Abstract
Anticoagulant treatment in patients with primary and metastatic brain cancer is a concern due to risk of intracranial hemorrhage (ICH). We performed a systematic review and meta-analysis to evaluate the risk of ICH in patients with primary or metastatic brain cancer treated with or without anticoagulants. Articles on ICH in patients with primary or metastatic brain cancer treated with or without anticoagulants published up to September 2021 were identified by searching PubMed, EMBASE, and Cochrane Library databases. The primary outcome of this analysis was ICH. Thirty studies were included. Rate of ICH was 13.0% in 1009 patients with metastatic brain cancer and 6.4% in 2353 patients with primary brain cancer (relative risk [RR], 3.26; 95% confidence interval [CI], 2.69-3.94; I2 = 92.8%). In patients with primary brain cancer, ICH occurred in 12.5% and 4.4% of patients treated with or without anticoagulants, respectively (11 studies, 659 treated and 1346 not treated patients; RR, 2.63; 95% CI, 1.48-4.67; I2 = 49.6%). In patients with metastatic brain cancer, ICH occurred in 14.7% and 15.4% (5 studies, 265 treated and 301 not treated patients; RR, 0.92; 95% CI, 0.43-1.93; I2 = 0%). ICH occurred in 8.3% of 172 treated with direct oral anticoagulants (DOACs) and in 11.7% of 278 treated with low-molecular weight heparin (LMWH) (5 studies; RR, 0.44; 95% CI, 0.25-0.79; I2 = 0%). Patients with metastatic brain cancer have a particularly high risk of ICH. Patients with primary brain cancer have an increased risk of ICH during anticoagulation. DOACs are associated with a lower risk of ICH than LMWH.
Introduction
It is estimated that 24 000 new cases of primary brain tumors and 200 000 new cases of brain metastatic cancers occurred in 2020 in the United States.1 Up to 20% to 25% of metastases spread to the central nervous system, with the highest incidence in patients with breast, melanoma, kidney, and non–small cell lung cancer. Spontaneous intracranial hemorrhage (ICH) is a common complication in patients with primary brain cancer and brain metastases.2 The clinical presentation of ICH is extremely heterogeneous, ranging from asymptomatic deposition of hemosiderin within the tumor seen on neuroimaging to large bleeds that cause clinical symptoms, either focal or related to intracranial hypertension.2,3 The incidence of ICH in patients with primary brain cancer and brain metastases varies according to the imaging diagnostic criteria and the cancer histotype, with rates as high as 50% in patients with brain metastases from melanoma, thyroid carcinoma, renal cell carcinoma, and choriocarcinoma.3-5
When, for any reason, an anticoagulant treatment is required, in patients with primary brain cancer and brain metastases, the conflict between the need for anticoagulation and the risk of bleeding is a challenge. In the context of a timely and debated medical issue, we report the results of a systematic review and meta-analysis in patients with primary brain cancer or brain metastases treated with or without anticoagulant therapy at therapeutic doses to provide summary estimates of ICH across studies, to evaluate these rates in patients with primary or metastatic brain cancer separately, and to investigate the impact of anticoagulation as well as of different anticoagulation strategies on ICH rates.
Materials and methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement6 (http://www.prisma-statement.org). This meta-analysis has been registered in PROSPERO.
Search strategy
We performed an unrestricted search in PubMed, EMBASE, and the Cochrane Library databases from inception to September 2021. The following search terms were used to search clinical trials, registers, and databases: “glioma, glioblastoma, oligodendroglioma, astrocytoma, oligoastrocytoma, brain metastasis” and “anticoagulant, heparin, low‐molecular‐weight heparin, vitamin K antagonist, oral anticoagulant, direct oral anticoagulant” and “hemorrhage, haemorrhage.” Additional studies were identified by hand searching of reference lists of the reviews and retrieved articles. Eligibility assessment was performed independently by two authors (G.P., M.G.), using a data extraction form, in an unblinded standardized approach. Study selection was initially performed by review of titles, and candidate abstracts were then reviewed. A third reviewer (M.M.) resolved disagreements between reviewers and any differences in study selection.
