Key Points
Long-term adult HCT survivors reported average cognitive quality of life compared with the general population.
Survivors with hearing issues and sleep impairments were more likely to report lower quality of life and impaired neurocognitive function.
Abstract
Survivors of hematopoietic cell transplant (HCT) are at risk for neurocognitive impairments, which can negatively affect quality of life. Given limited studies, we aimed to describe the neurocognitive outcomes in a cohort of long-term adult HCT survivors. Eligible survivors (age ≥21 years at HCT and alive ≥2 years following HCT) completed a 60-question survey of neurocognitive function and quality of life, which included the Neuro-Quality of Life Cognitive Function Short Form (Neuro-QoL) and the Childhood Cancer Survivor Study Neurocognitive Questionnaire (NCQ). Analyses of risk factors included univariate comparisons and multivariable logistic regression. Survivors (n = 1861, 47.7% female, 65.6% allogeneic HCT) were surveyed at a median age of 64.2 years (interquartile range [IQR], 56.8-70.5) and a median 12.0 years (IQR, 6.0-21.0) from HCT. Survivors reported average Neuro-QoL scores (50.0 allogeneic; 49.2 autologous survivors) compared with an expected mean of 50 in the general population. On the NCQ, 17.4% to 31.2% of survivors reported impairments (Z-score >1.28) in task efficiency, memory, emotional regulation, or organization, compared with an expected 10% in the general population (all P < .01). In multivariable regression analyses, impaired Neuro-QoL (T-score <40) was independently associated with hearing issues (odds ratio [OR], 2.13; 95% confidence interval [CI], 1.46-3.10) and sleep impairment (OR, 4.41; 95% CI, 2.80-6.94) among allogeneic survivors, with comparable associations in autologous survivors. Overall, long-term adult HCT survivors reported average cognitive quality of life compared with the general population. Subsets of survivors with hearing issues and sleep impairments were more likely to report lower quality of life and impaired neurocognitive function, which may facilitate targeted monitoring or interventions following HCT.
Introduction
Advances in transplantation practices and management have improved long-term survival for patients receiving allogeneic or autologous hematopoietic cell transplantation (HCT). Patients who survive at least 2 years after HCT now have long-term survival exceeding 75% at 15 years from HCT.1 Despite improving survival rates, HCT survivors remain at high risk for chronic health conditions that contribute to increased morbidity and mortality compared with non-HCT cancer survivors and the general population.1-3 Neurocognitive function is an expansive category encompassing memory, attention, concentration, planning, organization, and problem solving, among other abilities.4,5 Persistent deficits in these areas have been colloquially referred to as “chemo-brain” following cancer treatment.6,7 Neurocognitive impairments can complicate the post-HCT course with substantial effects on both specific cognitive abilities as well as overall quality of life.4,8,9 Despite these implications, few studies have characterized the late neurocognitive outcomes in HCT survivors.
Risk of neurocognitive dysfunction in adult HCT survivors has been associated with sex, age, education, receipt of total body irradiation (TBI), and chronic graft-versus-host disease (GVHD).4,10,11 Additionally, multiple clinical risk factors may have a cumulative effect on cognitive function.8 Studies of HCT survivors can be complicated by baseline cognitive impairments before transplant resulting from chemotherapy or other neurotoxic therapies,12,13 which may be more prevalent given the older age of patients now undergoing HCT.14 Time from HCT may also impact study results, with some prospective studies finding that neurocognitive function declines initially following HCT, with recovery in the majority of patients by the end of the first year after HCT.12,15 Although a previous meta-analysis of several small cohort studies found no significant changes in cognitive function following HCT,10 other studies have shown that neurocognitive dysfunction may persist long term in some HCT survivors.13,16
Overall, the incidence and characterization of neurocognitive dysfunction following HCT has been recognized as an understudied area.1,4,5 In this study, we aim to address this gap in knowledge by characterizing the late patient-reported neurocognitive outcomes in a cohort of long-term adult HCT survivors. We also examine the association between treatment variables and certain medical comorbidities with neurocognitive dysfunction in this patient population.
