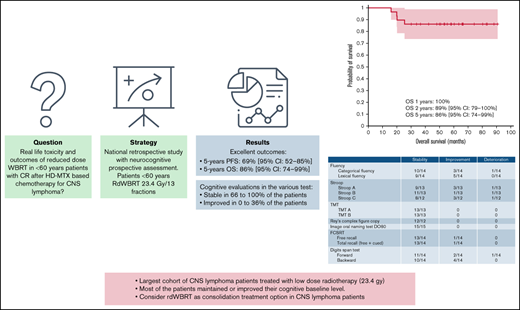
Key Points
After consolidation rdWBRT in a subset of patients, most of the patients exhibited sustained or improved cognitive function.
rdWBRT should be considered a strong consolidation treatment for PCNSL in patients aged <60 years showing CR after induction chemotherapy.
Abstract
The optimal consolidation strategy for primary central nervous system lymphoma (PCNSL) remains controversial. Preventing radio-induced neurotoxicity of consolidation treatment through reduced-dose whole-brain radiotherapy (rdWBRT) at a dose of 23.4 Gy is an interesting alternative to conventional WBRT in patients aged <60 years. From the LOC Network (Network for Oculo-cerebral Lymphomas) database, we retrospectively selected patients with PCNSL aged <60 years who showed complete (CR) or unconfirmed CR after high-dose methotrexate–based chemotherapy and had received consolidation rdWBRT as the first-line treatment. If available, prospective neuropsychological follow-ups were reported. Twenty-nine patients diagnosed between 2013 and 2018 met the study selection criteria. Nine (31%) patients experienced relapse during the follow-up, with a median time from radiotherapy to recurrence of 8.7 months (interquartile range, 4-11.5). Five of those patients received salvage treatment and consolidation with intensive chemotherapy and autologous stem cell transplantation. Progression-free survival rates were 89% (95% confidence interval [CI] 79%-100%), 72% (95% CI, 56%-88%), and 69% (95% CI, 52%-85%) at 1, 2, and 5 years, respectively. Overall survival rates were 100%, 89% (95% CI, 79%-100%), and 86% (95% CI, 74%-99%) at 1, 2, and 5 years, respectively, and were consistent with those observed for standard-dose WBRT (sdWBRT). No prognostic factor was identified. The results of the 36-month neuropsychological follow-up for a subset of patients appeared reassuring, with most patients exhibiting maintenance of or improvements in their baseline conditions. Our results, combined with phase 2 study results, support the use of rdWBRT instead of sdWBRT as a consolidation treatment in <60-year-old patients showing CR after induction treatment.
Introduction
Treatment for primary central nervous system lymphoma (PCNSL) has improved significantly over the past 20 years, and the 5-year overall survival (OS) for patients who receive high-dose methotrexate (HD-MTX)–based chemotherapy is ∼30%.1 However, regardless of age, the optimal treatment strategy for newly diagnosed PCNSL remains controversial. There is wide acceptance that HD-MTX-based chemotherapy, as an induction treatment, achieves high objective response rates and improves survival in this disease.2,3 However, a high rate of relapse occurs after HD-MTX–based chemotherapy when administered alone, but can be improved by the use of consolidation treatment.4-6 The first consolidation option used for the disease was whole-brain radiotherapy (WBRT). In the past 2 decades, the use of this therapy has been challenged by autologous stem cell transplant (ASCT) consolidation treatment for patients <60 to 70 years of age.7,8 However, the best consolidation strategy remains to be determined because of conflicting results of the 2 largest randomized phase 2 studies.7,8 The risk of radiation-induced, late-delayed neurotoxicity in patients who achieve long-term disease control constrains the use of consolidation standard-dose WBRT (sdWBRT).9 The detrimental effect of radiation on neural progenitor cells has been well documented in preclinical animal models, providing some explanation for the clinical neurotoxicity observed in humans treated with brain radiation.10 Clinical symptoms of neurotoxicity can range from mild short-term memory difficulties to more significant sequelae, such as gait disturbances, incontinence, or disabling dementia. The overall 5-year incidence of neurotoxicity is estimated to be 24%.9 Patients >60 years old are most vulnerable to the neurotoxic side effects of consolidative WBRT11 and show a rapid onset of severe symptoms. Patients aged <60 years may also be affected but less frequently and less severely, and they may be affected later. However, their quality of life can be altered, resulting in an incapacity in conducting their professional activities. Based on retrospective and prospective data, severe radiation-induced neurotoxicity affects 8% to 16% of patients <60 years of age.9,11-13 To reduce the presence of cognitive side effects, Morris et al proposed and reported a phase 2 study assessing the efficacy of rituximab, MTX, procarbazine, and vincristine (R-MPV) followed by consolidation reduced-dose WRBT (rdWBRT) and cytarabine. Patients showing complete response (CR) after R-MPVR-MPV received rdWBRT at a dose of 23.4 Gy in 13 fractions; otherwise, sdWBRT was offered (45 Gy, 25 fractions). The primary end point was 2-year progression-free survival (PFS). Thirty-one patients (60%) achieved CR after R-MPV and received rdWBRT. The 2-year PFS in this group was 77%, and the median PFS was 7.7 years.14 Of the 15 patients <60 years of age who received rdWBRT, 9 remained progression free and completed neuropsychological evaluations through up to 48 months. Among these patients, no evidence of significant cognitive decline was observed during the follow-up period. Thus, in a selected population, the long-term outcome achieved with R-MPV, rdWBRT, and consolidative HD arabinosylcytosine (Ara-C) was encouraging when compared with that realized with the historical MPV protocol.
