FOXO4 RNA expression levels of AML cells; or protein levels; or FOXOs nucleo-cytoplasmic distribution (Figure 3B; supplemental Figure 3A-B). These data indicate that FOXP1 did not change FOXOs expression. Because FOXOs are subjected to posttranslational modifications that change their activities, our RNA interference strategy was set up to inhibit FOXOs’ expression and to assess FOXOs’ regulatory activity on O2.− levels in myeloid cells. First, MV4-11 AML cells were used because among FOXOs, they only express FOXO3A (supplemental Figure 3C). FOXO3A KD, in contrast to FOXP1 KD, did not change O2.− levels of MV4-11 AML cells, suggesting no antioxidant activity of FOXO3A in such cells (Figure 3C). shRNA directed against FOXO1 was further selected, which exhibited cross-reactivity toward FOXO3A and FOXO4 and somehow mimicked FOXO-KD (supplemental Figure 4A-B). This shRNA reagent did not change O2.− levels in AML cells (data not shown); it did not impair O2.− levels of primary human CD34+ HSPCs (Figure 3D); however, both shFOXO1 and shFOXO3A triggered myeloid cell growth inhibition, as expected from the literature,11,12 further assessing proper biological efficiency (supplemental Figure 4C-D). To entirely rule out FOXO compensatory and redundant activities, we made use of the FOXO pharmacologic inhibitor AS1842856, which inhibits FOXO1/3A/4/6 DNA binding and transcriptional activities in multiple cell contexts, including human hematopoietic cells.28,29 As expected, human HSPC proliferation was impaired upon treatment with increasing concentrations (0.2-1 μM) of AS1842856 (supplemental Figure 4E). Such treatment, however, did not affect O2.− levels in human myeloid cells, as assessed via DHE labeling (Figure 3E). Similarly, treatment with carbenoloxone, another reported FOXO inhibitor,30 had no impact on DHE labeling (supplemental Figure 4F). Overall, these data indicate that FOXOs, as opposed to FOXP1, do not regulate O2.− levels of healthy human progenitors or AML cells. Thus, FOXP1 exhibit FOXO-independent antioxidant activity in these myeloid cells.
FOXOs have no impact on O2.− levels of HSPCs. (A) Kaplan-Meier survival curves of patients with CN-AML (GSE12417) based on FOXO3 and FOXO1 gene expression levels using median expression value cutoff. Log-rank (Mantel-Cox) test was used to determine statistical significance between groups. (B-C) MV4-11 AML cells were transduced with the indicated GFP- and shRNA-encoding lentiviral vector, or were left untreated (−). After 3 days, (B) protein expression was analyzed via western blot, using the indicated antibodies; β-actin was used as loading control. (C) Alternatively, oxidative stress was assessed in viable GFP+ cells using a DHE probe and flow cytometry. (D-E) CB CD34+ HSPCs were either transduced with the indicated GFP- and shRNA-encoding lentiviral vector and maintained in culture for 3 days (D), or maintained in culture for 3 days and incubated with the indicated drug during the last 18 hours (E). O2.−levels of CD34+ cells were then assessed using a DHE probe and flow cytometry in transduced GFP+ (D) or total (D,E) cells. H2O2 (350 μM) was used as a positive control; data are expressed as MFI (a.u.). H2O2, hydrogen peroxide.
FOXOs have no impact on O2.− levels of HSPCs. (A) Kaplan-Meier survival curves of patients with CN-AML (GSE12417) based on FOXO3 and FOXO1 gene expression levels using median expression value cutoff. Log-rank (Mantel-Cox) test was used to determine statistical significance between groups. (B-C) MV4-11 AML cells were transduced with the indicated GFP- and shRNA-encoding lentiviral vector, or were left untreated (−). After 3 days, (B) protein expression was analyzed via western blot, using the indicated antibodies; β-actin was used as loading control. (C) Alternatively, oxidative stress was assessed in viable GFP+ cells using a DHE probe and flow cytometry. (D-E) CB CD34+ HSPCs were either transduced with the indicated GFP- and shRNA-encoding lentiviral vector and maintained in culture for 3 days (D), or maintained in culture for 3 days and incubated with the indicated drug during the last 18 hours (E). O2.−levels of CD34+ cells were then assessed using a DHE probe and flow cytometry in transduced GFP+ (D) or total (D,E) cells. H2O2 (350 μM) was used as a positive control; data are expressed as MFI (a.u.). H2O2, hydrogen peroxide.