Methods
Participants
This study was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board. FHCRC maintains continuous follow-up of HCT survivors who consent to long-term follow-up via an annual patient-reported health survey, with the earliest transplant performed in 1971.17,18 Patients included in this analysis underwent HCT for both malignant and nonmalignant conditions, including immunodeficiencies and benign hematologic disorders. The following HCT survivors were eligible for this study: alive ≥ 2 years after HCT at FHCRC, age ≥ 21 years at time of transplant, and available current mailing address. Baseline demographic characteristics (including age, sex, race/ethnicity, and underlying diagnosis) and HCT details (including conditioning regimen, donor type, chronic GVHD status) were retrieved from the FHCRC research database.
Survey instruments
All FHCRC HCT survivors consenting to long-term follow-up receive an annual survey mailed on their transplant anniversary. The annual survey includes standardized questions on interval changes in health and presence of chronic GVHD or other health conditions. For this study, a 60-question supplementary module was added to the annual survey to collect information on neurocognitive function and perceived cognitive quality of life. This supplementary module included questions taken from several validated self-reported survey measures. The Neuro-Quality of Life Cognitive Function Short Form (Neuro-QoL) Version 2.0 is an 8-question self-reported measure addressing perceived difficulties in cognitive abilities, as well as application of these abilities to daily tasks reflecting cognitive quality of life.19 The Neuro-QoL was developed by the National Institute of Neurological Disorders and Stroke and validated in the adult general population.19,20 The Childhood Cancer Survivor Study Neurocognitive Questionnaire (NCQ) is validated for use in adult survivors of childhood cancer and consists of 33 questions divided into 4 domains corresponding to emotional regulation, task efficiency, memory, and organization.21 The factors of emotional regulation and organization are primarily measures of executive function, whereas task efficiency and memory address attention, processing speed, and both working and long-term memory.22 Normative data from noncancer controls are available for both questionnaires.
For medical comorbidities, we selected a priori sleep problems, hearing issues, and neurologic conditions (eg, previous strokes or seizures) to include in the survey because we hypothesized that these would be the most relevant comorbid conditions that would influence neurocognitive function. The Patient-Reported Outcomes Measure Information System (PROMIS) Sleep Disturbance Short Form 4a is a 4-question survey to assess perception of sleep quality and restfulness, and has been validated in the adult general population.23 An additional three questions were taken from the National Health and Nutrition Examination Survey 2011 Audiometry Questionnaire to screen for hearing issues (6-point Likert scale), need for hearing aid, or presence of tinnitus (5-point Likert scale).24 Six questions were included to assess for neurologic conditions predicted to affect neurocognitive function, including previous stroke or transient ischemic attack and epilepsy or seizures. These questions were adapted from the relevant questions from the Childhood Cancer Survivor Study questionnaires (yes/no/don’t know).25 Copies of the questionnaire are available on request. For this analysis, we used responses from surveys distributed from July 2018 to June 2019, with all results collected by November 2019. During this time, initial nonresponders were sent 2 follow-up survey requests for a total of 3 mailings.
Statistical analysis
Patient-reported surveys were scored and normalized to the general population (ie, T or Z-scores) according to the instructions provided by the individual test developers, including methods for handling any missing data. Responses for the Neuro-QoL were summed as total raw scores and converted to standardized T-scores with a mean score of 50 and a standard deviation of 10, with lower T-scores suggesting lower cognitive quality of life.19 The NCQ was scored by calculating a total raw score for each of the 4 domains, then converted to an age-adjusted Z-score, with higher Z-scores corresponding to worse neurocognitive scores.21 The PROMIS Sleep Short Form 4a was summed as a total raw score and then translated into a T-score with a mean of 50 and a standard deviation of 10, with higher T-scores signifying worse sleep quality.23 Hearing issues were defined as self-reported moderate/severe hearing trouble or deafness, current use of hearing aid, or moderate/severe tinnitus.
