Key Points
A DSG2-targeting HDAd5/3+ vector efficiently transduced mobilized HSCs in vivo in rhesus macaques.
Transient overexpression of cxcr4 in mobilized, in vivo transduced HSCs greatly increased their return to the bone marrow.
Abstract
We developed a new in vivo hematopoietic stem cell (HSC) gene therapy approach that involves only IV injections and does not require myeloablation/conditioning and HSC transplantation. In this approach, HSCs are mobilized from the bone marrow into the peripheral bloodstream and transduced with IV injected helper-dependent adenovirus (HDAd) vectors. A fraction of transduced HSCs returns to the bone marrow and persists there long term. Here, we report desmoglein 2 (DSG2) as a new receptor that can be used for in vivo HSC transduction. HDAd5/3+ vectors were developed that use DSG2 as a high-affinity attachment receptor, and in vivo HSC transduction and safety after IV injection of an HDAd5/3+ vector expressing green fluorescent protein (GFP) in granulocyte colony-stimulating factor/AMD3100 (plerixafor)-mobilized rhesus macaques were studied. Unlike previously used CD46-targeting HDAd5/35++ vectors, HDAd5/3+ virions were not sequestered by rhesus erythrocytes and therefore mediated ∼10-fold higher GFP marking rates in primitive HSCs (CD34+/CD45RA–/CD90+ cells) in the bone marrow at day 7 after vector injection. To further increase the return of in vivo transduced, mobilized HSCs to the bone marrow, we transiently expressed cxcr4 in mobilized HSCs from the HDAd5/3+ vector. In vivo transduction with an HDAd5/3+GFP/cxcr4 vector at a low dose of 0.4 × 1012 viral particles/kg resulted in up to 7% of GFP-positive CD34+/CD45RA–/CD90+ cells in the bone marrow. This transduction rate is a solid basis for in vivo base or prime editing in combination with natural or drug-induced expansion of edited HSCs. Furthermore, our study provides new insights into HSC biology and trafficking after mobilization in nonhuman primates.
Introduction
Our in vivo hematopoietic stem cell (HSC) transduction approach used helper-dependent adenovirus (HDAd) vectors. HDAd vectors are targeted to HSCs through their fiber protein and have a payload capacity of 35 kb, which allows for the insertion of large transcriptionally regulatory elements1 or multiple transgene cassettes.2 They can be formulated as episomal vectors (eg, for transient expression of genome editors3,4 ) or as integrating vectors (eg, by using a Sleeping Beauty transposase5 or homology-dependent repair6 ). HDAd vectors can be produced easily at low costs at very high yields and titers because, unlike recombinant adeno-associated virus vectors and lentivectors, no large-scale plasmid transfection is required.7
Previously, we tested our in vivo HSC transduction/selection approach in rhesus macaques using CD46-targeting HDAd5/35++ vectors designed for expression of human γ-globin in red blood cells (RBCs) to treat hemoglobinopathies.8 Although the study showed the safety of the approach, it also identified 2 factors limiting its efficacy. The first factor is the sequestration of injected HDAd5/35++ vectors by nonhuman primate (NHP) erythrocytes, which, in contrast to humans, are CD46-positive. Sequestration by erythrocytes decreases the vector dose that is available for transduction of mobilized HSCs and also complicates meaningful toxicology studies in NHPs. The second factor is the preferential return/survival of mobilized transduced HSCs to/in the spleen, a secondary, much smaller hematopoietic organ. Because the bone marrow is the major contributor to blood cells under physiological conditions, directing mobilized HSCs there can increase transgene marking rates in peripheral blood cells.
We searched for HSC-targeting receptors that are not present on erythrocytes and that are used by human adenoviruses. Desmoglein 2 (DSG2) is the primary attachment receptor for some species B human adenovirus serotypes, including Ad3, 7, 11, 14,9 and 55. It is mostly known to be an integral component of desmosomal epithelial junctions. In polarized epithelial tissues, DSG2 is trapped in lateral junctions and not accessible to ligands.9,10 More recently, DSG2 has gained recognition for its ability to exist outside of desmosomes and to regulate additional biological processes; for example, it plays an important role in the acquisition of pluripotency in somatic cells during reprogramming to induced pluripotent stem cells.11 DSG2 expression in multiple myeloma cells is thought to support their adhesion to endothelial cells.12 Ebert et al13 reported that DSG2 is almost ubiquitously expressed by CD34+CD90+CD117+CD38– stem cells in normal human bone marrow, with expression being progressively downregulated in more differentiated hematopoietic cell subsets. This, together with inaccessibility in normal epithelial tissues, makes DSG2 an attractive HSC-targeting receptor.
To improve the return of granulocyte colony-stimulating factor (G-CSF)/AMD3100–mobilized HSCs to the bone marrow, we focused on CXCR4. It is known from HSC transplantation studies that CD34+ cells that express high levels of cxcr4 allow for faster recovery.14 The cell surface expression of cxcr4 is therefore considered a major determinant in the migration and repopulation capacity of HSCs.15,16 Along this line, Kahn et al17 showed that ectopic overexpression of the cxcr4 gene in human CD34+ cells, achieved by lentivirus transduction, increased their proliferation, migration, and NOD/SCID repopulation. Here, we tested whether short-term cxcr4 overexpression on mobilized HSCs would increase the percentage of transduced HSCs in the bone marrow.
Materials and methods
Reagents
G-CSF (Neupogen, Amgen, Thousand Oaks, CA), AMD3100 (MilliporeSigma, Burlington, MA), and dexamethasone sodium phosphate (Fresenius Kabi USA, Lake Zurich, IL) were used. Recombinant human DSG-2 was from Leinco Technologies (Fenton, MO; catalog no. D340) and recombinant CD46 from Sino Biological (Beijing, China; catalog no. 12239-H08H). Both recombinant proteins were produced in mammalian cells as Fc fusion protein (DSG2) or with His tag (CD46).
Adenovirus vectors
For the production of HDAd5/35++ vectors, corresponding plasmids were linearized with PmeI and rescued in 116 cells18 with AdNG163-5/35++, an Ad5/35++ helper vector containing chimeric fibers composed of the Ad5 fiber tail, the Ad35 fiber shaft, and the affinity-enhanced Ad35++ fiber knob.19 HDAd5/35++ vectors were amplified in 116 cells as described in detail elsewhere.18 For the production of HDAd5/3+ vectors, corresponding plasmids were rescued and propagated in 116 cells with Ad5/3+ helper virus. Helper virus contamination levels were found to be <0.05%. Titers were 1 to 5 × 1012 viral particles (vp)/mL.