Study selection
Inclusion criteria of this meta-analysis were: (1) randomized clinical trials or observational studies; (2) patients aged ≥18 years with primary brain cancer and/or brain metastases treated with or without therapeutic doses of anticoagulants; and (3) availability of number of patients who experienced ICH. No language, publication date, or publication status restrictions were imposed. Review articles not reporting original data, case reports and case series with <10 patients, abstracts, editorials/letters, and studies not involving humans were excluded. Studies reporting on the rate of ICH in patients with primary brain cancer or brain metastases who received thromboprophylaxis for venous thromboembolism (VTE) were also excluded. The inter-reviewer agreement for study selection was assessed by the κ statistic, which measures agreement beyond chance.7
Study objectives and outcomes
The primary objective of this meta-analysis was to evaluate the overall incidence of ICH in patients with primary brain cancer or brain metastases treated with or without anticoagulants at therapeutic doses. The secondary objectives were to evaluate: (1) the rate of major and fatal ICH; (2) the rate of ICH in relationship to anticoagulant therapy; and (3) the rate of ICH according to the type of anticoagulant treatment. The primary outcome was ICH. Secondary outcomes were major ICH and fatal ICH.
Data extraction
The following data were extracted from each included trial: (1) general data (study design and year of publication); (2) characteristics of trial participants (number, mean age, sex, site of cancer, and cancer histotype); (3) type of anticoagulant (agent, dose, duration, and daily dosing); and (4) study outcomes (primary outcome, secondary outcomes, and length of follow-up). The quality of the studies was evaluated by using the Newcastle-Ottawa quality assessment scale (range, 1-9 [1-3 indicates low quality, 4-6 indicates moderate quality, and 7-9 indicates high quality]).
Statistical analysis
Pooled outcome event rates were calculated by using the logit transformed proportion and corresponding sampling variances. The rates of ICH were pooled by using random effects models and are presented with the corresponding 95% confidence interval (CI). Heterogeneity was assessed by the I2 test. I2= 0 was considered to indicate no heterogeneity; I2 < 25%, 25% to 75%, and >75% were considered to indicate low, moderate, and high degrees of heterogeneity, respectively.13 To evaluate publication bias, both Egger’s test and funnel plots of the logit transformed proportion vs standard error were computed. If the Egger’s test confirmed asymmetry, the Duval and Tweedie’s trim-and-fill procedure was used to compute an unbiased estimate of the effect size. A mixed effects meta-regression analysis was performed to test differences among subgroups according to the median length of follow-up and the median length of anticoagulation therapy. We also determined pooled relative risk (RR) and 95% CI using a random effects model for ICH in patients with primary brain cancer or brain metastases treated with or without anticoagulant therapy and according to the anticoagulant type. For studies presenting zero cells, 0.5 was added for a correct estimation of risk measures. The following prespecified sensitivity analyses were performed: (1) rate of ICH in primary brain cancer only; (2) rate of ICH in brain metastases only; (3) rate of major ICH in primary brain cancer or brain metastases; (4) rate of ICH according to the type of anticoagulant treatment; (5) rate of fatal ICH; and (6) rate of ICH in patients with VTE.
The statistical analyses, forest plots, and publication bias analyses were produced with Comprehensive Meta-Analysis version 3.0 (Biostat Inc.). P values <.05 were considered statistically significant.