Descriptive statistics, including frequency distributions, medians, and interquartile ranges (IQR) were calculated for demographic and treatment variables. Primary outcome variables, including Neuro-QoL and individual NCQ domain scores, were further dichotomized into impaired vs not impaired. Consistent with previous studies,26 impairment was defined as T-score <40 for the Neuro-QoL (corresponding to 1 standard deviation below the standardized mean)27 and Z-score >1.28 for the NCQ (corresponding to the worst 10th percentile of scores based on healthy control age-adjusted norms).21 Scores were compared using χ2 test for categorical variables and analysis of variance or t tests as appropriate for continuous variables. Statistical significance was considered at the level of P < .01 (2-tailed), given multiple analyses. These results were not further adjusted for multiple comparisons but were used to help identify the variables to be included in the multivariable analyses. To assess the potential influence of nonresponders on the reported group averages, we also applied inverse probability sampling weights28 accounting for sex, race/ethnicity, current age, and type of transplant. After weighting for these features, the outcomes measurements were recalculated to represent the responses of the entire eligible population. Finally, the strength of the associations between certain clinical features and impairment on the Neuro-QoL or NCQ domains were examined using multivariable logistic regression and reported as odds ratios (ORs) with 95% confidence intervals (CIs). Based on our univariate analyses, variables included in the multivariable logistic regression were the following: sex, time since transplant (continuous), current age (<50 years, 50-59 years, 60-70 years, and ≥70 years), hearing issues, history of stroke or seizures, and sleep impairment. All analyses were completed using Stata (Version 16; StataCorp, College Station, TX).
Results
Of 3521 eligible adults who had survived ≥2 years from HCT, 1861 participated (52.9%). At the time of survey, participants had attained a median age of 64.2 years (IQR, 56.8-70.5) and a median 12.0 years (IQR, 6.0-21.0) since transplant (Table 1). The majority of patients received an allogeneic HCT (65.6%), with 10.4% of patients receiving more than 1 transplant. The majority of patients in the cohort received a myeloablative transplant (91.7%), including all of the patients who received autologous HCT. Most patients underwent transplantation for malignant conditions, including acute leukemia (22.5%), chronic leukemia (19.5%), and lymphoma (22.0%). Across all transplants, 47.9% of patients underwent conditioning with TBI, with 29.5% receiving ≥1000 cGy. The majority of patients (66.6%) who received allogeneic HCT reported a diagnosis of chronic GVHD. The prevalence of moderate/severe hearing loss or tinnitus and history of stroke or seizures was 30.4% and 9.7%, respectively. Compared with respondents, nonrespondents were more likely to be male, non-White, and younger at the time of the survey (supplemental Table 1).
Respondents reported average Neuro-QoL scores (50.0 in allogeneic HCT survivors and 49.2 in autologous HCT survivors) compared with an expected mean score of 50 in the general population (Table 2). These values were similar after accounting for inverse probability weights, with mean Neuro-QoL score of 49.6 (supplemental Table 2). For both allogeneic and autologous HCT survivors, characteristics associated with lower Neuro-QoL scores included female sex, hearing issues, and sleep disturbances (P < .01). Time since transplant was not associated with a change in Neuro-QoL scores. History of stroke/seizure was generally associated with lower Neuro-QoL scores, although this association was statistically significant among allogeneic HCT survivors only. Older survivors appeared to report relatively higher cognitive quality of life compared with younger survivors. For both groups of survivors, higher educational achievement (defined as college completion) was associated with higher Neuro-QoL scores, whereas no significant differences in Neuro-QoL scores were seen based on race/ethnicity, underlying diagnosis, number of transplants, cumulative TBI exposure, or presence of chronic GVHD (results not shown).
On the NCQ, 43.1% of allogeneic HCT survivors reported impairments in 1 or more domains, with 27.7% reporting impairments in 2 or more domains. By individual domain, 31.2% of allogeneic survivors reported problems with task efficiency, 26.0% with memory, 20.9% with organization, and 17.4% with emotional regulation, compared with an expected 10% in the general population (all P < .01; Table 3). In unweighted analyses, female allogeneic survivors reported more issues in all domains relative to males, although only memory impairments rose to the level of statistical significance. Characteristics associated with impairments in most or all NCQ domains included hearing issues, history of stroke/seizure, and self-reported sleep disturbances. Survivors with history of stroke/seizures reported worse scores in task efficiency and memory. Similar to our Neuro-QoL findings, fewer impairments were reported by older participants relative to age-matched norms, particularly in emotional regulation and memory.