The guidelines of the LOC network (“Réseau Expert National pour les Lymphomes Oculo-Cérébraux”), which is the French national expert network on PCNSL, were updated after the end of inclusion of the PRECIS trial and the results from Morris phase II study.7 PRECIS was a phase 2 randomized study evaluating the use of WBRT and ASCT as consolidation treatments after induction chemotherapy consisting of 2 cycles of R-MBVP (rituximab, methotrexate, BCNU, VP16, and prednisone) followed by 2 cycles of R-AraC in immunocompetent patients aged <60 years. Beginning in 2014, the guidelines of the French LOC network offered 2 consolidation options for patients aged <60 years showing a CR after HD-MTX-based chemotherapy: intensive chemotherapy with autologous stem cell transplantation (IC-ASCT) or 23.4-Gy rdWBRT. In these guidelines, rdWBRT was not considered to be an option for patients aged >60 years, considering that there were no published safety data regarding rdWBRT in the elderly (in the Morris study, 11 patients >60 years of age received rdWBRT, but only 3 benefited from a neuropsychological follow-up).14
The purpose of this retrospective study was to analyze the toxicity and outcomes of rdWBRT in patients <60 years of age who had a CR after HD-MTX–based chemotherapy in a real-life setting.
Patients and methods
Population and inclusion criteria
Patients were selected from the LOC Network database, a nationwide database that contains centralized information on patients with newly diagnosed PCNSL treated in 10 expert centers in France since 2011. Patients were retrospectively selected according to the following criteria: (1) pathological diagnosis of diffuse large B-cell PCNSL; (2) age range, >18 and <60 years; (3) immunocompetent status; (4) use of first-line induction treatment based on HD-MTX (at least >1.5 g/m2 per injection); and (5) CR (confirmed or unconfirmed [uCR]) according to the International PCNSL Collaborative Group criteria after first-line induction treatment.15 All cerebral magnetic resonance images (MRIs) obtained at the end of induction treatment were reviewed by a neuro-oncologist (C.H.), and (6) rdWBRT at a dose of 23.4 Gy in 13 fractions of 1.8 Gy was performed as consolidation treatment after first-line induction treatment. Other reduced-dose irradiation schedules were not considered. Patients treated with a sequential focal boost and those with isolated primary vitreoretinal lymphoma were excluded from the study.
The study was approved by the Institutional Ethical Committee of the coordinating center and by the French Commission Nationale de l’Informatique et des Libertés. All patients provided informed consent.
Treatment and assessment of therapeutic response
After the completion of HD-MTX–based induction chemotherapy, all patients received rdWBRT according to the LOC Network guidelines. The patient was positioned supine on a couch and immobilized with a commercial mask fixation system. All irradiations were conducted with a 2- to 3-mm-thick slice dosimetric computed tomographic scan. Three-dimensional conformational radiotherapy was delivered with 2 opposite lateral fields as follows: 23.4 Gy in 13 fractions of 1.8 Gy, with 5 fractions per week. The 95% isoline was designed to encompass the inner table of the skull. Clinical target volumes included the whole brain extending to the second cervical vertebral body. The posterior one-third of the orbits were included in the irradiated volume. Early response corresponded to the first MRI evaluation after 2 months of induction treatment. During the follow-up, clinical and MRI evaluations were performed 1 to 2 months after the end of rdWBRT and then every 3 months for 2 years, followed by every 6 months until relapse or progression.
The Fazekas score was used to evaluate the white matter disease induced by rdWBRT. The median periventricular and deep white matter Fazekas scores were assessed at the last follow-up.16 The Fazekas score was calculated only for patients from centers for which neuropsychological testing was available. Fluid-attenuated inversion recovery MRI sequences were reviewed for white matter changes and rated as per the modified Fazekas scale as follows: grade 0, no white matter change; grade 1, minimal patchy white matter foci; grade 2, start of confluence of white matter disease; grade 3, large confluent areas; grade 4, confluence of white matter changes with cortical and subcortical involvement; and grade 5, diffuse leukoencephalopathy with widespread and diffuse white matter disease.
End points
Responses were assessed according to IPCG recommendations.15 OS and PFS were calculated from the date of histologic diagnosis. PFS events included lymphoma progression or death from any cause. Patients were evaluated at the last follow-up. Adverse events related to irradiation were assessed according to Common Terminology Criteria for Adverse Events (CTCAE) 4.0 criteria. Outcomes were compared with those of arm A (induction chemotherapy and sdWBRT consolidation) of the PRECIS trial.
Neuropsychological analysis
Neuropsychological evaluations were not available from all LOC network centers. Neuropsychological evaluations included the assessment of 4 cognitive domains by trained neuropsychologists. The following domains were explored with 1 or more tests:
- 1.