Results
The search of PubMed, EMBASE, and Cochrane Library databases provided a total of 692 articles, while 3 of them were identified through other sources. After removal of duplicates, as well as an additional 530 articles that did not meet the inclusion criteria at abstract review, the full text of the remaining 64 articles was examined in detail. Of these, 34 studies did not meet the inclusion criteria. Thus, 30 studies were included in the systematic review.3 -5,8 ,,-12,14 ,,,,,,,,,,,,,,,,,, -35 No unpublished relevant studies were found. The flow diagram of the literature search is shown in supplemental Figure 1, and the main features of the studies are reported in Table 1 and supplemental Tables 1 and 3. The inter-reviewer agreement for study selection was very good (κ statistic, 0.85).
The studies selected for the review included 3893 patients (range for individual studies, 16-364 patients), and all were retrospective. Fifteen studies included patients with primary brain cancer only,4,9,11,12,15,17,21,22,24,28,30,31,33 -35 six studies included patients with brain metastases only,3,5,10,16,18,27 and nine studies included both patients with primary brain cancer and patients with brain metastases.8,14,19,20,23,25,26,29,32 Overall, this analysis includes 2353 patients with primary brain cancer and 1009 with brain metastases. Seven studies (531 patients) did not separately report ICH occurring in primary cancer or brain metastases.17,19,23,25,26,29,32
Mean age varied from 51 to 72 years, and men were slightly more represented than women. Seventeen studies (2896 patients) included both patients treated (1072 patients) and not treated (1824 patients) with anticoagulants, whereas 13 studies (997 patients) included only those treated with anticoagulants. The main indication for anticoagulant treatment was acute VTE (25 studies, 3313 patients), followed by atrial fibrillation (2 studies, 268 patients), cerebral vein thrombosis (2 studies, 187 patients), or any indication for anticoagulant treatment (1 study, 125 patients) (Table 1).
The anticoagulant agent was heparin in 21 studies (1338 patients),3 -5,8 -12,14 -25 warfarin in 13 studies (475 patients),4,5,12,17,22 -24,30 ,,,-35 and direct oral anticoagulants (DOACs) in 5 studies (172 patients).8 -10,14,16
The median patient’s observation period was 125 days, ranging from to 27 days and 240 days. The median duration of anticoagulant treatment at the time of ICH was 8.1 months.
The quality of studies assessed according to the Newcastle-Ottawa quality assessment scale was poor in 22 studies and good in 8 studies. The quality assessment is reported in supplemental Table 2.
Rates of ICH in primary or metastatic brain cancer
Overall, the weighted incidence rates in patients with primary brain cancer or brain metastases estimated by using a random effects model was 7.7% (95% CI, 5.1-11.5; I2 = 92.8%; 445 events in 3893 patients) (Table 2). Egger’s tests revealed the presence of publication bias (t = 5.90; P < .001). After using the trim-and-fill procedure, 5 studies were trimmed, and the ICH adjusted rate was 9.1% (95% CI, 6.2-13.2). Bias assessment plots are reported in supplemental Figure 2A.
Rates of ICH in patients with primary brain cancer were reported in 18 studies and ranged from 1.1% to 25.4%.4,9 -12,16,18 -21,23,27,28,30,31,33 -35 The weighted incidence rate was 6.4% (95% CI, 4.1-9.9; I2 = 84.4%; 156 events in 2353 patients) (supplemental Figure 3A), and the risk of publication bias was significant (t = 2.39; P = .03) (supplemental Figure 2B). Adjusted value after the trim-and-fill procedure was 7.5% (95% CI, 4.9-11.3).
Rate of ICH in patients with metastatic brain cancer was reported in 9 studies and ranged from 2.7% to 47.6%.3,5,9,12,14 -16,18,26 In these studies, the weighted incidence rate of ICH was 13.0% (95% CI, 6.5-24.2; I2 = 93.7%; 218 events in 1009 patients) (supplemental Figure 3B). The risk of publication bias was significant (t = 5.10; P = .001); after the trim-and-fill procedure, 2 studies were trimmed, and the adjusted rate of ICH was 17.5% (95% CI, 9.6-29.8) (supplemental Figure 2C).