NHP studies
Animals.
Four female rhesus macaques (Macaca mulatta) from the Oregon National Primate Research Center were used for the studies. Their weights ranged from 7.1 to 9.7 kg. The studies were performed by the Washington National Primate Research Center Research Support Team. All experiments were conducted in accordance with the institutional guidelines set forth by the University of Washington. The studies were approved by the University of Washington Institutional Animal Care and Use Committee (Protocol No. 3108-04). After implantation of an IV catheter, animals were housed individually.
Antibiotics.
Ceftazidime (Tazicef, Hospira, Inc., Lake Forest, IL) was injected IV at 150 mg/kg on the day of surgery and continued through the study as long as tether once daily. Fluconazole (NorthStar Rx, Memphis, TN) was administered orally at a 50-mg flat dose per animal starting on day –5 and then once daily. Acyclovir (AuroMedics Pharma, Windsor, NJ) was delivered IV at 10 mg/kg starting on day –5, and then once daily.
Mobilization.
Filgrastim/G-CSF (Neupogen, Amgen) was injected subcutaneously at 50 μg/kg in the morning from day –5 to day 0 (once daily). AMD3100/plerixafor (Calbiochem) was given subcutaneously at 5 mg/kg at midnight on day 0 (8 hours before HDAd dosing).
Cytokine prophylaxis.
Dexamethasone (Fresenius Kabi USA) was injected IV (4 mg/kg) at 2 pm on day –1 and then at 7:30 am and 2:30 pm on day 0. Anakinra (Kineret, Swedish Orphan Biovitrum, Stockholm, Sweden) was administered subcutaneously at a 50-mg flat dose, 2 doses each on days –1 and 0 (1 hour before and 6 hours after HDAd injection). Tocilizumab (Actemra, Genentech Inc., South San Francisco, CA) was injected IV at 8 mg/kg, 2 doses each on days –1 and 0 (1 hour before and 6 hours after HDAd injection) via diluted infusions (50 mL each).
HDAd vector infusion.
For infusion, HDAd preps (dialyzed overnight against phosphate-buffered saline [PBS]) were thawed, diluted with PBS (room temperature), and infused within 30 to 60 minutes after preparation. A low dose (5 × 1010 vp/kg) in 5 mL of PBS was given over 10 minutes followed by the therapeutic dose (here, 0.4 × 1012 vp/kg) in 20 mL of PBS infused over 20 minutes.
Antiemetic/hypotension prophylaxis.
Lactated Ringer’s solution bolus was injected IV (8 mL/kg) after HDAd infusion over 15 minutes. Maropitant (Cerenia; Zoetis, Florham Park, NJ) was given IV at 1 mg/kg on day 0 before HDAd infusions. Ondansetron (Hikma Pharmaceuticals, London, United Kingdom) was delivered IV at 2 mg/kg on day 0 before HDAd infusion.
Necropsy.
Animals were sedated and then injected IV with an overdose of pentobarbital. Blood was flushed out from the body with 5 L of PBS using an external perfusion pump.
Statistical analyses
Data are presented as mean ± standard error of the mean. For comparisons of multiple groups, one-way and two-way analyses of variance with Bonferroni posttesting for multiple comparisons were used. Statistical analysis was performed by using GraphPad Prism version 6.01 (GraphPad Software Inc., La Jolla, CA). *P ≤ .05, **P ≤ .0001. A P value <.05 was considered significant. The NHP study was conducted with one animal per HDAd vector. The standard deviation of technical replicates was consistently <10%.
A detailed description of the following methods is provided in the supplemental Methods: adenovirus vector construction, CD34+ cell isolation and culture, colony-forming cell assay, RBC competition, gating of green fluoresnt protein (GFP)-positive CD34+/CD45RA–/CD90+ cells, GFP immunofluorescence analysis on CD34+ cells, measurement of vector copy number (VCN), real-time reverse transcription polymerase chain reaction, cytometric bead array, and anti-HDAd and anti-GFP serum antibodies.
Results
Targeting HSCs through DSG2
Flow cytometry revealed DSG2 expression on ∼90% of human CD34+ cells isolated from peripheral blood of mobilized healthy donors (Figure 1A). DSG2 expression largely overlapped with CD46, the receptor used in our previous studies.8 For HSC transduction via DSG2, we generated helper-dependent adenovirus HDAd5/3+ vectors. The capsid of these vectors contained the DSG2-targeting Ad3 fiber knob domain with the affinity-enhancing Y239D mutation20 (Figure 1B). We compared HDAd5/3+ vectors with our previously developed HDAd5/35++ vectors that use CD46 as a receptor. Both HDAd5/3+ and HDAd5/35++ vectors contained identical transgene cassettes. The mgmtP140K/GFP cassette under the control of the ubiquitously active EF1α promoter allows for tracing of transduced cells and, if needed in future studies, for in vivo selection with O6BG/BCNU.21 A second set of vectors had an additional cytomegalovirus (CMV) promoter-cxcr4 expression cassette (described below). Usage of the corresponding receptors, CD46 and DSG2, by HDAd5/35++GFP and HDAd5/3+GFP vectors was shown in transduction studies with soluble receptor proteins (sCD46 or sDSG2) as competitors (Figure 1C). This study also showed that the percentage of GFP+ cells was comparable for both vectors.
Analysis of DSG2 expression on HSCs. (A) Flow cytometry for CD46 and DSG2 on human CD34+ cells from 2 different healthy G-CSF–mobilized donors. (B) Schematic of HDAd vector capsids and genomes. The chimeric capsids contain either affinity-enhanced Ad35 or Ad3 fiber knob domains. Both vector genomes contain an EF1α-mgmtp140k/GFP expression cassette used before.21 Both proteins are linked via a self-cleaving picornavirus 2A peptide. One set of vectors has an additional CMV promoter–human cxcr4 expression cassette. The 4 vectors used in this study are HDAd5/35++GFP, HDAd5/3+GFP, HDAd5/35++GFP/cxcr4, and HDAd5/3+GFP/cxcr4. (C) In vitro CD34+ cell transduction studies. Cells were infected with HDAd5/35++-GFP and HDAd5/3+-GFP virus at a multiplicity of infection of 2000 vp/cell with and without pre-incubation with recombinant soluble CD46 or DSG2 (10 μg/mL) for 1 hour. GFP expression was analyzed 24 hours’ postinfection. (D) CD46 and DSG2 flow cytometry on human and rhesus peripheral RBCs stained with antibodies that recognize the receptors in both species (anti-human CD46 mAb clone M177 from Santa Cruz Biotechnology [Dallas, TX] and anti-human DSG2 polyclonal antibody AF947 from R&D Systems [Minneapolis, MN]). (E) Transduction of 293 cells with HDAd5/35++GFP and HDAd5/3+GFP in the absence and presence of human (huRBCs) and rhesus (rhRBCs) RBCs (details are provided in the Materials and methods). Shown is the percentage of GFP-positive cells measured 2 days after transduction.