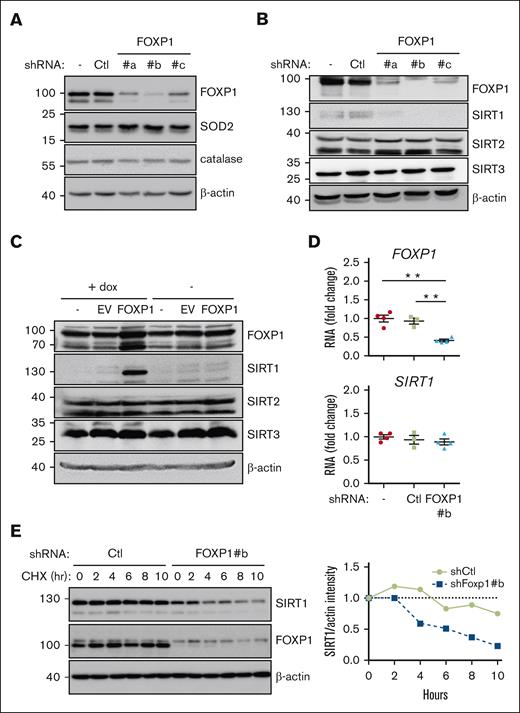
FOXP1 regulates SIRT1 gene expression after transcription. MV4-11 (A-C) or MOLM-14 (D-E) AML cells were transduced with the indicated shRNA- (A-E) or complementary DNA–encoding (C) lentiviral vectors and maintained in culture for 3 days; as indicated, cells were then incubated with cycloheximide for increasing time (E), or with doxycycline for 15 hours (C). (A-C,E) Protein expression was assessed via western blot; β-actin was used as a loading control; as indicated (E) (right), SIRT1 protein expression was quantified, normalized to β-actin level, and expressed relative to levels observed before cycloheximide addition; (D) FOXP1 and SIRT1 RNA levels were assessed via reverse transcription quantitative polymerase chain reaction and expressed relative to mean level of control untransduced (−) cells (n = 4). Ctl, shControl (luciferase); EV, empty vector.
FOXP1 regulates SIRT1 gene expression after transcription. MV4-11 (A-C) or MOLM-14 (D-E) AML cells were transduced with the indicated shRNA- (A-E) or complementary DNA–encoding (C) lentiviral vectors and maintained in culture for 3 days; as indicated, cells were then incubated with cycloheximide for increasing time (E), or with doxycycline for 15 hours (C). (A-C,E) Protein expression was assessed via western blot; β-actin was used as a loading control; as indicated (E) (right), SIRT1 protein expression was quantified, normalized to β-actin level, and expressed relative to levels observed before cycloheximide addition; (D) FOXP1 and SIRT1 RNA levels were assessed via reverse transcription quantitative polymerase chain reaction and expressed relative to mean level of control untransduced (−) cells (n = 4). Ctl, shControl (luciferase); EV, empty vector.