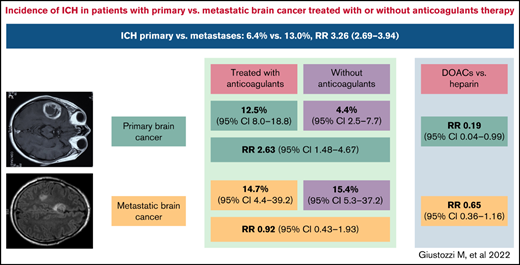
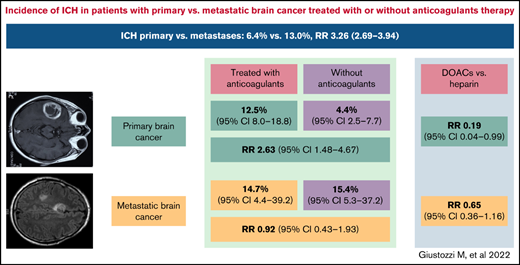
Risk of ICH was significantly higher in patients with metastatic brain cancer than in patients with primary brain cancer (RR, 3.26; 95% CI, 2.69-3.94; I2 = 92.8%).
At meta-regression analysis, the median length of a patient’s observation period (P = .99; I2 = 87.7%) and the median length of anticoagulant therapy before ICH (P = .763; I2 = 92.4%) did not influence the rate of ICH.
For patients with VTE, the overall weighted incidence rate of ICH was 7.1% (95% CI, 4.4-11.5; I2 = 93.7%; 25 studies, 384 events in 3313 patients)3 -5,8,9,11,12,14,15,20 ,,,,,,,,,,,, -35 (Table 2). Specifically, the weighted incidence rate of ICH was 6.1% (95% CI, 3.7-9.7%; I2 = 85.3%; 17 studies) and 13.6% (95% CI, 5.6-29.2; I2 = 94.1%; 6 studies) in patients with primary and metastatic brain cancer, respectively. Rates of major and fatal ICH in patients with primary or metastatic brain cancer are reported in Table 2.
Rates of ICH in patients treated with or without anticoagulant therapy
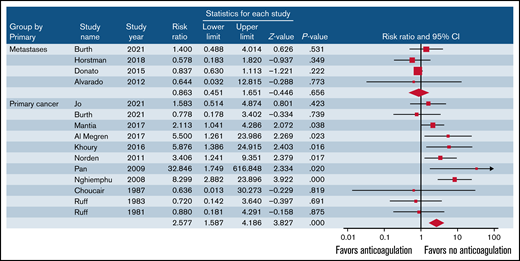
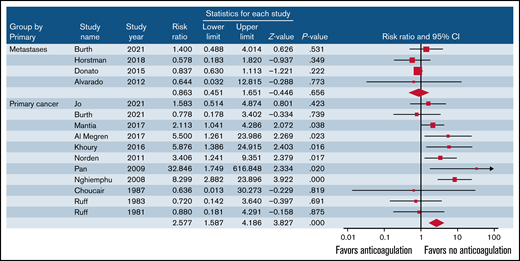
Overall, rates of ICH in patients treated with anticoagulants was 11.5% (95% CI, 7.4-17.6; I2 = 83.7%; 152 events in 1072 patients) and 6.0% in those not treated with anticoagulants (95% CI, 3.0-11.5; I2 = 92.2%; 177 events in 1824 patients) (RR, 1.81; 95% CI, 1.15-2.84; P = .001; I2 = 60.3%) (Table 3). No publication bias was observed (t = 1.41; P = 0.17). In patients with primary brain cancer, anticoagulant therapy was associated with an increased risk of ICH and of major ICH (Figure 1; Table 3). In patients with metastatic brain cancer, anticoagulant therapy was not associated with an increased rate of ICH. Fatal ICH while on anticoagulant therapy was reported in 11 studies, and the weighted incidence rate was 2.7% (95% CI, 1.6-4.5; I2 = 0%; 13 events in 764 patients).4,13,14,18,21,25,26,30,32,34,35
Risk of ICH in patients with primary or metastatic brain cancer treated with or without anticoagulants.
