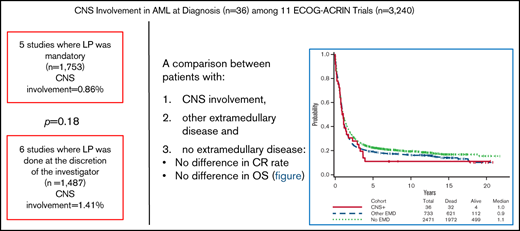
Key Points
There was no significant difference in CR rate and OS among patients with CNS involvement, other EMD, or no EMD.
The incidence of CNS involvement of newly diagnosed AML is low, irrespective of whether an LP is mandatory or not.
Abstract
Central nervous system (CNS) involvement in patients with newly diagnosed acute myeloid leukemia (AML) is rare, and systematic data regarding outcome are scarce. This retrospective study summarized data from 11 consecutive Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) clinical trials for patients with newly diagnosed AML. In all, 3240 patients with AML were analyzed, and 36 (1.11%) were found to have CNS involvement at diagnosis. The incidence of CNS disease among the 5 studies with per protocol mandatory lumbar puncture (LP) was similar to the incidence among studies in which LP was performed at the discretion of the investigator (0.86% vs 1.41%; P = .18). There was no significant difference in the rate of complete remission (CR) among patients with CNS involvement and those with other extramedullary disease (EMD) sites or those with no EMD (52.8% vs 59.3%-60%). The median overall survival (OS) for patients who were CNS positive, who had other EMD, or who had no EMD was 11.4, 11.3, and 12.7 months, respectively. There was no difference in OS among patients with CNS involvement, those with other EMD (hazard ratio [HR], 0.96; adjusted P = .84), and those with no EMD (HR, 1.19; adjusted P = .44). In conclusion, the reported incidence of CNS involvement in patients with newly diagnosed AML is low (1.1%), irrespective of whether an LP is mandatory or not. The presence of CNS disease at diagnosis in and of itself does not seem to portend a poor prognosis for achieving an initial CR or for OS.
Introduction
Extramedullary disease (EMD) is a known manifestation of acute myeloid leukemia (AML) with an overall reported incidence ranging between 2.5%1 and 30%,2 depending on, among other things, the precise definition of EMD. Its rate is highest among patients with monocytic AML3 and in those with t(8;21).4,5 Its prognostic impact is controversial.2,5-9
Data regarding central nervous system (CNS) involvement in patients with newly diagnosed AML are scarce, and the prognostic implication of CNS involvement is controversial.10-12 There is also no current agreement regarding whether lumbar puncture (LP) should routinely be performed in every patient with newly diagnosed AML, similar to that performed in patients with acute lymphoblastic leukemia or in pediatric patients with AML13 instead of being reserved for certain clinical scenarios of adult patients with AML at higher risk for CNS involvement or when neurologic signs or symptoms are present. In this retrospective study, a very large database of 11 consecutive clinical trials of patients with newly diagnosed AML was reviewed. The focus was on 3 issues: first, whether the incidence of CNS involvement at diagnosis was higher among the 5 studies in which an LP was mandatory for all patients (n = 1753) than in studies in which patients received an LP only if neurologic symptoms were present and/or at the discretion of the physician (n = 1487). The second issue was to describe the characteristics of patients with CNS involvement compared with patients without any EMD or with EMD other than in the CNS. The third issue was to report the prognosis of patients with CNS involvement compared with that of patients with other or no EMD.
Methods
Patient population
Between 1980 and 2008, 3522 patients age 15 years or older with untreated AML were enrolled on 11 consecutive, phase 2 or phase 3 clinical trials led by Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN).14-23 The treatment protocols, their activation dates, and accrual numbers are summarized in Table 1. Of the 3522 enrolled patients, 282 were excluded because of diagnosis of acute promyelocytic leukemia (n = 168) or leukemia other than AML (n = 29), no EMD evaluation at baseline (n = 41), ineligibility for retrospective central review (n = 24) or no survival data (n = 20). Each protocol was approved by the institutional review boards, and all patients signed a written informed consent.