FOXP1 increases SIRT1 expression of myeloid cells
We wished to delineate the molecular mechanism(s) that mediate(s) FOXP1 antioxidant activity. Increased expression of superoxide dismutases (SODs) and catalase reportedly mediates FOXO antioxidant activities in a wide range of cell contexts including murine HSPCs.1,10,31 In concordance with the differential activity observed between FOXOs and FOXP1, FOXP1-KD had no impact on SOD1, SOD2, or catalase expression in all AML cell lines tested (MV4-11, MOLM-14, OCI-AML2, or KASUMI-1); it similarly did not change SOD1/2 expression in healthy primary human CD34+ progenitors (Figure 4A; supplemental Figure 5A,C). Sirtuins (SIRTs), also referred as class 3 histone deacetylases, are nicotinamide adenine dinucleotide–dependent protein deacetylases. SIRT1 regulates numerous cellular processes, including aging, genome stability, DNA repair, metabolism, and oxidative stress responses.32,33 Growing evidence has indicated that SIRT1 acts as a sensor of oxidative stress response in AML cells.34,35 In the hematopoietic system, SIRT1 plays an important role in maintaining HSPC self-renewal and differentiation under stress conditions.36-39 We therefore wondered whether SIRT1 contributes to FOXP1 antioxidant activity. FOXP1 KD decreased SIRT1 expression in all AML cell line tested (MV4-11, MOLM-14, OCI-AML2, and KASUMI-1), whereas SIRT2 or SIRT3 expressions were unchanged (Figure 4B). Similarly, FOXP1 KD inhibited SIRT1 expression in primary AML cells and healthy primary human CD34+ cells (supplemental Figure 5B-C). Conversely, inducible overexpression of FOXP1 transgene by lentiviral transduction triggered a parallel and quick increase of SIRT1 protein level in MV4-11 with no consequences to SIRT2/3 expression (Figure 4C); similar results were obtained using MOLM-14 cell line (supplemental Figure 5D). These data show that FOXP1 regulated SIRT1 protein expression. Surprisingly, despite the well-known FOXP1 transcriptional activities, SIRT1 transcript level did not change upon FOXP1 KD (Figure 4D). These observations suggest that FOXP1 regulates SIRT1 protein levels after transcription. Indeed, SIRT1 protein half-life was reduced in FOXP1-KD cells, as assessed upon cycloheximide treatment (Figure 4E). These results indicate that FOXP1 upregulates SIRT1 expression by enhancing SIRT1 protein stability.
FOXP1 expression level correlates with increased AML cell resistance to cytotoxic chemotherapies
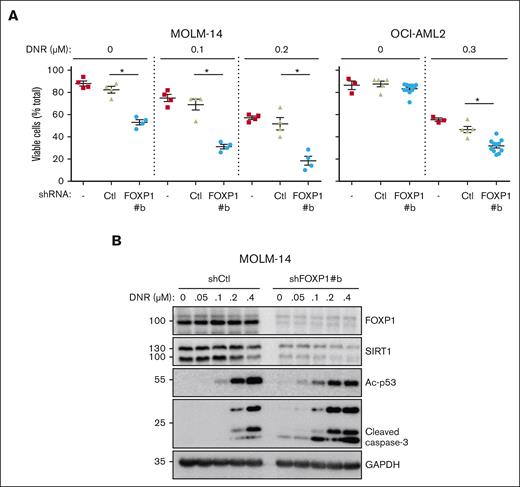
To determine SIRT1 contribution to FOXP1 antioxidant activity directly, SIRT1 complementary DNA was introduced in AML cells to overexpress SIRT1 via an inducible lentiviral vector; FOXP1-KD was induced by shRNA. Despite SIRT1 transduction, FOXP1-KD strongly inhibited SIRT1 expression in AML cells (supplemental Figure 5E). These data helped confirm FOXP1-dependent posttranscriptional regulation of SIRT1. Furthermore, these data directly ruled out rescue experiments to assess SIRT1 contribution to FOXP1 antioxidant activity. We assessed FOXP1/SIRT1 connection indirectly. SIRT1 expression reportedly correlates with increased resistance to the targeted therapy of FLT3-mutated AML cells, counteracts oncogene-induced stress; prevents genotoxic stress–induced p53 acetylation and subsequent activation, limits leukemic cell sensitivity to chemotherapies via restoration of p53 activity,34,35 and contributes to characteristic metabolic reprogramming and autophagy of drug-resistant AML cells.40 Thus, we tested whether FOXP1 exhibited similar activities. shFOXP1- and shCtl-expressing AML cells were treated with increasing concentrations of the cytotoxic drug daunorubicin, and cell viability was assessed and compared via flow cytometry. The combination of FOXP1-KD and daunorubicin treatment strongly enhanced AML cell death, as compared with individual AML cell treatments, as illustrated in FLT3-mutated MOLM-14 or DNMT3A-mutated OCI-AML2 cells (Figure 5A). Moreover, FOXP1 KD enhanced acetyl-p53, in agreement with FOXP1-dependent SIRT1 loss, and further increased caspase-3 cleavage (Figure 5B). These observations indicate that FOXP1 activity protects AML cells against cytotoxic treatments, which is obviated through FOXP1 inhibition.