Risk of ICH in patients with primary or metastatic brain cancer treated with or without anticoagulants.
Rate of ICH according to the type of anticoagulant treatment
In patients with primary brain cancer or brain metastases, DOACs were associated with a lower risk of ICH than low-molecular weight heparin (LMWH) (8.3% vs 11.7%; RR, 0.44; 95% CI, 0.25-0.79; I2 = 0%; 5 studies in 450 patients) (supplemental Figure 4A).8 -10,14,17 In patients with primary brain cancer, DOACs were associated with a reduced risk of ICH (RR, 0.19; 95% CI, 0.04-0.99; I2 = 0%; 0 events in 69 DOAC-treated patients and 19 events in 95 LMWH-treated patients, 4 studies). In patients with brain metastases, 12 events were observed in 103 DOAC-treated patients and 52 events in 183 LMWH-treated patients (RR, 0.65; 95% CI, 0.36-1.16; I2 = 0%; 3 studies). When considering only studies in patients receiving anticoagulants for VTE, the RR for ICH was 0.36 (95% CI, 0.18-0.71; I2 = 0%; 4 studies) in patients treated with DOACs (8 events in 131 patients) compared with LMWH (64 events in 223 patients).9,11,14,16 Risk of ICH was not significantly different with LMWH vs warfarin (4 studies, 15 events in 211 LMWH-treated patients vs 8 events in 198 warfarin-treated patients) (RR, 1.45; 95% CI, 0.56-3.79; I2 = 0%) (supplemental Figure 4B).17,22,23,27
Discussion
The current meta-analysis provides the following findings: (1) the rate of ICH and major ICH is higher in patients with metastatic brain cancer compared with those with primary brain cancer; (2) anticoagulant therapy is associated with an increase in ICH and major ICH in patients with primary brain cancer but not in those with brain metastases; and (3) the risk of ICH is lower in patients with primary or metastatic brain cancer treated with DOACs compared with those treated with LMWH.
According to international guidelines, presence of an intracranial primary or metastatic brain cancer is not an absolute contraindication for anticoagulation.36 Nevertheless, limited data support the use of anticoagulant therapy in these patients. In this context of uncertainty, our results may be relevant and timely for clinical decision-making and design of future clinical trials. Of potential clinical interest, our analysis provides data on brain cancer overall as well as separately in patients with primary or metastatic brain cancer. Data are also provided concerning the rate of ICH in patients treated with or without anticoagulants.
In this meta-analysis, the rates of ICH and major ICH were higher in patients with metastatic brain cancer than in patients with primary brain cancer. The safety profile of anticoagulant therapy seems to be different in patients with primary or metastatic brain cancer. Of clinical relevance, in patients with primary brain cancer, therapeutic anticoagulation was associated with an increased risk of ICH and major ICH. In contrast, in patients with metastatic brain cancer, the administration of anticoagulant therapy was not associated with an increased rate of ICH. Although ICH from metastatic brain cancers is a relatively common clinical observation, its pathogenesis has not been fully elucidated. Several biomarkers involved in angiogenesis reportedly contribute to the vascular instability of brain metastases.37-39 Our meta-analysis was not able to elucidate the risk of bleeding associated with specific tumor histotypes. Future studies are needed to definitively establish rates of bleeding and the safety of anticoagulation in malignancies associated with high ICH rates, including metastatic melanoma, choriocarcinoma, and renal cell carcinoma.
Due to the high bleeding risk, patients with brain cancer should be, as a priority, the target for future studies with new, potentially safer anticoagulant agents, including the anti–factor XI inhibitors. These agents have been recently shown to be associated with a lower risk of bleeding than LMWH40,41 when given for thromboprophylaxis in patients who underwent major orthopedic surgery.