Among autologous HCT survivors, 46.2% reported impairments in 1 or more NCQ domains, with 32.4% reporting impairments in 2 or more domains. Autologous HCT survivors reported impairments in all NCQ domains at higher rates compared with the general population (34.7% with task efficiency, 31.1% with memory, 24.3% with organization, 17.4% with emotional regulation; all P < .01; Table 4). Similar to allogeneic survivors, hearing issues and self-reported sleep disturbances were consistently associated with lower NCQ scores among autologous HCT survivors. In evaluation of all survivors combined, NCQ results were similar after application of inverse probability weights (supplemental Table 2).
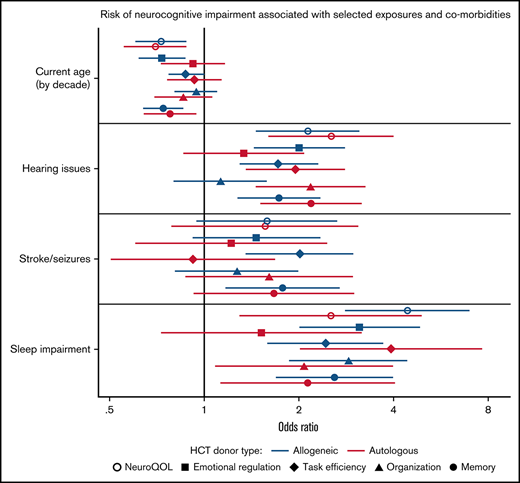
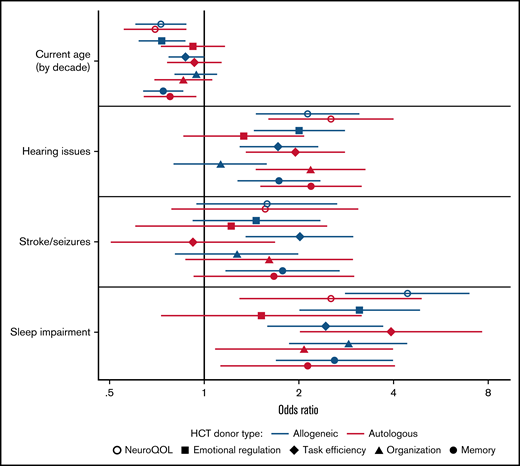
In multivariable regression analysis adjusted for sex and time since transplant, older age at time of survey was independently associated with improved cognitive quality of life (OR, 0.73; 95% CI, 0.60-0.88) for every decade increase in age for allogeneic HCT survivors, with similar values in autologous HCT survivors (Figure 1; supplemental Table 3). In contrast, impaired Neuro-QoL was associated with hearing issues (OR, 2.13; 95% CI, 1.46-3.10) and sleep impairment (OR, 4.41; 95% CI, 2.80-6.94) among allogeneic HCT survivors, with comparable associations in autologous HCT survivors. For NCQ outcomes, sleep impairment was independently associated with impairments in almost all domains among both allogeneic and autologous HCT survivors. Among allogeneic HCT survivors, older attained age was associated with a reduced impact on emotional regulation and memory Z-scores compared with younger survivors. Hearing issues were associated with impaired emotional regulation, task efficiency, and memory, whereas a history of stroke or seizures was associated with impaired task efficiency and memory. For autologous HCT survivors, older individuals were also less likely to report issues with memory, whereas hearing issues were associated with worse task efficiency, organization, and memory.
Risk of neurocognitive impairment associated with selected exposures and comorbidities using multivariable logistic regression adjusted for sex and time since transplant. Model includes all variables listed (current age by decade, hearing issues, stroke/seizures, sleep impairment) and additionally adjusted for sex and time since transplant (continuous).
Risk of neurocognitive impairment associated with selected exposures and comorbidities using multivariable logistic regression adjusted for sex and time since transplant. Model includes all variables listed (current age by decade, hearing issues, stroke/seizures, sleep impairment) and additionally adjusted for sex and time since transplant (continuous).
Discussion
Our study found that adult HCT survivors, surveyed at a median of 12.0 years after HCT, reported average cognitive quality of life compared with general population norms. Despite reported problems with specific cognitive abilities reflected as impairments on the NCQ, these cognitive problems were not perceived to interfere with daily functioning nor impact quality of life, as reflected on the Neuro-QoL. This may reflect compensatory strategies, adaptation of expectations, or a degree of resiliency to maintain well-being and quality of life despite treatment-related complications and ongoing cognitive deficits. Our study is consistent with several other studies showing that long-term survivors of HCT report an overall quality of life comparable with age and sex-matched healthy controls.11,29 While we analyzed allogeneic and autologous HCT survivors separately, we found generally similar outcomes in cognitive quality of life and neurocognitive impairments between the 2 groups.