Analysis of DSG2 expression on HSCs. (A) Flow cytometry for CD46 and DSG2 on human CD34+ cells from 2 different healthy G-CSF–mobilized donors. (B) Schematic of HDAd vector capsids and genomes. The chimeric capsids contain either affinity-enhanced Ad35 or Ad3 fiber knob domains. Both vector genomes contain an EF1α-mgmtp140k/GFP expression cassette used before.21 Both proteins are linked via a self-cleaving picornavirus 2A peptide. One set of vectors has an additional CMV promoter–human cxcr4 expression cassette. The 4 vectors used in this study are HDAd5/35++GFP, HDAd5/3+GFP, HDAd5/35++GFP/cxcr4, and HDAd5/3+GFP/cxcr4. (C) In vitro CD34+ cell transduction studies. Cells were infected with HDAd5/35++-GFP and HDAd5/3+-GFP virus at a multiplicity of infection of 2000 vp/cell with and without pre-incubation with recombinant soluble CD46 or DSG2 (10 μg/mL) for 1 hour. GFP expression was analyzed 24 hours’ postinfection. (D) CD46 and DSG2 flow cytometry on human and rhesus peripheral RBCs stained with antibodies that recognize the receptors in both species (anti-human CD46 mAb clone M177 from Santa Cruz Biotechnology [Dallas, TX] and anti-human DSG2 polyclonal antibody AF947 from R&D Systems [Minneapolis, MN]). (E) Transduction of 293 cells with HDAd5/35++GFP and HDAd5/3+GFP in the absence and presence of human (huRBCs) and rhesus (rhRBCs) RBCs (details are provided in the Materials and methods). Shown is the percentage of GFP-positive cells measured 2 days after transduction.
In agreement with previous reports,22 CD46 flow cytometry on peripheral RBCs revealed CD46 receptor absence on human RBCs and presence on rhesus RBCs (Figure 1D). Importantly, DSG2 was absent on RBCs for both species, implying that the DSG2-targeting HDAd5/3+ vectors will not be critically sequestered by NHP RBCs. This was confirmed in an in vitro transduction study with HDAd5/35++GFP and HDAd5/3+GFP in the presence or absence of human or rhesus RBCs (Figure 1E). Although HDAd5/35++GFP transduction was blocked by rhesus RBCs, HDAd5/3+GFP transduction was not.
To increase homing of in vivo transduced mobilized HSCs to the bone marrow, we wanted to overexpress cxcr4 on the surface of HSCs while they were circulating in the periphery or while they were trafficking from the spleen to the bone marrow.23 We therefore produced HDAd5/35++ and HDAd5/3+ vectors that, in addition to the EF1α-mgmtP140/GFP cassette, contained the human cxcr4 complementary DNA under control of the human CMV promoter. Human and rhesus CXCR4 share 99% homology at the amino acid level (supplemental Figure 1A). The CMV promoter was chosen for 2 reasons. Its strength can provide high-level cxcr4 expression while it would be only transiently active. Silencing of the CMV promoter by cytosine guanine dinucleotide (CpG) methylation24,25 has been reported regardless of the vector system, the transduction method, and the species26 in muscle,27 liver,28,29 fibroblasts,30 and lung.31 Furthermore, we noticed in human CD34+ cell transduction studies with an HDAd5/35-CMV-GFP vector that a large fraction of vector DNA–containing cells did not express GFP.32 Along this line, Ngai et al33 found that lentivirus vectors carrying the GFP reporter gene under the CMV promoter were subjected to transgene silencing after transduction of HSC. In preparation for our in vivo studies, we validated cxcr4 expression from HDAd5/35++GFP/cxcr4 and HDAd5/3+GFP/cxcr4 vectors in in vitro transduction studies (supplemental Figure 1B-C).
Studies in rhesus macaques
Rhesus macaques are an adequate model to study the efficacy and safety of our in vivo HSC gene therapy approach. We first evaluated receptor expression and in vitro HDAd transduction of rhesus CD34+ cells. Using antibodies that reacted with both human and rhesus CD46 and DSG2, we showed that 98% of rhesus CD34+ cells were positive for CD46 and 33% were positive for DSG2 (supplemental Figure 2A). About one-half of CD46+ cells were also positive for DSG2. In vitro transduction of rhesus CD34+ cells with HDAd5/35++GFP and HDAd5/3+GFP was less efficient compared with human CD34+ cells (supplemental Figure 2B). This could be due to small structural differences between the human and rhesus orthologs (supplemental Figure 3) and small differences in HSC biology, specifically regarding DSG2 expression.