Executive functions: the Stroop test,17 the Trail Making Test,18 and a test of verbal fluency, including lexical and categorical fluency.19
- 2.
Language: the Image Oral Naming Test DO8020 and a test of verbal fluency including lexical and categorical fluency.19
- 3.
Visuoconstructive functions: Rey's Complex Figure Copy.21
- 4.
Memory: episodic memory assessed by the Free and Cued Selective Reminding Test,22,23 and working memory by the Wechsler Adult Intelligence-IV battery digit span test.24
Neuropsychological test scores were converted to standardized z scores (Stroop test, verbal fluency test, Image Oral Naming Test DO80, and Battery Digit Span Test) or percentiles (TMT, Rey’s complex figure copy, and Free and Cued Selective Reminding Test). The conversion of test scores to z scores or percentiles was performed according to sex, age, and socioeconomic status. The pathological threshold corresponded to a z score ≤ −1.65 or a percentile ≤5, and the borderline threshold corresponded to a z score of (min;max) −1.65; −1 and a percentile of 5; 16. Test results above a z score of −1 and those above a percentile of 16 were considered normal.25-27
Neuropsychological evaluations were performed at baseline (from 1 month before to 3 months after radiotherapy) and then at 18 months (±6 months), 36 months (±6 months), 48 months (±6 months), and 60 months (±6 months) in patients who maintained a CR.
In this article, we report only data from patients evaluated at least at baseline and once more at 36 months or thereafter.
Statistics
Descriptive analyses were performed using frequency parameters expressed as percentages and median parameters expressed with their interquartile range [IQR] 25-75. The Kaplan-Meier method was used to assess OS and PFS. Univariate analysis was performed using the log-rank test for categorical variables and the univariate Cox model for quantitative continuous variables. Multivariate analysis was not performed because of the sample size and low number of events. P < .05 was the threshold for statistical significance.
Statistical analysis was performed with XLstat 2016 (Addinsoft).
Results
Population characteristics
Between 2013 and 2018, 63 patients received WBRT as consolidation treatment. Thirty-four patients in the database were excluded because they received sdWBRT or alternative rdWBRT (20 30 Gy in 10-15 fractions). Finally, 29 patients diagnosed between 2013 and 2018 met the study selection criteria.
The patient characteristics are indicated in Table 1. The median age was 52 years (IQR, 46-55), and the median Karnofsky Performance Status (KPS) at diagnosis was 80 (IQR, 70-90). Twenty (69%) patients exhibited multifocal disease at diagnosis, with cerebral spinal fluid (CSF) involvement in 6 cases and ophthalmic involvement in 4 cases. Patients received a median number of 8 (IQR, 6-8) infusions of IV HD-MTX combined with rituximab in 100% of cases. Twenty-five patients (25 of 29; 86%) received MTX doses equal to or higher than 3 g/m2, and all patients received high-dose cytarabine (1 or 2 cycles). At the beginning of the rdWBRT regimen, 21 patients (72%) showed CR, 8 patients showed uCR (28%), and the median KPS was 90 [IQR 80-90]. All patients completed the planned radiotherapy regimen. The median delay from the end of chemotherapy to the start of irradiation was 40 days (minimum-maximum [min-max], 19-87.
Patient characteristics
| Patients, N | 29 |
| Median age, y (IQR, 25-75) | 52 (46-55) |
| Sex ratio M/F | 12/17 (0.7) |
| KPS | |
| Median at diagnosis: median (IQR, 25-75) | 80 (70-90) |
| Median before rdWBRT (IQR, 25-75) | 90 ([80-90) |
| Unifocal disease, n (%) | 9 (31) |
| Multifocal disease, n (%) | 20 (69) |
| Meningeal involvement, n | |
| Yes, n (%) | 6 (21%) |
| No | 18 |
| Unknown | 5 |
| Ophthalmic involvement, n | |
| Yes, n (%) | 4 (14%) |
| No | 22 |
| Unknown | 3 |
| Induction chemotherapy regimen, n | |
| R-MPV + R-A | 12 |
| R-MBVP + R-A | 10 |
| Other | 7 |
| Median HD-MTX infusions, n (IQR, 25%-75%) | 8 [6-8] |
| MTX dose >3 g/m2, n | |
| Yes | 25 |
| No | 2 |
| Unknown | 2 |
| Surgery (excluding biopsy), n | 0 |
| Neuropsychological follow-up, n (%) | 16 (55) |
| Early response to induction chemotherapy, n (%) | |
| CR/uCR | 7/4 (38) |
| PR | 18 (62) |
| Final response to induction chemotherapy, n (%) | |
| CR | 21 (72) |
| uCR | 8 (28) |
| Patients, N | 29 |
| Median age, y (IQR, 25-75) | 52 (46-55) |
| Sex ratio M/F | 12/17 (0.7) |
| KPS | |
| Median at diagnosis: median (IQR, 25-75) | 80 (70-90) |
| Median before rdWBRT (IQR, 25-75) | 90 ([80-90) |
| Unifocal disease, n (%) | 9 (31) |
| Multifocal disease, n (%) | 20 (69) |
| Meningeal involvement, n | |
| Yes, n (%) | 6 (21%) |
| No | 18 |
| Unknown | 5 |
| Ophthalmic involvement, n | |
| Yes, n (%) | 4 (14%) |
| No | 22 |
| Unknown | 3 |
| Induction chemotherapy regimen, n | |
| R-MPV + R-A | 12 |
| R-MBVP + R-A | 10 |
| Other | 7 |
| Median HD-MTX infusions, n (IQR, 25%-75%) | 8 [6-8] |
| MTX dose >3 g/m2, n | |
| Yes | 25 |
| No | 2 |
| Unknown | 2 |
| Surgery (excluding biopsy), n | 0 |
| Neuropsychological follow-up, n (%) | 16 (55) |
| Early response to induction chemotherapy, n (%) | |
| CR/uCR | 7/4 (38) |
| PR | 18 (62) |
| Final response to induction chemotherapy, n (%) | |
| CR | 21 (72) |
| uCR | 8 (28) |
PR, partial response; R-A, rituximab, cytarabine; R-MBVP, rituximab, methotrexate, BCNU, VP16, prednisone.