Cytogenetic risk classification
Cytogenetic risk was classified as favorable, intermediate, unfavorable, or undetermined after central review by the ECOG-ACRIN Leukemia Cytogenetics Subcommittee, according to the definitions established by the Southwest Oncology Group (SWOG) and ECOG-ACRIN.24 Only minimal cytogenetic information was available for patients enrolled on earlier protocols E1479, E1490, E3480, E3483, or PC486.
EMD assessment
In all 11 trials, bone marrow (BM) leukemic involvement was an eligibility criterion, meaning that patients with an isolated extramedullary myeloid sarcoma, including isolated CNS leukemia, without BM involvement were not included. The presence of EMD at baseline was defined clinically by physical examination and radiology without necessarily requiring a biopsy.9
Diagnosis and treatment of CNS involvement
An LP was mandatory in 5 trials (E3483, PC486, E3489, E1490, E3993) and recommended for patients with high blast count in 2 trials (E1479, E3480) or if CNS signs or symptoms were present in 4 trials (E4995, E3997, E3999, E1900). The presence of any unequivocal blasts in the cerebrospinal fluid (CSF) was considered as CNS involvement. For all studies, the intended postremission systemic chemotherapy was not altered by the initial presence of CNS disease or any EMD. In several trials, high-dose cytarabine (HiDAC), which can penetrate into the CSF, was part of the regular protocol but not as a supplement for CNS-positive patients.
The treatment of CNS involvement was not uniform among the different trials. In 7 trials (E1479, E3480, E3483, PC486, E1490, E3993, E1900), intrathecal methotrexate (IT MTX) was mandatory, and in another trial (E3997) that used HiDAC as part of induction as well as consolidation therapy, it was optional. In 2 studies (E1479, E3480), cranial radiotherapy was optional; in E3483, it was recommended for patients who were randomly assigned to receive only maintenance therapy; and in E3993, it was recommended for patients with persistent leukemic cells in the CSF after consolidation therapy. In 3 studies (E1490, E3993, E3999), HiDAC was given to all of the patients as part of consolidation, and in 2 studies (E4995, E3997), it was given as part of induction therapy. In 2 studies (E4995, E1900), HiDAC was given as consolidation only to patients who did not undergo transplantation, and in E3483, it was given as consolidation in only 1 arm of randomly assigned patients. In E3489, patients with CNS leukemia went off study. In this latter trial, because the CSF was examined only after full blast clearance, patients went off study only after induction, at which point they were observed for survival.
Statistical analysis
Descriptive statistics were used for patient demographics and disease characteristics. Wilcoxon 2-sample tests (for continuous variables) and Fisher’s exact tests (for categorical variables) were used to explore potential differences between groups. The Kaplan-Meier method was used to estimate median overall survival (OS). Univariable and multivariable Cox proportional hazard models were used to evaluate the effect of CNS involvement on OS, with variables significant at the 0.10 level in univariable analyses adjusted as covariates in the multivariable models. Because of the exploratory nature of this study, no statistical adjustments were made for multiple comparisons. A 2-sided P value of .05 was considered statistically significant.
Results
Incidence of CNS involvement
Of the 3240 patients included in this analysis, 36 patients had CNS involvement (CNS-positive) at the time of diagnosis. The overall incidence was 1.11%, but it varied among the different studies (from 0% to 4.2%; Table 2). The incidence of CNS disease among all patients in the 5 studies that mandated an LP was similar to the incidence among all patients in trials in which LP was performed solely on the basis of neurologic symptoms and/or at the discretion of the attending physician (0.86% vs 1.41%; P = .18).
Characteristics of patients with CNS involvement
Half of the CNS-positive patients were males, the median age was 44.5 years (range, 17-79 years), 38.9% had an ECOG performance status (ECOG PS) of 2 or higher, and 55.6% had FAB (French-American-British classification of AML for acute myelomonocytic leukemia) -M4 disease. Among the 39 patients, cytogenetic analysis was available in only 9 (which included 5 patients with normal karyotype and 1 patient each with t(8;21)(q22;q22) with –Y, t(6;9)(p23;q34), del (16)(q22), or +2mar with –Y).