The schematic of the NHP in vivo transduction protocol is shown in Figure 2A. The 4 different HDAd vectors (Figure 1B) were injected into 4 rhesus macaques (N = 1). To reduce variables, all test animals were female and of comparable age and weight. Animals were mobilized by 5 subcutaneous injections of G-CSF and one injection of AMD3100. HDAd vectors were injected IV 8 hours after AMD3100 administration. This timing was based on the HSC mobilization kinetics determined earlier.8 Compared with our previous study, animals here received only 1 round of HDAd injection at a dose of 0.4 × 1012 vp/kg, a dose that was 4-fold lower than the total HDAd5/35++ dose in the study by Li et al.8 Animals injected with HDAd5/35++GFP and HDAd5/3+GFP were observed for 7 days. At day 7, these 2 animals underwent a necropsy. HDAd5/35++GFP/cxcr4– and HDAd5/3+GFP/cxcr4–injected animals were observed for 8 weeks and then returned to the colony. Longer monitoring of GFP expression would not have been practical because of strong anti-GFP immune responses (supplemental Figure 4) that can lead to T cell–mediated elimination of bone marrow and blood cells expressing heterologous transgene products (GFP, human mgmtP140K).8,34
In vivo HSC transduction studies in rhesus macaques with HDAd5/35++GFP, HDAd5/3+GFP, HDAd5/35++GFP/cxcr4, and HDAd5/3+GFP/cxcr4. (A) Schematic of the experiment showing the injection time of the mobilization drugs (G-CSF and AMD3100) and cytokine prophylaxis drugs (dexamethasone, anakinra, and tocilizumab), as well as the injection time of the HDAd vector at a dose of 0.4 × 1012 vp/kg. The time point of HDAd injection is taken as “day 0, 0 hours.” HDAd5/35++GFP– and HDAd5/3+GFP–injected animals were monitored for 7 days. HDAd5/35++GFP/cxcr4– and HDAd5/3+GFP/cxcr4–injected animals were observed for 8 weeks to capture late effects of cxcr4 expression. Bone marrow aspirates (BMAs) were performed at days 3, 7, and (for HDAd5/35++GFP/cxcr4 and HDAd5/3+GFP/cxcr4) at weeks 4 and 8. Blood samples were taken at 0 hours (before HDAd injection) and 2, 6, 9, and 12 hours on day 0; on days 1, 2, 3, and 7; and then weekly. (B) HSC mobilization. Shown are numbers of primitive (CD34+/CD45RA–/CD90+) HSCs in peripheral blood. HDAd vectors were IV injected at day 0, 0 hours. Notably, there was no correlation between the weight of the animals and the efficacy of mobilization. The weights were 9.70 kg (HDAd5/35++GFP), 6.64 kg (HDAd5/3+GFP), 7.13 kg (HDAd5/35++GFP/cxcr4), and 7.19 kg (HDAd5/3+GFP/cxcr4). (C) Vector clearance from blood. HDAd vector genome copies in serum samples were measured by using quantitative polymerase chain reaction.
In vivo HSC transduction studies in rhesus macaques with HDAd5/35++GFP, HDAd5/3+GFP, HDAd5/35++GFP/cxcr4, and HDAd5/3+GFP/cxcr4. (A) Schematic of the experiment showing the injection time of the mobilization drugs (G-CSF and AMD3100) and cytokine prophylaxis drugs (dexamethasone, anakinra, and tocilizumab), as well as the injection time of the HDAd vector at a dose of 0.4 × 1012 vp/kg. The time point of HDAd injection is taken as “day 0, 0 hours.” HDAd5/35++GFP– and HDAd5/3+GFP–injected animals were monitored for 7 days. HDAd5/35++GFP/cxcr4– and HDAd5/3+GFP/cxcr4–injected animals were observed for 8 weeks to capture late effects of cxcr4 expression. Bone marrow aspirates (BMAs) were performed at days 3, 7, and (for HDAd5/35++GFP/cxcr4 and HDAd5/3+GFP/cxcr4) at weeks 4 and 8. Blood samples were taken at 0 hours (before HDAd injection) and 2, 6, 9, and 12 hours on day 0; on days 1, 2, 3, and 7; and then weekly. (B) HSC mobilization. Shown are numbers of primitive (CD34+/CD45RA–/CD90+) HSCs in peripheral blood. HDAd vectors were IV injected at day 0, 0 hours. Notably, there was no correlation between the weight of the animals and the efficacy of mobilization. The weights were 9.70 kg (HDAd5/35++GFP), 6.64 kg (HDAd5/3+GFP), 7.13 kg (HDAd5/35++GFP/cxcr4), and 7.19 kg (HDAd5/3+GFP/cxcr4). (C) Vector clearance from blood. HDAd vector genome copies in serum samples were measured by using quantitative polymerase chain reaction.
HSC mobilization was measured based on the number of primitive HSCs (CD34+/CD45RA–/CD90+)35 present in peripheral blood (Figure 2B). As expected from studies in humans,36 mobilization efficacy varied between animals, with the animals that received HDAd5/3+GFP and HDAd5/3+GFP/cxcr4 having the lowest numbers of mobilized HSCs at the time of HDAd injection. Numbers of HSCs in the periphery were above premobilization levels for up to 3 days. Virus clearance from the serum was measured by quantitative polymerase chain reaction for HDAd vector genomes using mgmtP140K primers (Figure 2C). At 1 hour after injection, HDAd5/35++ vector genome concentrations were about one order of magnitude lower compared with those of HDAd5/3+ vector genomes, which was most likely due to immediate sequestration of HDAd5/35++ particles by RBCs (via CD46). Also, for HDAd5/35++ genomes, there was a second peak around day 1, which we previously attributed to virions released from RBCs.8
Analyses of peripheral blood cells
We then measured the presence of HDAd genomes, mgmtP140K messenger RNA (mRNA), and cxcr4 mRNA expression in peripheral blood cells. Notably, up to day 3 after HDAd injection, peripheral blood contains mobilized HSCs, and HSCs are thought to express higher target receptor levels than differentiated blood cells.13 The data, therefore, to some degree, reflect the transduction of mobilized HSCs. The VCN was measured in peripheral blood mononuclear cells (PBMCs) and, for HDAd5/35++GFP/cxcr4 and HDAd5/3+GFP/cxcr4, in total blood cells (which are 99.9% RBCs) (Figure 3A). A number of conclusions can be made: (1) vector DNA declined in PBMCs to nearly undetectable levels by day 3, indicating that transduced cells disappeared from the periphery either due to HSC homing to bone marrow and spleen, or natural turnover of differentiated cells; (2) VCN levels were the lowest in the HDAd5/3+GFP–treated animal (ie, the animal for which mobilization was least efficient); (3) the VCN measured in total blood (RBCs) was higher in the HDAd5/35++GFP/cxcr4–treated animal, whereas it was comparable in PBMCs and RBCs for HDAd5/3+GFP/cxcr4, consistent with HDAd5/35++ sequestration by RBCs; and (4) for the HDAd5/35++GFP/cxcr4 vector–injected animal, a second VCN peak occurred around day 7. We speculate that this is still RBC-derived vector DNA, possibly from hepatic or splenic erythrocytes. The concentrations of mgmtP140K and cxcr4 mRNAs in transduced PBMCs mirrored, to a large degree, the VCN data. As expected, cxcr4 mRNA concentrations were higher than mgmtP140K mRNA concentrations, most likely due to the strong CMV promoter (Figure 3B). Puzzling, however, is the second peak of mgmtP140K and cxcr4 mRNA in the HDAd5/35++GFP/cxcr4–treated animal.