OS and PFS
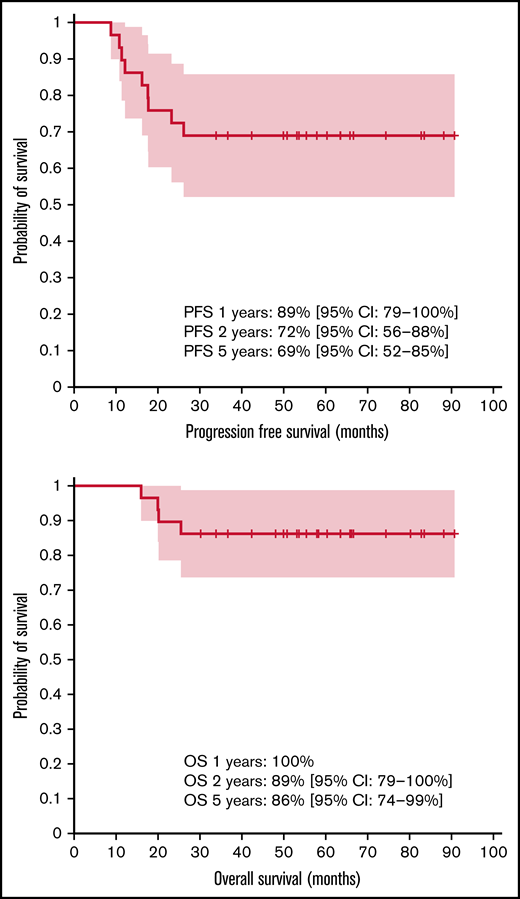
The median follow-up durations from initial diagnosis and from radiotherapy were 55 months (range, 16-91) and 47 months (range, 6-85), respectively. The PFS rates were 89% (95% CI, 79%-100%), 72% (95% CI, 56%-88%), and 69% (95% CI, 52%-85%) at 1, 2, and 5 years, respectively. The OS rates were 100%, 89% (95% CI, 79%-100%), and 86% (95% CI, 74%-99%) at 1, 2, and 5 years, respectively (Figure 1). At the time of analysis, 4 of the 29 patients had died (13.8%), with all deaths caused by relapse of CNS lymphoma.
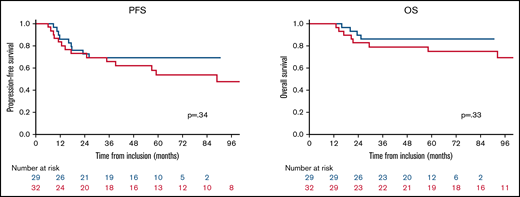
We compared the results of our series with results obtained from the subgroup of patients included in the WBRT arm of the PRECIS trial, 7 of whom showed CR/uCR after induction treatment (n = 32). Both cohorts were well balanced in age and functional status at diagnosis and before WBRT (the χ2 score was applied for the following criteria: age >50 years vs ≤50 years and KPS <70 or ≥70). There was no difference between the 2 populations in PFS (P = .34) or OS (P = .33; Figure 2).
Comparison of outcomes between the rdWBRT cohort (blue curve) and WBRT cohort in the PRECIS trial (red curve).
Comparison of outcomes between the rdWBRT cohort (blue curve) and WBRT cohort in the PRECIS trial (red curve).
Patterns of recurrence
Only 1 patient presented progressive disease immediately after the end of rdWBRT, whereas the 28 remaining patients maintained CR or uCR. A total of 9 of 29 (31%) patients experienced relapse, with a median time from radiotherapy to recurrence of 8.7 months (IQR, 4-11.5). All recurrences except 1 were observed outside the initially involved site(s), and 56% of tumors recurred as multifocal disease (Table 2). There was no case of systemic recurrence. Of the 6 patients who had a lumbar puncture at relapse, 2 experienced CSF relapse (concomitant with cerebral recurrence). Both of those patients exhibited CSF involvement at baseline. At relapse, all patients received salvage treatment (Table 2), followed by thiotepa-based IC-ASCT consolidation in 5 cases. Four of the 5 patients were still disease free at the last follow-up, including 3 who never relapsed. None of the patients received additional irradiation. Four patients did not receive ASCT consolidation at relapse: 1 patient refused the strategy, and salvage induction treatment was not effective in the 3 remaining patients, who died rapidly.