Survivors reported persistent impairments in specific NCQ domains of emotional regulation, task efficiency, memory, and organization. Our results corroborate with several studies demonstrating the presence of pervasive cognitive deficits in patients post-HCT.8,11,16 These persistent deficits have also been referred to as “chemo-brain” in previous studies.7 In a small study of long-term HCT survivors (range 2-7 years after HCT), survivors were more likely to have objective impairments in attention, processing speed, and memory on cognitive testing, whereas 27.5% and 17.5% of patients reported subjective moderate-severe problems in memory and attention, respectively.11 We did not find any differences in neurocognitive outcomes based on time since transplant in our cohort of survivors (median, 12.0 years; range, 2-47 years after HCT). Similarly, in a meta-analysis based on 11 studies comparing pre- and post-HCT neurocognitive assessments, time since transplant (ranging from mean 35 days to almost 9 years) was not associated with changes in cognitive function.10 In contrast, several smaller studies found that cognitive function improves after the immediate post-HCT period.15,30 In a previous longitudinal study at our institution, Syrjala et al found that cognitive impairments were greatest in the immediate posttransplantation period, with partial recovery by 1 year15 and continued improvement by 5 years after HCT16 ; however, 41.5% of survivors at 5 years after HCT still demonstrated overall cognitive impairment compared with 19.7% of matched non-HCT controls.16 Although the vast majority of our patients received myeloablative conditioning regimens, a previous longitudinal study suggested that recipients of reduced-intensity allogeneic HCT have neurocognitive outcomes comparable with healthy controls initially after HCT, with increased impairments noted after 3 years.13
Older age was consistently associated with greater cognitive quality of life and fewer impairments in specific neurocognitive domains in our cross-sectional study, even after adjustment for time since transplant. Although NCQ results were scaled based on the current age of the respondent (up to age 50 years),21 NeuroQoL scoring did not account for age, so the effect of current age on neurocognitive outcomes relative to the general population may be obscured. Although aging is generally associated with cognitive declines, particularly in executive function and working memory,31 this process is often variable and accompanied by compensatory mechanisms.32 In other studies specifically in the HCT population, a small study of allogeneic survivors found that older age (≥ 65 years) was associated with worse verbal memory and verbal fluency compared with younger patients in the first year posttransplantation.14 Because our study focused on long-term survivors, this suggests that although older patients may be more susceptible to acute neurocognitive toxicities, this effect may be mitigated with increased time from transplant. Interestingly, a small study in adult HCT survivors at 6 months after HCT found that older age was associated with more impairments in objective cognition tests; however, younger patients made more subjective cognitive complaints (most commonly in the domains of remote memory, attention/concentration, and language).33
In our study, subsets of HCT survivors were more likely to report lower quality of life and impaired neurocognitive function, including those with hearing conditions (moderate to severe hearing loss or more bothersome tinnitus), history of stroke or seizures, or sleep disturbances. Although studies specific to adult HCT survivors are limited, previous studies in adult survivors of pediatric cancers demonstrated the association of hearing and visual deficits with impaired emotional regulation and organization,34 whereas history of stroke was associated with worse health-related quality of life and neurocognitive function, particularly task efficiency and memory.35 Similarly, both hearing issues and history of stroke or seizures were associated with neurocognitive dysfunction and worse quality of life in our group’s previous study of adult survivors of pediatric HCT.26 Hearing loss in particular is a leading cause of global disability36 and associated with cognitive dysfunction in the general adult population,37 with effective interventions, from hearing devices to rehabilitation, available to potentially improve quality of life and other health outcomes.36 In terms of other risk factors, similar to other studies, we did not find that history of TBI10,12 or chronic GVHD12 were associated with neurocognitive outcomes. While we were unable to examine the additive effect of multiple comorbidities, patients with more clinical risk factors in the acute setting (including transplant-related factors and complications) have been reported to exhibit worse neuropsychological performance at 6 months after HCT and less evidence of recovery at 12 months after HCT.8 Identification of vulnerable subgroups of patients at higher risk for post-HCT complications is critical to improve preventive care measures and screening.18