Analysis of transduction in peripheral blood cells. (A) VCN per cell in PBMCs (black lines) and total blood cells (RBCs+PBMCs, red lines, measured only for HDAd5/35++GFP/cxcr4– and HDAd5/3+GFP/cxcr4–injected animals). (B) Analysis of transgene mRNA levels relative to mRNA levels of the housekeeping gene GAPDH. Human mgmtP140K mRNA data are shown by black lines; human cxcr4 mRNA levels are shown by red lines. Note that the scale of the y-axis for the HDAd5/3+GFP/cxcr4–injected animal is different. (C) Percentage of GFP+ HSCs in peripheral blood white cells. The scale of the y-axis for the HDAd5/3+GFP/cxcr4–injected animal is different.
Analysis of transduction in peripheral blood cells. (A) VCN per cell in PBMCs (black lines) and total blood cells (RBCs+PBMCs, red lines, measured only for HDAd5/35++GFP/cxcr4– and HDAd5/3+GFP/cxcr4–injected animals). (B) Analysis of transgene mRNA levels relative to mRNA levels of the housekeeping gene GAPDH. Human mgmtP140K mRNA data are shown by black lines; human cxcr4 mRNA levels are shown by red lines. Note that the scale of the y-axis for the HDAd5/3+GFP/cxcr4–injected animal is different. (C) Percentage of GFP+ HSCs in peripheral blood white cells. The scale of the y-axis for the HDAd5/3+GFP/cxcr4–injected animal is different.
We then measured GFP expression in mobilized HSCs (CD34+/CD45RA–/CD90+ cells) (Figure 3C). The number of GFP+ circulating HSCs was lower in the animals injected with HDAd5/35++, underscoring again that RBC sequestration of this vector greatly diminished the transduction of the target cells. Transduction rates of mobilized HSCs reached up to 23% for the animals injected with the HDAd5/3+ vectors. For all animals, the percentage of GFP+ HSCs sharply declined by day 7, similar to the VCN kinetics.
Analyses of bone marrow cells
Again, our aim was to increase the number of transduced HSCs in the bone marrow. First, we measured the percentage of CD34+/CD45RA–/CD90+ cells in total bone marrow mononuclear cells (MNCs) to assess the effect of HSC mobilization (Figure 4A). As expected, only about 15% to 20% of mobilized primitive HSCs returned to the bone marrow, as seen in both animals that were injected with HDAd vectors that did not express cxcr4. Cxcr4 expression in mobilized HSCs greatly increased the number of HSCs that returned to the bone marrow, reaching levels that were even higher than pretreatment levels. The latter finding might indicate that HSCs mobilized from the spleen also migrated to the bone marrow.
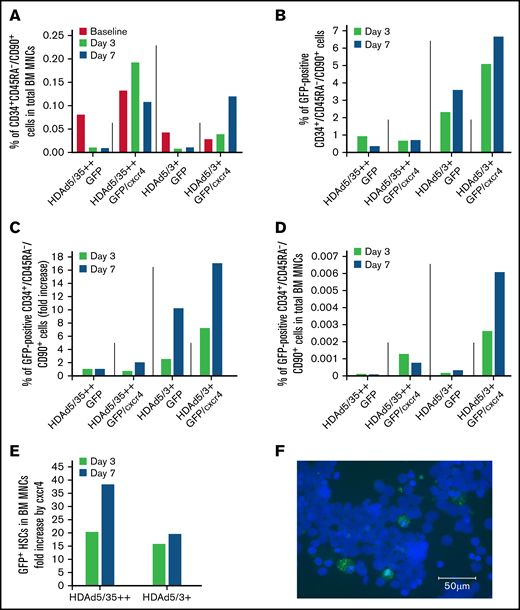
GFP expression in bone marrow (BM) HSCs and MNCs. (A) Return of mobilized HSCs to the BM. Shown is the percentage of primitive (CD34+/CD45RA–/CD90+) HSCs in total BM MNCs measured in BM samples at days 3 and 7. (“Day 0” is the day of HDAd injection.) (B) In vivo transduced primitive HSCs that returned to the BM. Shown is the percentage of GFP-positive CD34+/CD45RA–/CD90+ HSCs in BM aspirates collected at days 3 and 7. (C) Shown are the data from panel B as fold increase, taking transduction with the HDAd5/35++GFP vector as “1.” (D) Percentage of GFP-positive CD34+/CD45RA–/CD90+ HSCs relative to total BM MNCs. (E) Data extracted from panel D showing the effect of cxcr4 expression on the GFP+ HSCs in the BM (comparison of HDAd5/35++GFP vs HDAd5/35++GFP/cxcr4 and HDAd5/3+GFP vs HDAd5/3+GFP/cxcr4). (F) Representative GFP immunofluorescence on a BM CD34+ cytospin of the HDAd5/3+GFP/cxcr4–injected animal. CD34+ cells were isolated from a BM aspirate (after RBC lysis) by human CD34 magnetic-activated cell sorting, spun on a glass slide, and then stained with a GFP–fluorescein isothiocyanate antibody. GFP signals are green. Nuclei were stained with 4′,6-diamidino-2-phenylindole and appear in blue. Scale bar = 50 μm.
GFP expression in bone marrow (BM) HSCs and MNCs. (A) Return of mobilized HSCs to the BM. Shown is the percentage of primitive (CD34+/CD45RA–/CD90+) HSCs in total BM MNCs measured in BM samples at days 3 and 7. (“Day 0” is the day of HDAd injection.) (B) In vivo transduced primitive HSCs that returned to the BM. Shown is the percentage of GFP-positive CD34+/CD45RA–/CD90+ HSCs in BM aspirates collected at days 3 and 7. (C) Shown are the data from panel B as fold increase, taking transduction with the HDAd5/35++GFP vector as “1.” (D) Percentage of GFP-positive CD34+/CD45RA–/CD90+ HSCs relative to total BM MNCs. (E) Data extracted from panel D showing the effect of cxcr4 expression on the GFP+ HSCs in the BM (comparison of HDAd5/35++GFP vs HDAd5/35++GFP/cxcr4 and HDAd5/3+GFP vs HDAd5/3+GFP/cxcr4). (F) Representative GFP immunofluorescence on a BM CD34+ cytospin of the HDAd5/3+GFP/cxcr4–injected animal. CD34+ cells were isolated from a BM aspirate (after RBC lysis) by human CD34 magnetic-activated cell sorting, spun on a glass slide, and then stained with a GFP–fluorescein isothiocyanate antibody. GFP signals are green. Nuclei were stained with 4′,6-diamidino-2-phenylindole and appear in blue. Scale bar = 50 μm.