The data regarding the relative safety of anticoagulation in patients with brain metastases seem to be reassuring. Thus, exclusion of these patients from clinical trials on the treatment of cancer-associated VTE should no longer be supported, although caution should remain for patients with primary brain cancer. It should be recommended that patients with primary or metastatic cancer, respectively, should be subjected to a priori stratification before study randomization.
Interestingly, although the number of patients on DOACs in our analysis was relatively low, we found that treatment with DOACs was associated with a lower risk of ICH than treatment with LMWH in patients with primary or metastatic brain cancer, with no heterogeneity across studies. These results are in agreement with recent studies suggesting the safety profile of DOACs in terms of ICH rates in patients with brain cancer42,43 and make these agents an attractive strategy in particular for VTE treatment. Indeed, in our study, anticoagulants were mostly used for the treatment of VTE, and DOACs were associated with one-third the risk of ICH compared with that of LMWH.
The overall rates of ICH varied considerably, ranging from 1.4% to 47.6%. Specifically, among patients receiving anticoagulant therapy, the rate of ICH in patients with primary and metastatic brain cancer ranged from 1.4% to 29.0% and from 2.7% to 47.6%, respectively. Several reasons can justify these large ranges: the retrospective design of the included studies, the heterogeneity in ICH monitoring, the definition of ICH and imaging modalities used, cancer histotype, and the type of the adopted anticoagulant therapeutic strategy.
Our meta-analysis had several limitations, including the fact that none of the studies in the analysis was prospective. Furthermore, for different subgroup analyses, there was a significant heterogeneity due to differences in study target populations or targeted effects, protocol-scheduled imaging, types and duration of anticoagulant therapy, and/or analytical methods, including covariate adjustments. Moreover, the definition of major and nonmajor was not formally standardized, although the same definition was consistently reported in different studies.3,4,8 , -12 Finally, due to the paucity of data, we were not able to distinguish rates of ICH in patients with brain cancer treated with or without chemotherapy or radiotherapy.
Nevertheless, our meta-analysis has several strengths, including: (1) an extensive search of available data that makes this meta-analysis the most updated reported so far; (2) the punctual estimation of the rates of ICH and major ICH in large series of patients with primary or metastatic brain cancer; and (3) the assessment of RRs in patients with primary or metastatic brain cancer treated with or without anticoagulants.
In summary, our study confirms the not-negligible risk of ICH in patients with primary brain cancer or brain metastases. Anticoagulation is associated with an increase in the risk of ICH and major ICH in patients with primary brain cancer. This increase in risk does not seem to occur in patients with brain metastases. DOACs seem to be associated with a lower risk of ICH than LMWH. Prospective controlled studies on new anticoagulant strategies, potentially associated with a reduced risk of bleeding, are needed in patients with brain cancer, with these patients and clinical settings as a priority in the unmet clinical need-based clinical research.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship
Contribution: M.M. and M.G. were responsible for study conception and design; G.P., M.G., and M.M. were responsible for data acquisition; and M.G. and C.B. performed the statistical analysis; and all authors contributed to interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and gave final approval of the manuscript.
Conflict-of-interest disclosure: C.B. reports lecture fees and consultancies for Bayer Health Care, Bristol Myers Squibb, and Daiichi Sankyo outside the submitted paper. G.A. reports lecture fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, and Daiichi Sankyo outside the submitted paper. M.M. received honoraria for participation at advisory boards from Novartis, BMS, MSD, Pierre Fabre, and Sanofi; reports lecture fees from Novartis, BMS, MSD, Pierre Fabre, and Sanofi; and reports grants from Roche and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Mario Mandalà, Unit of Medical Oncology, University of Perugia, Santa Maria della Misericordia Hospital, Perugia, Italy; e-mail: mario.mandala@unipg.it.
References
Author notes
Requests for data sharing may be submitted to the corresponding author (mario.mandala@unipg.it).
The full-text version of this article contains a data supplement.