Characteristics of patients with relapses and rescue treatments
| Patients, n (%) | 9 (31) |
| Median time to recurrence (from irradiation) (mo): median (IQR, 25-75] | 8.7 [4-11.5] |
| Pattern of recurrence, n (%) | |
| Unifocal | 4 (44) |
| Multifocal | 5 (56) |
| Site of recurrence, n (%) | |
| Initial | 1 (12) |
| Distant | 8 (88) |
| Second-line chemotherapy, n | |
| R-ICE | 5 |
| Other (IVA, ibrutinib, R-lenalidomide, R-MBVP) | 4 |
| IC-ASCT, n (%) | 5 (55) |
| At first recurrence | 2 |
| Later | 3 |
| Patients, n (%) | 9 (31) |
| Median time to recurrence (from irradiation) (mo): median (IQR, 25-75] | 8.7 [4-11.5] |
| Pattern of recurrence, n (%) | |
| Unifocal | 4 (44) |
| Multifocal | 5 (56) |
| Site of recurrence, n (%) | |
| Initial | 1 (12) |
| Distant | 8 (88) |
| Second-line chemotherapy, n | |
| R-ICE | 5 |
| Other (IVA, ibrutinib, R-lenalidomide, R-MBVP) | 4 |
| IC-ASCT, n (%) | 5 (55) |
| At first recurrence | 2 |
| Later | 3 |
IVA, ifosfamide, vincristine, actinomycin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
Prognostic factors
No factors, most notably, age or KPS, were significantly associated with PFS or OS (supplemental Data 1).
Early toxicity of rdWBRT
No acute grade 3 or 4 toxicity related to rdWBRT was reported. Specifically, none of the patients presented grade 3 or 4 intracranial hypertension, nausea or vomiting, hearing loss, dysphagia, or tinnitus.
Late toxicity of rdWBRT
Of the 29 patients included in the series, 18 were treated in centers where neuropsychological testing was available. Two of those patients were not tested because of an insufficient level of French fluency. Of the 16 patients who benefited from a neuropsychological follow-up, 14 were assessed both at baseline and at least 36 months after rdWBRT or thereafter and were thus included in the final analysis, and 2 patients were excluded from the final analysis because of progression before 36 months (the consent diagram is available in supplemental Data 2). Nine of the 14 patients had their baseline evaluations performed after WBRT, whereas 5 were evaluated at baseline before WBRT. Five patients had their last evaluation performed at 36 months after rdWBRT, 6 were last evaluated at 48 months, and 3 were last evaluated at 60 months. The results are reported in Tables 3 and 4. At baseline, the median scores of all neuropsychological tests were normal except for the median score of the Backward Digit Span Test, which exhibited a borderline result. None of the median scores of the various tests deteriorated between the baseline and the last follow-up, and there was even a slight improvement in the median scores for executive functions. The individual results assessing the follow-up of each patient are reported in Table 4. During the follow-up, deteriorations occurred in only 1 case each for the Stroop test and Forward Digit Span Test and categorical fluency (different patients in each test, aged 54, 53, and 46 years with KPS values before rdWBRT of 90, 90, and 80, respectively), whereas other cognitive functions were not affected. The results remained stable in 66% to 100% of patients and improved in 0% to 36% of patients in the various tests. None of the 20 patients who never relapsed developed impaired balance or sphincter disorders (fecal or urinary incontinence or urinary urgency), as assessed during the follow-up.