The GFP analyses in bone marrow CD34+/CD45RA–/CD90+ cells revealed a number of important findings (Figures 4B-C). First, the percentage of GFP+ HSCs was higher for the HDAd5/3+GFP–treated animal compared with HDAd5/35++GFP (in spite of the poor mobilization and in vivo transduction). The percentage increased from day 3 to 7 for HDAd5/3+GFP, reaching 3.6% (Figure 4B; supplemental Figure 5). This corresponds to a 10-fold greater in vivo HSC transduction rate with HDAd5/3+GFP compared with HDAd5/35++GFP (Figure 4C). This difference was also seen when the percentage of GFP+ HSCs in total bone marrow MNCs was plotted (Figure 4D). Second, cxcr4 overexpression from the HDAd vectors increased the percentage of GFP+ HSCs in both the HDAd5/35++GFP/cxcr4– and the HDAd5/3+GFP/cxcr4–transduced animals. Peak GFP marking levels in HSCs (6.7% for HDAd5/3+GFP/cxcr4) corresponded to an approximately 18-fold increase over HDAd5/35++GFP (ie, the capsid that was used in our previous NHP studies8 ). This pattern was also observed when data were plotted as %GFP+ HSCs in total bone marrow HSCs. The effect of cxcr4 expression from HDAd5/35++ and HDAd5/3+ vectors on the percentage of GFP+ HSCs in bone marrow MNCs, a 20- to 38-fold increase, is separately shown in Figure 4E. Again, in vivo HSC transduction of a rhesus macaque with HDAd5/3+GFP/cxcr4 at a dose of 0.4 × 1012 vp/kg resulted in 6.7% of GFP-positive primitive HSCs present in the bone marrow. This was confirmed by an independent method (ie, GFP immunofluorescence on ∼7% of CD34+ cells that were isolated by magnetic-activated cell sorting from bone marrow of the HDAd5/3+GFP/cxcr4–transduced animal at day 7) (Figure 4F).
For both HDAd5/35++ and HDAd5/3+ vectors, mgmtP140K and cxcr4 mRNA expression levels were higher in bone marrow CD34+ cells compared with total bone marrow MNCs, indicating preferential HSC transduction upon mobilization (supplemental Figure 6), which is in agreement with our previous study.8 As shown in the GFP analyses, mgmtP140K and cxcr4 mRNA levels were the highest in the HDAd5/3+GFP/cxcr4–transduced animal. No detectable mRNA expression was seen in bone marrow aspirates taken at weeks 4 and 8 after in vivo transduction, most likely because rapid HSC and progenitor cell proliferation resulted in the loss of episomal vector genomes. Notably, G-CSF+AMD3100 not only mobilized HSCs from the bone marrow but also differentiated HSC progeny cells as shown by white blood cell counts (supplemental Figure 7A) and analyses of CD3+ lineage cells in PBMCs (supplemental Figure 7B).
Importantly, the presence of ∼7% in vivo transduced, primitive HSCs in the bone marrow is a solid basis for further in vivo HSC transduction studies.
Safety of in vivo transduction with new HDAd vectors
A major focus of our work is the evaluation of the safety of our approach. Mobilization and IV HDAd injection after cytokine prophylaxis (anakinra, tocilizumab, and dexamethasone) was well tolerated in all 4 mobilized rhesus macaques. No changes in behavior, general health, or weight were noted during the observation period.
Pro-inflammatory cytokines.
Compared with our previous study, fewer cycles of anakinra and tocilizumab were given, and the route of anakinra was changed from an IV to a subcutaneous injection.8 Serum cytokines (interleukin-2 [IL-2], IL-4, IL-5, IL-6, tumor necrosis factor α [TNFα], and interferon γ) were measured by using a highly sensitive cytometric bead array. Only IL-6 (Figure 5A) and TNFα (Figure 5B) were detectable, and their levels were below what is considered concerning in humans37 and NHPs38 (15 000 pg/mL for IL-6 and 100 pg/mL for TNFα). It is notable, however, that the peak of serum IL-6 and TNFα occurred ∼2 hours later in the animals injected with HDAd5/3+ compared with those injected with HDAd5/35++, suggesting a potentially different virus uptake/sensing by the reticulo-endothelial system.39
Safety of IV HDAd injection into mobilized rhesus macaques. (A) Serum IL-6 levels measured by cytometric bead array. HDAd injections (day 0, 0 hours) are indicated by red arrows. (B) Serum TNFα levels. (C) Selected hematologic parameters. The normal range is indicated by the gray shading. THOU, thousands.
Safety of IV HDAd injection into mobilized rhesus macaques. (A) Serum IL-6 levels measured by cytometric bead array. HDAd injections (day 0, 0 hours) are indicated by red arrows. (B) Serum TNFα levels. (C) Selected hematologic parameters. The normal range is indicated by the gray shading. THOU, thousands.
Hematologic parameters and organ histology.
Most hematologic parameters were unremarkable, including liver transaminase levels (Figure 5C; supplemental Figures 8-10). As expected, white blood cells, specifically neutrophils, were transiently elevated due to G-CSF/AMD3100 mobilization (supplemental Figures 8 and 10). Of note is a thrombocytopenia that lasted up to day 4 after HDAd injection (Figure 5C), the degree of which, however, did not require blood transfusion. At necropsy (day 7), no treatment-related abnormalities were found.
Off-target HDAd transduction: blood/bone marrow.
Virus transduction does not occur below a threshold density of receptors on cells40 (ie, CD46 and DSG2). As outlined earlier, CD46 and DSG2 levels are lower on differentiated blood cells than on HSCs. After IV injection into mobilized macaques, for both HDAd5/35++ and HDAd5/3+ vectors, low level (∼3%) GFP+ CD14+ cells were detected in PBMCs early after infusion (Figure 6A). These could represent directly transduced mobilized neutrophils. GFP+CD14+ cells disappeared by day 2 from the circulation. In bone marrow, GFP+ lineage-positive cells were <1% (Figure 6B). These are most likely transduced peripheral blood cells that returned to the bone marrow because direct transduction of cells within the bone marrow is very inefficient due to physical barriers.19
Percentage of GFP-positive cells in lineage–positive cells. Percentage of GFP+ CD3+, CD14+, and CD20+ lineage cells in PBMCs (A) and bone marrow MNCs (B).
Percentage of GFP-positive cells in lineage–positive cells. Percentage of GFP+ CD3+, CD14+, and CD20+ lineage cells in PBMCs (A) and bone marrow MNCs (B).
Overall, the hematologic, histologic, and biodistribution studies suggest an acceptable safety profile for DSG2-targeting HDAd5/3+ vectors. Further attention is needed regarding the transient thrombocytopenia.