FOXP1 regulates AML cell resistance to chemotherapy. AML cells were transduced or not (−), with control (Ctl) shRNA- or shFOXP1-expressing vector (#b). Daunorubicin (DNR) was added 2 days later for 24 hours; (A) alive cells were identified by flow cytometry forward and side scatter fluorescence and expressed as percentage of total cell events; difference between control shRNA- and shFOXP1#b-expressing cells were analyzed for significance using a Mann-Whitney test (n = 4). (B) Cells were lysed and protein expression was assessed using western blot, with GAPDH as a loading control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
FOXP1 regulates AML cell resistance to chemotherapy. AML cells were transduced or not (−), with control (Ctl) shRNA- or shFOXP1-expressing vector (#b). Daunorubicin (DNR) was added 2 days later for 24 hours; (A) alive cells were identified by flow cytometry forward and side scatter fluorescence and expressed as percentage of total cell events; difference between control shRNA- and shFOXP1#b-expressing cells were analyzed for significance using a Mann-Whitney test (n = 4). (B) Cells were lysed and protein expression was assessed using western blot, with GAPDH as a loading control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
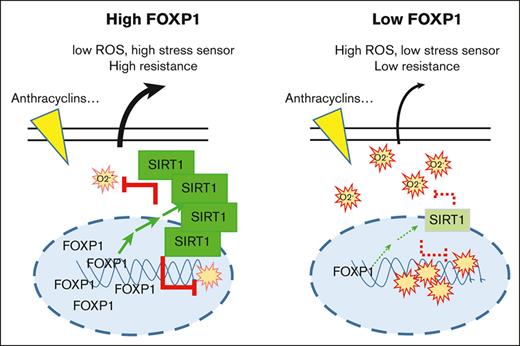
In this study we demonstrate that the transcription factor FOXP1 limits oxidative stress of healthy progenitors and myeloid leukemia cells by decreasing regulatory intracellular O2.− levels, an activity that was not shared by FOXOs. Our data further identify a novel FOXP1/SIRT1 pathway in AML, which drives cytoprotection and contributes to leukemic cell resistance to cytotoxic chemotherapies. These results propose FOXP1 as a novel prognostic marker in CN-AML and as a new promising therapeutic target for patients with AML.
FOXP1 was initially discovered as a transcription factor that contributes to late B-lymphoid cell differentiation. Although widely expressed, FOXP1 was later extensively studied in the context of diffuse large B–cell lymphoma, in which its utility as a prognostic and subtype marker of mature B–cell lymphoma was identified, along with its genetic aberrations and its emerging functional role in lymphoma biology, as reviewed elsewhere.41 Furthermore, it contributes to pre–B acute lymphoid leukemia cell growth and to their resistance to chemotherapeutics.42 In the context of myeloid malignancies, FOXP1 was recently reported as an adverse prognosis factor for patients with AML who were intensively treated and received auto-SCT as a consolidation treatment.21 It was earlier described, in a case report, to be involved in the progression from myelodysplastic syndrome to AML.43 Our data strengthen and extend these observations by revealing that FOXP1 contributes to chemoresistance in patients with AML who are starting conventional therapeutic treatments; this observation may help in choosing appropriate therapeutic strategies based on risk index. Unexpectedly, our study data reveal that FOXOs, as opposed to FOXP1, do not regulate O2.− levels in healthy HSPCs and AML cells in vitro. These data were not anticipated, knowing their well-reported antioxidant properties detected in murine HSC–enriched populations ex vivo.1,10,27 Within the hematopoietic niches of mice with deleted FOXO, these data question the possible long-term impact of FOXO loss on cell development and/or adaptation at organ level. Another possibility exists that FOXOs regulate the oxidative stress of myeloid progenitors by limiting ROSs besides O2.−. Interestingly, antioxidant activities of FOXOs were always linked to the induction of SODs and catalase genes,8 which was not observed upon FOXO-KD in our experimental settings in vitro (data not shown), questioning the true antioxidant properties of FOXOs in human AML cells and highlighting the key role of cell context in gene regulatory pathways.