Population analysis of neuropsychological assessments
| . | Median score at baseline (min-max) . | Median score at last follow-up (min-max) . |
|---|---|---|
| Fluency | ||
| Categorical | −0.55 (−1.37-0.71) | −0.02 (−1.01-0.83) |
| Lexical | −0.58 (−2.53-1.71) | 0.05 (−1.86-3.07) |
| Stroop | ||
| A | −0.16 (−2.08-0.67) | 0.08 (−4.52-0.88) |
| B | −0.44 (−2.84-1.4) | −0.34 (−3.44-1.3) |
| C | −0.13 (−1.87-0.88) | 0.02 (−1.57-1.53) |
| TMT, percentile (range) | ||
| A, rote memory | 50 (25-90) | 75 (25-90) |
| B, executive functioning | 50 (25-90) | 75 (25-90) |
| Rey’s Complex Figure Copy | 75 (20-90) | 75 (50-90) |
| Image Oral Naming Test DO80 | 0.7 (−0.17-0.7) | 0.7 (−0.59-0.7) |
| FCSRT | ||
| Free recall, percentile (range) | 75 (5-90) | 75 (5-90) |
| Total recall (free + cued), percentile (range) | 50 (5-75) | 75 (5-75) |
| Digit Span Test | ||
| Forward | −0.7 (−2.1-0.6) | −0.7 (−1.7-0.6) |
| Backward | −1.1 (−1.7-0.6)* | −0.7 (−1.7-0.6) |
| . | Median score at baseline (min-max) . | Median score at last follow-up (min-max) . |
|---|---|---|
| Fluency | ||
| Categorical | −0.55 (−1.37-0.71) | −0.02 (−1.01-0.83) |
| Lexical | −0.58 (−2.53-1.71) | 0.05 (−1.86-3.07) |
| Stroop | ||
| A | −0.16 (−2.08-0.67) | 0.08 (−4.52-0.88) |
| B | −0.44 (−2.84-1.4) | −0.34 (−3.44-1.3) |
| C | −0.13 (−1.87-0.88) | 0.02 (−1.57-1.53) |
| TMT, percentile (range) | ||
| A, rote memory | 50 (25-90) | 75 (25-90) |
| B, executive functioning | 50 (25-90) | 75 (25-90) |
| Rey’s Complex Figure Copy | 75 (20-90) | 75 (50-90) |
| Image Oral Naming Test DO80 | 0.7 (−0.17-0.7) | 0.7 (−0.59-0.7) |
| FCSRT | ||
| Free recall, percentile (range) | 75 (5-90) | 75 (5-90) |
| Total recall (free + cued), percentile (range) | 50 (5-75) | 75 (5-75) |
| Digit Span Test | ||
| Forward | −0.7 (−2.1-0.6) | −0.7 (−1.7-0.6) |
| Backward | −1.1 (−1.7-0.6)* | −0.7 (−1.7-0.6) |
Data are median scores (min-max), unless otherwise noted. Test results above a z score of −1 or a percentile of 16 were considered normal.
The pathological threshold corresponds to a z score ≤ −1.65 or a percentile ≤5.
FCSRT, Free and Cued Selective Reminding Test; TMT, Trail Making Test.
The borderline threshold corresponds to a z score of −1.65; −1 and a percentile of 5; 16.
Individual analysis of neuropsychological assessments
| . | Stability . | Improvement . | Deterioration . |
|---|---|---|---|
| Fluency | |||
| Categorical | 10/14 | 3/14 | 1/14 |
| Lexical | 9/14 | 5/14 | 0/14 |
| Stroop | |||
| A | 9/13 | 3/13 | 1/13 |
| B | 11/13 | 1/13 | 1/13 |
| C | 8/12 | 3/12 | 1/12 |
| TMT | |||
| TMT A, rote memory | 13/13 | 0 | 0 |
| TMT B, executive functioning | 13/13 | 0 | 0 |
| Rey’s Complex Figure Copy | 12/12 | 0 | 0 |
| Image Oral Naming Test DO80 | 15/15 | 0 | 0 |
| FCSRT | |||
| Free recall | 13/14 | 1/14 | 0 |
| Total recall (free + cued) | 13/14 | 1/14 | 0 |
| Digit Span Test | |||
| Forward | 11/14 | 2/14 | 1/14 |
| Backward | 10/14 | 4/14 | 0 |
| . | Stability . | Improvement . | Deterioration . |
|---|---|---|---|
| Fluency | |||
| Categorical | 10/14 | 3/14 | 1/14 |
| Lexical | 9/14 | 5/14 | 0/14 |
| Stroop | |||
| A | 9/13 | 3/13 | 1/13 |
| B | 11/13 | 1/13 | 1/13 |
| C | 8/12 | 3/12 | 1/12 |
| TMT | |||
| TMT A, rote memory | 13/13 | 0 | 0 |
| TMT B, executive functioning | 13/13 | 0 | 0 |
| Rey’s Complex Figure Copy | 12/12 | 0 | 0 |
| Image Oral Naming Test DO80 | 15/15 | 0 | 0 |
| FCSRT | |||
| Free recall | 13/14 | 1/14 | 0 |
| Total recall (free + cued) | 13/14 | 1/14 | 0 |
| Digit Span Test | |||
| Forward | 11/14 | 2/14 | 1/14 |
| Backward | 10/14 | 4/14 | 0 |
Data are the number of patients qualifying for each item/total group. For each patient, the results of each test at baseline and at last follow-up were classified as normal, borderline, or pathological, as explained in “Patients and methods.” If the results remained in the same category, they were classified as stable; otherwise, the results were classified as improved or deteriorated.
FCSRT, Free and Cued Selective Reminding Test; TMT, Trail Making Test.
The Fazekas score was calculated at the last MRI in 17 of the 18 patients with neuropsychological follow-up (missing data for 1 patient). The median periventricular and deep white matter scores were 1(0;3) and 0 (0;3), respectively. The 3 patients who experienced an isolated deterioration in 1 neuropsychological test had a deep white matter Fazekas score of 0. Two of them had a periventricular score of 0, and 1 had a score of 1.