Discussion
We show here that DSG2 can be used as a receptor for safe in vivo HSC transduction in rhesus macaques. In 2011, we identified DSG2 as a receptor for species B adenoviruses,9 and this led to research that was mostly focused on its role as an epithelial junction protein.9,10,20,41 , -45 The presence of DSG2 on HSCs was first reported by Ebert et al13 and then further elucidated in this study. The ubiquitous presence of DSG2 on HSCs, together with the finding that DSG2 is also elevated on endothelial progenitor cells, points toward a potential role of DSG2 in linking HSCs and bone marrow endothelial cells, thereby retaining HSCs in the bone marrow. There is 87% to 90% and 50% overlap in CD46 and DSG2 expression on human and rhesus CD34+ cells, respectively. The subfraction of CD46+/DSG2– CD34-positive cells still has to be characterized functionally. Important for our study is that both DSG2-targeting HDAd5/3+ and CD46-targeting HDAd5/35++ vectors efficiently transduced human CD34+ cells. It is noteworthy, however, that transduction of rhesus CD34+ cells was less efficient than human CD34+ cells. We attribute this to species-related structural differences in the corresponding receptors.
Rhesus macaques are still the only relevant species for studying DSG2-targeting HDAds. A human DSG2 transgenic mouse strain that we developed earlier adequately modeled the presence and function of DSG2 in epithelial tissues.43 However, human DSG2 was only expressed at low levels on lineage-negative bone marrow cells, making DSG2-transgenic mice a poor model for in vivo HSC transduction studies.
First, we showed in rhesus macaques that targeting DSG2 vs CD46 on HSCs in vivo increases the HDAd transduction rate of primitive HSCs about 10-fold. Several lines of evidence suggest that this is due to the avoidance of sequestration by RBCs (vector serum clearance) (Figure 3C), VCN in total blood cells (Figure 4A), and percentage of circulating GFP+ HSCs (Figure 4C). Notably, the superiority of the HDAd5/3+ vector was obvious despite lower DSG2 receptor presence (compared with CD46) on rhesus HSCs and relatively inefficient HSC mobilization in the animal that received the HDAd5/3+GFP vector.
Second, transient overexpression of human cxcr4 on mobilized HSCs increased the percentage of GFP+ HSCs in the bone marrow regardless of the receptor usage (ie, for both HDAd5/35++ and HDAd5/3+). For the animal injected with HDAd5/3+, cxcr4 expression in circulating HSCs reached a peak at ∼12 hours after production. This finding is consistent with our in vitro studies showing that expression from the CMV promoter appears as early as 3 hours after in vitro transduction (data not shown). After appearance of the CXCR4 receptor at the cell surface, the next step in trafficking could be the direct translocation of CXCR4high HSCs to the bone marrow, or it could involve the spleen as a transitory organ.17 We hypothesize that chemotaxis of mobilized, transduced HSCs and homing to the bone marrow involves stromal cell–derived factor 1, the CXCR4 ligand. Stromal cell–derived factor 1/CXCR4 interactions are also critical in retention of HSCs in the bone marrow.16 The bone marrow–homing effect of CXCR4 protein could be seen at day 3 and was more pronounced at day 7. cxcr4 expression was still detectable on bone marrow HSCs at day 7. Additional studies with non-immunogenic transgene products are required to determine how long cxcr4 expression on HSCs lasts and whether the CMV promoter driving cxcr4 is indeed silenced. Alternative approaches to increase HSC homing include the drug cilostazol46 and iron chelators.47
A main result of this study was the finding that in vivo HSC transduction with the HDAd5/3+GFP/cxcr4 vector at a low dose of 0.4 × 1012 vp/kg resulted in ∼7% of transduced primitive HSCs in the bone marrow at day 7. Based on our mouse studies, expression of sickle mutation–correcting genome editors in 7% of HSCs would give the edited HSC progeny cells a survival/proliferation advantage that would result in an amelioration or correction of the sickle cell disease phenotype.48
Clearly, a key question is the safety of our approach, specifically side effects from systemic HDAd vector administration. All procedures, including mobilization and IV HDAd injection, were well tolerated in the 4 animals. This adds to the list of 20 rhesus macaques in which the procedure was performed safely. It is also in agreement with good safety data from clinical trials in which cancer patients received IV infusion of oncolytic Ad5/3 or Ad3 viruses that also target DSG2.49-51
Activation of pro-inflammatory cytokines in hepatic and splenic reticulo-endothelial system cells after IV Ad infusion can lead to critical acute toxicity. We therefore implemented a prophylactic regimen consisting of anakinra, tocilizumab, and dexamethasone to block cytokine activation. However, transient thrombocytopenia in the days that followed HDAd infusion was still observed. In part, this is due to side effects from dexamethasone pretreatment. It is thought, however, that interactions between the Ad5 penton capsid protein and cellular αvβ3/5 integrins contribute to thrombocytopenia.52 Small structural changes within the Ad capsid or the use of other serotypes could address this problem. Overall, there were no other remarkable, treatment-related abnormalities in hematologic, pathologic, or histopathologic analyses.
The transduction profile of non-HSCs after IV injection was acceptable, based on our experience with other viral vectors. Because the receptor density (DSG2 and CD46) was lower on differentiated blood cells than on mobilized HSCs, both vector platforms preferentially transduced HSCs. Furthermore, in the context of Investigational New Drug–enabling NHP studies with a modified recombinant Ad3 fiber knob (aka JO4) that also targets DSG2, we showed that DSG2 in normal epithelial tissues, which display a strict apical basal polarization, is trapped in lateral junctions and not accessible to IV injected ligands.10,53 This would imply that our DSG2-targeting HDAd5/3+ vectors would be safer compared with HDAd5/35++ vectors that target the ubiquitously expressed CD46 receptor. Furthermore, in contrast to HDAd5/35++ vectors, the use of HDAd5/3+ vectors would allow for adequate toxicology studies in NHPs, a prerequisite for the clinical translation of our approach for the treatment of nonmalignant diseases such as hemoglobinopathies.
There are additional improvements to the HDAd5/3+/cxcr4 vector system that we could consider; for example, switching the main body of the capsid from Ad5 to a less prevalent serotype to avoid neutralization by anti-Ad serum antibodies. Furthermore, instead of Ad3 fibers, fibers from other DSG2-interacting species Ad serotype that are rare in the human population (eg, Ad5554 ) could be used.
Overall, this study describing a new vector platform, HDAd5/3+/cxcr4, is a step toward the clinical translation of our in vivo HSC gene therapy approach. In addition, the ability to genetically mark mobilized HSCs in vivo by HDAd vector transduction can help in studying their fate.