The underlying molecular mechanisms of FOXP1 oncogenicity are mostly unknown. FOXP1 cooperates with nuclear factor κB or Wnt pathways to enhance B-cell survival in some models, and it mediates the transcriptional repression of important regulators of plasma cell gene signature. Also, FOXP1 was shown to regulate oxidative stress in a few nonhematopoietic cell models, such as glomerular mesangial cells or hairy cells, through altered NADPH oxidase (NOX)2/4 or thioredoxin expression, respectively.19,20 Our studies reveal that FOXP1 contributes to leukemogenesis and chemotherapy sensitivity of myeloid progenitors by enhancing the expression of a new target, the wide cellular stress sensor and deacetylase SIRT1. SIRT1 is a well-known negative regulator of p53 activity, which promotes cell survival under stress conditions in many cells,44 including AML35 and chronic myeloid leukemia (CML) leukemia stem cells.45 Furthermore, SIRT1 works as a cellular metabolism sensor that activates the transcriptional coactivator PGC-1α and also autophagy actors,46 which play a critical role in stress–induced hematopoiesis, metabolic reprogramming, and maintenance of AML/CML leukemic stem progenitor cell regenerative potential selectively; it contributes to the maintenance of CML stem progenitor cells after targeted therapy.6,40,47,48 Whether a similar FOXP1/SIRT1 axis exists in acute lymphoid leukemia cells and sustains leukemic cell resistance to chemotherapy will be worth studying.
SIRT1 overexpression was observed in a number of AML34,35 and CML47 cells; its intensity correlated with poor prognosis, especially in FLT3-ITD AML samples, which exhibited strong SIRT1-dependent cell survival and genotoxic stress protection mediated by p53 inhibition. By inhibiting p53 acetylation, SIRT1 was shown to regulate expression of p21 CDKN1A and p27CDKNIB cell-cycle regulators.49-52 Thus, our data reveal that FOXP1-dependent regulation of SIRT1 expression further contribute to FOXP1-dependent regulation of leukemia cell growth in addition to sensing cell oxidative stress. Interestingly, previous studies in both AML and CML34,35,47 indicated that SIRT1 protein overexpression did not correlate with significant differences in SIRT1 messenger RNA levels in the AML samples/cell lines that harbor activating mutations in oncogenic signaling pathways (FLT3, KIT, KRAS, or NRAS genes). Instead, the increased SIRT1 expression observed was linked to a myc-dependent induction of USP22 deuibiquitinase, leading to reduced SIRT1 ubiquitination and increase SIRT1 stability in leukemic cells.34 Our data extended these observations and indicated that SIRT1 stability is under the control of another transcription factor, namely FOXP1. Of note, we did not observe any change in c-myc or USP22 expression in healthy and malignant myeloid cells. Moreover, SIRT1 expression was unaffected upon various proteasome inhibitor treatments (MG132, bortezomid, and lactacystin) that each lowered the global level of ubiquitylated proteins of shFOXP1-expressing cells (data not shown). Recently, an autophagy-lysosome pathway was reported to regulate SIRT1 stability during aging of several tissues related to immune and hematopoietic systems in mice.53 However, using the same inhibitors, namely, VPS34, PIK inhibitor, and chloroquine, at concentrations that blocked the autophagy/lysosomal pathway of AML cells did not reverse SIRT1 expression upon FOXP1 KD (data not shown). These observations indicate the existence of a different FOXP1-dependent regulatory pathway that modulates SIRT1 half-life. Mechanisms that fine tune SIRT1 expression are known to be quite diverse in mammalian cells.54 The ones that mediate SIRT1 stabilization by FOXP1 in myeloid cells await further investigation.
In addition to AML cells, FOXP1 antioxidant activity, and its associated SIRT1 overexpression, were selectively detected in human-committed, but not HSC-enriched, myeloid progenitors. Interestingly, SIRT1 activity was, however, reported to differentially affect leukemia progenitors compared with normal HSPCs in the steady state: SIRT1-deficient leukemia cells showed a selective and significant reduced basal and maximal mitochondrial respiration, which was not observed in healthy progenitors.47 Numerous studies have demonstrated that ROS-generating agents may serve as enhancers of chemotherapy through elevated rates of oxidative damage and dysregulation of mitochondrial metabolism.6,7,55 Interestingly, FOXP1 did not regulate O2.− levels in mitochondria, which suggest that the O2.− cytosolic pool must be the actual target of FOXP1. Whether FOXP1 regulates NOX activities in AML cells selectively, remains to be studied.