Discussion
Although therapeutic options for PCNSL have been expanding over the years, the best consolidation strategy for younger patients has yet to be defined. Two randomized phase 2 trials compared the use of full-dose WBRT (40 Gy in 20 fractions in the PRECIS trial and 36 Gy in 20 fractions in the whole brain) with the use of IC-ASCT.8,28 The results indicated that sdWBRT and IC-ASCT are feasible and effective as consolidation therapies after HD-MTX–based chemoimmunotherapy in patients with primary CNS lymphoma <70 years (IESLG32) or 60 years (PRECIS trial) of age. There was no significant difference between the 2 arms in PFS in the IELSG trial (2-year PFS, 80% [95% CI, 70-90] in the sdWBRT arm and 69% (95% CI, 59-79) in the IC-ASCT arm [hazard ratio, 1.50; 95% CI, 0.83-2.71; P = .17]), but PFS was significantly better in the IC-ASCT arm of the PRECIS trial (2-year PFS: 58%; 95% CI, 47%-71%) in the sdWBRT arm and 70% (95% CI, 59%-82%) in the IC-ASCT arm, whereas OS (2-year OS) was similar in the 2 arms in both trials: 75% (95% CI, 65%-87%) vs 66% (95% CI, 55%-79%) in the PRECIS trial and 85% (95% CI, 75%-95%) vs 71% (95% CI, 60%-82%) in the IESLG32 trial. However, delayed cognitive decline after sdWBRT, which limits the routine use of sdWBRT,7 was described in both studies, In contrast, IC-ASCT was associated with a risk of acute toxic death, which occurred in 6% and 11% of patients in the IELSG32 and PRECIS trials, respectively. The risks and implications of each consolidation strategy should therefore be considered at the time of therapeutic decision-making. In this setting, reducing the WBRT dose could be a promising option, as it reduces the risk of neurotoxicity.
In this retrospective study, we report for the first time in a real-life setting that rdWBRT is an efficient and safe consolidation treatment for <60-year-old patients with PCNSL showing CR after HD-MTX–based chemotherapy.
To our knowledge, although this study was retrospective, this is the largest cohort of patients <60 years old treated with rdWBRT as a consolidation strategy ever reported. A few retrospective or phase 2 studies have been published, but they included very small sample numbers (n < 10) and produced conflicting results, making it difficult to draw conclusions.29,30 In a phase 2 study30 of dose-adapted WBRT, there was no difference observed between rdWBRT (n = 9) and sdWBRT concerning cognitive outcomes, but in univariate analysis, the use of sdWBRT (45 Gy) led to significantly improved outcomes. In contrast, in a retrospective study,29 survival outcomes after rdWBRT (n = 10; 3-year OS, 100%; 3-year PFS, 80%) were comparable to our results.
With 2- and 5-year PFS rates of 72% and 69%, respectively, and 2- and 5-year OS rates of 89% and 86%, respectively, our results confirm the good efficacy reported by Morris et al in their phase 2 trial, although they reported a slightly better 2-year PFS of 94% for patients <60 years of age, but only in a small number of 15 patients.14 The Radiation Therapy Oncology Group (RTOG) is currently conducting a randomized phase 2 study (registered at https://clinicaltrials.gov as #NCT01399372) comparing the R-MPV regimen with and without the use of reduced-dose WBRT. This study includes a prospective neuropsychological evaluation, the collection of neuroimaging, and the evaluation of other potential biomarkers predicting efficacy and neurotoxicity, which could ultimately guide the individualization of treatment choices to achieve optimal outcomes. The main hypothesis of this trial was that patients in the rdWBRT arm would experience improved PFS compared with the chemotherapy-alone arm. The first data were reported in 2020,31 demonstrating that the addition of rdWBRT to R-MPV-A improved PFS in newly diagnosed PCNSL without increasing neurotoxicity. After a median follow-up of 55 months, the 2-year PFS was 54% in the chemotherapy arm and 78% in the rdWBRT consolidation arm. The preliminary results in the rdWBRT arm are comparable with ours. Furthermore, regarding the efficacy, our results did not differ from those of the sdWBRT arm of the PRECIS trial in patients showing CR or uCR after induction HD-MTX chemotherapy. As in the PRECIS trial, more than half of the patients benefitted from IC-ASCT in the case of relapse, which may have also contributed to the excellent results in OS. It is worth noting that all relapses occurred early after rdWBRT (ie, during the first year after rdWBRT) in all but 1 patient, raising a question about the quality of the CR after induction treatment. We hypothesize that patients who relapsed early after WBRT had residual disease at the end of the induction treatment that we were not able to detect with the neuroimaging and biomarker techniques currently available. The median delay of relapse was similar to those observed in prospective trials with sdWBRT, such as PRECIS (Figure 2).