Acknowledgments
The study was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL128288 and R01HL141781), by a grant from Ensoma Bio, and by a grant from the Bill & Melinda Gates Foundation (OPP1212391). Under the grant conditions of the Bill & Melinda Gates Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The Washington National Primate Research Center is supported by grant P51 OD010425 from the National Institutes of Health Office of Research Infrastructure Programs.
Authorship
Contribution: A.L. provided the conceptual framework for the study; H.W. designed the experiments and performed vector production, in vitro studies, and all NHP sample analyses; A.G. performed all NHP procedures; S.G., J.K. and C.L. performed experiments; and H.-P.K. provided critical comments on the manuscript.
Conflict-of-interest disclosure: A.L. and H.-P.K. are scientific cofounders of Ensoma Bio. H.-P.K. is a paid advisor for Ensoma Bio. The remaining authors declare no competing financial interests.
Correspondence: André Lieber, University of Washington, Box 357720, Seattle, WA 98195; e-mail: lieber00@uw.edu.
References
Author notes
Requests for data sharing may be submitted to the corresponding author (e-mail: lieber00@uw.edu).
The full-text version of this article contains a data supplement.


![Analysis of DSG2 expression on HSCs. (A) Flow cytometry for CD46 and DSG2 on human CD34+ cells from 2 different healthy G-CSF–mobilized donors. (B) Schematic of HDAd vector capsids and genomes. The chimeric capsids contain either affinity-enhanced Ad35 or Ad3 fiber knob domains. Both vector genomes contain an EF1α-mgmtp140k/GFP expression cassette used before.21 Both proteins are linked via a self-cleaving picornavirus 2A peptide. One set of vectors has an additional CMV promoter–human cxcr4 expression cassette. The 4 vectors used in this study are HDAd5/35++GFP, HDAd5/3+GFP, HDAd5/35++GFP/cxcr4, and HDAd5/3+GFP/cxcr4. (C) In vitro CD34+ cell transduction studies. Cells were infected with HDAd5/35++-GFP and HDAd5/3+-GFP virus at a multiplicity of infection of 2000 vp/cell with and without pre-incubation with recombinant soluble CD46 or DSG2 (10 μg/mL) for 1 hour. GFP expression was analyzed 24 hours’ postinfection. (D) CD46 and DSG2 flow cytometry on human and rhesus peripheral RBCs stained with antibodies that recognize the receptors in both species (anti-human CD46 mAb clone M177 from Santa Cruz Biotechnology [Dallas, TX] and anti-human DSG2 polyclonal antibody AF947 from R&D Systems [Minneapolis, MN]). (E) Transduction of 293 cells with HDAd5/35++GFP and HDAd5/3+GFP in the absence and presence of human (huRBCs) and rhesus (rhRBCs) RBCs (details are provided in the Materials and methods). Shown is the percentage of GFP-positive cells measured 2 days after transduction.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/15/10.1182_bloodadvances.2022007975/4/m_advancesadv2022007975f1.png?Expires=1767892519&Signature=cwvPbRmxlyQU2PBWNmVb5c03IV87dtqK5bYt1zsA1NvqutPlftKzEngOWJkMNRXbG-JClWMxICTKGXWfVf7~QxhTylIwSNuFdWb1RFiLihLfUQRSJBsX6RFfbqug7ve2Rlgz8TbIkMcrJuXPWJTb4MLbp9ddpdefMZR3hOq3FrFSUMlhs02D1JRDF~pJGfSjH4XOI7t~oDG-1xaWG1Q~hnUcOKr9un45B1nxXAQcYYUgdn9ePaFsmL2DqOpGsYsYGK9bkBuMFKKIPQIcYIWEE6yFNG6m66LAVGX7DxR2bXO~DmGbsN5j-~Tn9f3bPGQpMdvBXm3Wqtac633LF2MSSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)







![Analysis of DSG2 expression on HSCs. (A) Flow cytometry for CD46 and DSG2 on human CD34+ cells from 2 different healthy G-CSF–mobilized donors. (B) Schematic of HDAd vector capsids and genomes. The chimeric capsids contain either affinity-enhanced Ad35 or Ad3 fiber knob domains. Both vector genomes contain an EF1α-mgmtp140k/GFP expression cassette used before.21 Both proteins are linked via a self-cleaving picornavirus 2A peptide. One set of vectors has an additional CMV promoter–human cxcr4 expression cassette. The 4 vectors used in this study are HDAd5/35++GFP, HDAd5/3+GFP, HDAd5/35++GFP/cxcr4, and HDAd5/3+GFP/cxcr4. (C) In vitro CD34+ cell transduction studies. Cells were infected with HDAd5/35++-GFP and HDAd5/3+-GFP virus at a multiplicity of infection of 2000 vp/cell with and without pre-incubation with recombinant soluble CD46 or DSG2 (10 μg/mL) for 1 hour. GFP expression was analyzed 24 hours’ postinfection. (D) CD46 and DSG2 flow cytometry on human and rhesus peripheral RBCs stained with antibodies that recognize the receptors in both species (anti-human CD46 mAb clone M177 from Santa Cruz Biotechnology [Dallas, TX] and anti-human DSG2 polyclonal antibody AF947 from R&D Systems [Minneapolis, MN]). (E) Transduction of 293 cells with HDAd5/35++GFP and HDAd5/3+GFP in the absence and presence of human (huRBCs) and rhesus (rhRBCs) RBCs (details are provided in the Materials and methods). Shown is the percentage of GFP-positive cells measured 2 days after transduction.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/15/10.1182_bloodadvances.2022007975/4/m_advancesadv2022007975f1.png?Expires=1767892520&Signature=bmHZSFr6saOtV6IGg6kbJHQ7y2Jyw3qM0OJsS0bgUN7ykLHAjxSzNh87vWv0Esjy2VyMMQWoC3Yt2CjC9wLGXd4VNVhGgn6S4KZC1yyL7xlTtM7XYbTqxEyaEAzbN3yw6vNqsCZXPuizryQS-Ep3wiKUDig-DSxxbD51sZyqoJbWYHwpznAdLthy6~1MwlKC5h7zTs9K5OY6FgsLWuEORsl-54wBlRBf9mfo2xJ4nkK5lEfWAEILExd51CH66wx4czgGuVImHvhk7speYLbdmCWIgvIEJS8UzG6Vcn5kpqAALzrUYeaSk-cjgikzsQ-q4ovooimHwTBU1NOWw7hI6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)