FOXP1 inhibition stops AML cell growth and results in cell death, as we have previously reported.17 Here, we provide evidence that FOXP1 loss further enhances AML cell sensitivity toward chemotherapeutic treatments. Of note, various pharmacologic inhibitors that block FOXO-DNA binding on their selective DNA binding motifs have been identified. Such inhibitors were as active as FOXO-KDs at regulating cell proliferation and metabolic activation; these inhibitors were proposed as new tools in anti–HIV-1 viral therapy.29 Whether similar inhibitors, selectively dedicated to FOXP1-DNA binding inhibition, will contribute to eradicate AML cells will be worth testing.
Altogether, our data from this study have identified FOXP1 antioxidant activity as a new risk factor in AML that contributes to chemotherapy sensitivity; the data propose FOXP1 gene expression as a new risk indicator to classify patients with CN-AML and to design appropriate therapeutic approaches for risk-adapted remission induction.
Acknowledgments
The authors acknowledge the Cytometry and Immunobiology Facility of Institut Cochin/INSERM U1016, and the Obstetric Unit from Orsay Hospital and Cell Therapy Center from St-Louis Hospital (Paris, France) for CB samples.
This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Université de Paris, and Ligue contre le Cancer (ELFUZ17337). The funders had no role in study design, data collection and analysis, publishing decision, or manuscript preparation.
Authorship
Contribution: F.L., S.O., E.L., and I.D.-F. designed the research study and analyzed data; F.L., S.O., T.Z., and D.R. performed experiments; I.D.-F. wrote the paper and original draft; I.D.F., E.L., O.K., M.F., F.P., and D.R. wrote, reviewed, and edited the manuscript; F.L., S.O., E.L., and I.D.-F. created the figures; and D.B., E.L., and I.D.-F. acquired/provided funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Isabelle Dusanter-Fourt, Institut Cochin-INSERM U1016, 27 rue du Fg St Jacques, F-75014 Paris, France; e-mail: isabelle.dusanter@inserm.fr; and Evelyne Lauret, Institut Cochin-INSERM U1016, 27 rue du Fg St Jacques, F-75014 Paris, France; e-mail: evelyne.lauret@inserm.fr.
References
Author notes
∗F.L. and S.O. are joint first authors.
†E.L. and I.D.-F. are joint senior authors.
All data and protocols are available upon request to the corresponding authors, Isabelle Dusanter-Fourt (isabelle.dusanter@inserm.fr) and Evelyne Lauret (evelyne.lauret@inserm.fr).
The full-text version of this article contains a data supplement.

![FOXP1 loss enhances HSPC oxidative stress. CD34+ HSPCs were isolated from healthy human CB samples and transduced with the indicated GFP- and shRNA-encoding lentiviral vectors directed against FOXP1 (#a, #b, and #c) or luciferase as a control (Ctl). After 3 days, FOXP1 (A; bottom) and NRF2 (B) protein expression was assessed using western blot; β-actin was used as a loading control; size markers (kDa) are indicated on the left (1 representative experiment; n ≥ 3); (A [top],C) O2.− levels of total (−, untreated) or GFP+ cells were assessed using a DHE probe and flow cytometry in total CD34+ (A) (n = 7), and CD34+CD38+ and CD34+CD38− (C) (n = 3) cell populations. Data are expressed as MFI (a.u.); 1 spot represents 1 independent sample from independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/13/10.1182_bloodadvances.2022008585/2/m_blooda_adv-2022-008585-gr2.jpeg?Expires=1769146021&Signature=D0Dhpl2yxKfknhLOaj65Xy2YxVfD4zM5zXRjUXzI-X5ibrSxuwZXi5nD4G2YSCrscEtbf6aIJN5ed94nYrdhZjNxAZk5~JBQBTBNUkKmZLTlaJMM3qj39WDRIisHtkhfvx50oFmjRVUbJWqr~Tgyo3gzQ-h6lkKZ3ik8PvgnrcHH5itqKAsK9Oz1VhTY9Kqu1MqVBlb0ZVgGkFn6AKyVuwHd1rvp462qX1PsKB6pG81LajFEZpQv2McQnCCA8T97vVh4pIyDAsOe6mE5kyDK743fTe2ZWk~rcTKFTt4x4GxILbRH0cBWbbRBHNXJKIdrMrIrI24Z5sECEx0RH8Yoeg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)