Finally, we found that self-reported sleep disturbances were significantly associated with worse cognitive quality of life and greater impairments in all NCQ domains. Given the cross-sectional nature of our study, we were unable to determine if sleep issues had a causal effect on neurocognitive function, although sleep impairment is generally associated with worse quality of life and frequently identified as a significant concern following HCT.38-41 Sleep impairment is common in the posttransplant period but generally improves/stabilizes by 1 year after HCT.38,42 In a previous short-term study, adult HCT survivors with sleep problems at 1 year after HCT reported greater cognitive dysfunction, even after controlling for depressive symptoms, fatigue, and pain.43 Our study corroborates the results from other long-term studies that sleep continues to be an ongoing issue for a subset of HCT survivors,39 with considerable impact on neurocognitive function. Using similar neurocognitive measures as our study, long-term adult survivors of pediatric cancer and HCT who reported sleep disturbances exhibited greater impairments in all NCQ domains.26,44 Routine assessments of sleep, fatigue, and circadian rhythm are critical as potentially modifiable risk factors, although interventional studies specifically targeting sleep in HCT survivors have not yet been successful.38 In cancer survivors with insomnia, sleep education and group cognitive behavioral therapy have been shown to be effective therapies.45
Although our study benefited from a long average follow-up duration and one of the largest samples examining neurocognitive outcomes in long-term HCT survivors, there were several limitations. Our study was conducted at a single center and limited by lower response rate as well as limited racial/ethnic diversity. Thus, our results may not be fully representative of the overall population of adult HCT survivors. Nonrespondents were more likely to be male, non-White, and younger. It is unclear how potential response bias would have influenced these results, as those with lower cognitive function may have been less likely to respond; conversely, these patients may have been more likely to participate given their interest in the survey. However, when inverse probability weighting was applied to represent the entire cohort, we did not find a meaningful change in our results. We were also unable to account for preexisting cognitive deficits or pre-HCT exposures that may increase neuropsychological risk factors, such as history of cranial irradiation or receipt of intrathecal chemotherapy. Although we lacked correlative objective neuropsychological testing, a recent study demonstrated a modest correlation between self-reported cognitive impairment and objective cognitive test results for allogeneic HCT survivors, although no correlation was seen in autologous HCT survivors.46 In the absence of a direct comparison group, we used self-reported measures that had been validated in and normalized to the general population. Additionally, comorbid medical or psychiatric conditions were not included in the questionnaire, and it is possible that those conditions may have influenced our results. Finally, given the cross-sectional nature of this study, limited conclusions about the causality of the findings could be made.
Conclusions
Long-term adult survivors of HCT, at a median of 12.0 years following transplant, reported average cognitive quality of life compared with the general population. However, survivors reported persistent impairments in specific neurocognitive domains of emotional regulation, task efficiency, memory, and organization. Subsets of HCT survivors with hearing issues or sleep impairment were more likely to report lower quality of life and neurocognitive dysfunction. These comorbid conditions may represent potential targets of intervention and early identification may help mitigate long-term HCT-associated risk factors.
Acknowledgments
The authors thank the patients who completed the LTFU surveys for participating in this study, as well as Kara Cushing-Haugen for statistical assistance.
This work was supported in part by National Institutes of Health National Cancer Institute grants CA15704 and CA018029, as well as T32 training grant 5T32CA009351-41 (N.L.W.).
Authorship
Contribution: P.A.C., L.S.C.-S., M.E.F., E.F.K., M.U.O., and S.J.L. helped with patient data and materials; K.R.K., S.J.L., and E.J.C. contributed to study conception and design; N.L.W., A.I.P., K.R.K., K.L.S., and E.J.C. analyzed the data; N.L.W. wrote the first draft of the manuscript; all authors commented on previous versions of the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Natalie L. Wu, University of California San Francisco Benioff Children’s Hospital, 747 52nd St, Oakland, CA 94609; e-mail: natalie.wu@ucsf.edu.
References
Author notes
For original data and copies of the survey, please contact LTFU@fredhutch.org.
The full-text version of this article contains a data supplement.