Regarding toxicity, the acute toxicity profile of rdWBRT appeared excellent, without any reported acute grade 3 to 4 side effects. Fourteen of the 20 patients who maintained CR throughout the follow-up period benefitted from prospective neuropsychologic testing. In contrast, in the prospective study by Morris et al, only 9 patients <60 years of age completed neuropsychological evaluations through up to 48 months. The medium-term neuropsychological follow-up appeared reassuring, with most patients maintaining their baseline levels and a subgroup of patients even improving their scores, notably in working memory and executive functions, whereas worsening occurred in only 1 case each for 3 tests.14 In both the PRECIS and IELSG trials, cognitive deterioration was noted at the 2-year follow-up. The patients in our cohort also did not develop other symptoms of neurotoxicity, such as balance or sphincter disorders. However, these results must be confirmed in larger series and, above all, through a longer follow-up period, as younger patients are known to develop neurotoxicity after combined HD-MTX chemotherapy and WBRT later than elderly patients.32 Correa et al recently reported cognitive functions in patients with PCNSL achieving long-term remission after rdWBRT. Fourteen patients with a median age of 58 years (range, 49-76 years) completed cognitive assessments at diagnosis, after R-MPV, before rdWBRT and at yearly intervals up to 5 years after rdWBRT. They described continuous improvement in cognitive functions from baseline up to year 3. However, after the third year, significant cognitive decline was observed in the TMT A, Hopkins Verbal Learning Test-R-Learning, and HVLT-R Delayed-Recall tests, indicating that radiation-induced toxicity was delayed in this population.32 Interestingly, similar delayed neurotoxicity was also observed in the group of patients receiving IC-ASCT, suggesting that chemotherapy alone can be involved in cognitive impairment. In fact, the role of chemotherapy in cognitive decline has been confirmed for many cancers, including lymphoma.33,34 It was not possible to confirm the results reported by Correa et al in our study because only 6 patients had neuropsychological assessments performed through up to 48 months, and only 3 patients were assessed through up to 60 months.
The neuroimaging follow-up results were also reassuring. No white matter deterioration was observed. Even if the Fazekas score could not be used to discriminate MTX- or radiation-induced white matter damage, it remained very low in most cases; the median periventricular and deep white matter scores were 1 (min-max, 0;3) and 0 (0;3), respectively.
In addition to reducing the irradiation dose, other strategies to decrease the neurotoxicity of radiotherapy, including focal irradiation with radiosurgery or rdWBRT followed by a sequential focal boost, have been reported in small retrospective series.35-37 However, PCNSL should be considered a diffuse process. The patterns of failure reported herein found that 100% of relapses occurred outside the initially involved site. Therefore, as the first-line treatment, a whole-brain field remains the standard technique for ensuring adequate disease coverage. Likewise, patterns of failure do not truly support irradiation strategies combining WBRT and stereotactic focal boost.38
Hippocampal-avoidance WBRT has not yet been evaluated for the treatment of PCNSL. This technique, which is mainly used in patients with brain metastases who are not eligible for radiosurgery and without hippocampal involvement, reduces levels of cognitive impairment and could be an interesting option.39 However, dosimetric and pattern-of-recurrence studies did not support this strategy of irradiation. It seems that only patients whose primary tumor was located more than 15 mm from the hippocampus at the time of diagnosis could be eligible for hippocampal-avoidance WBRT.40
Our study has limitations, related to the small number of patients included and all of the biases inherent in its retrospective nature. One of the major limitations concerns the neurocognitive evaluations. The sample size was small, as only 50% of patients were assessed by a full cognitive exploration, a few patients had their baseline evaluations performed 3 months after the end of irradiation, and follow-ups were limited. This limitation should encourage caution in interpreting the data, and because of all these reasons, the cognitive deterioration rate could have been underestimated. The main reason for the lack of data are that the number of neuropsychologists in our country remains very low, and furthermore, the use of cognitive evaluations is not supported by the French health care system. Consequently, there was heterogeneity between the different centers with patients included in the database, depending on the local availability of neuropsychologist resources.
Conclusion
To our knowledge, this cohort study represents the largest study to evaluate consolidation rdWBRT in young patients with PCNSL. In a real-life setting, rdWBRT appeared effective and well tolerated. Our results, combined with previously reported results from smaller retrospective or phase 2 studies,14,31,32 indicate that rdWBRT could represent a valuable option as a consolidation treatment in <60-year-old patients showing CR after induction treatment, given the apparent similar efficacy profile to that of sdWBRT and no medium-term signs of neurotoxicity. Further studies are needed to establish the role of this strategy compared with IC-ASCT consolidation, but rdWBRT can already be considered a valuable option in patients who are not eligible for or refuse IC-ASCT. Patients who relapse after consolidation rdWBRT could still benefit from rescue IC-ASCT.
Acknowledgment
The authors thank all members feeding the LOC database.
No funding was received in support of the study.
Authorship
Contribution: P.L., C.H., K.H.-X., G.D., and C.S. wrote the manuscript, analyzed the data, and designed the research study; L.F., D.S., V.R., A.S., O.C., M.F., P.A., C.M.-C., S.C., A.A., D.D., S.C., L.N., K.M., B.M., and S.D. contributed to the research design and the acquisition of data; and all authors approved the final submitted version.
Conflict-of-interest disclosure: S.C. has served on the scientific advisory boards of Gilead, Novartis, Roche France, AbbVie, Sandoz, Sanofi, Janssen, Celgene-BMS, Takeda, and Atara. P.L. has served on the scientific advisory boards of Astra Zeneca and BMS. The remaining authors declare no competing financial interests.
Correspondence: Paul Lesueur, Radiation Therapy Department, Centre François Baclesse, 14000 Caen, France; e-mail: paul.lesueur89@gmail.com.
References
Author notes
The original data are available by e-mail request to the corresponding author (paul.lesueur89@gmail.com).
The full-text version of this article contains a data supplement.