The characteristics of CNS-positive patients were compared with those of patients with EMD other than CNS (n = 733) and with those without EMD (n = 2471) (Table 3). The rate of ECOG PS 2 to 4 was highest among CNS-positive patients (38.9%), intermediate among patients with other EMD sites (22.9%), and lowest among patients without EMD (14%). Similar grading, respectively, was found regarding other characteristics such as the rate of FAB-M4 classification (55.6%, 38.2%, and 26%, respectively) and the median initial white blood cell (WBC) count (36.2, 31.7, and 8.6 × 103/μL, respectively).
Compared with patients without EMD, CNS-positive patients were younger (median age. 44.5 vs 52 years; P = .07) and had a higher median WBC count (P = .0004). The rate of CNS involvement was significantly higher among patients with WBC ≥50 × 103/µL compared with those with WBC <50 × 103/μL (2.32% vs 0.79%; P = .002). A similar pattern was seen when comparing CNS involvement in patients with WBC counts above or below 100 × 103/μL (2.99% vs 0.97%; P = .01).
Response and survival by CNS involvement
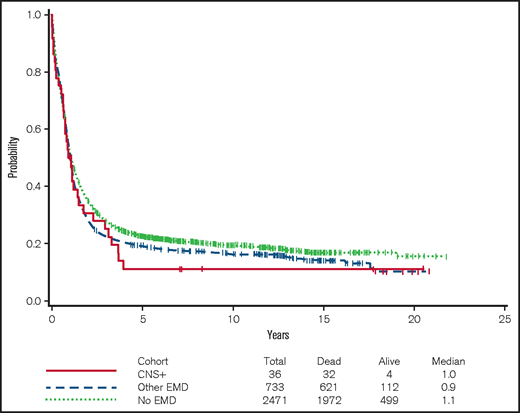
The rate of complete remission (CR) among CNS-positive patients was similar to that in the other groups (52.8% vs 59.3%-60%; P = .49). The median OS was 11.4 months (95% confidence interval [CI], 7.2-17.7 months) among CNS-positive patients, 11.3 months (95% CI, 10.4-12.8 months) among patients with other EMD, and 12.7 months (95% CI, 12.1-13.7 months) among those without EMD (Figure 1). By univariable analysis, the differences among these groups were not significant. The hazard ratios (HRs) for OS of CNS-positive patients compared with the other EMD patients was 1.07 (95% CI, 0.75-1.53; P = .70) and 1.22 (95% CI, 0.86-1.74) compared with patients without EMD (P = .26). In multivariable analysis, adjusting for covariates (listed in Table 4), there were also no significant differences in OS between patients with CNS involvement and those with other EMD (HR, 0.96; 95% CI, 0.66-1.41; adjusted P = .84) or no EMD (HR, 1.19; 95% CI, 0.77-1.84; adjusted P = .44).
Among the 36 patients who were CNS-positive, there was no significant difference in the CR rate between patients with other EMD (n = 23) and those with CNS only (n = 13) (60.9% vs 38.5%; P = .30). Similarly, no significant difference in OS was observed between the CNS-positive patients with other EMD and the CNS only groups (HR for other EMD vs CNS only, 1.95; 95% CI, 0.89-4.30; P = .10). The same conclusion remains using multivariable analysis (P = .26).
Discussion
CNS involvement in adults with newly diagnosed AML is a rare phenomenon with limited published data.10-12 The need for and clinical impact of a routine LP have remained unclear.10,12 Among pediatric patients, CNS assessment is part of the routine evaluation but the prognostic impact of CNS involvement remains controversial.2,6,13,25 This study examined a very large database of 11 ECOG-ACRIN consecutive clinical trials that had a total of 3240 patients with newly diagnosed AML to gain more information about this phenomenon, focusing on the relationship between CNS involvement and other sites of EMD, its incidence with or without a routine LP, and its overall prognostic impact. The incidence of CNS involvement at diagnosis was 1.1%, similar to the 0.9% that was published relatively recently by the MD Anderson Cancer Center (MDACC) group10 or the 2.2% published by an Italian group.26 The rate of FAB-M4 disease, incidence of ECOG PS 2 to 4, and median level of initial WBC count were highest in CNS-positive patients, intermediate in patients with EMD other than CNS, and lowest in patients without EMD. Although the differences in PS may be related to the debilitating effects of CNS involvement, the higher rate of FAB-M4 and higher WBC counts places CNS involvement and other EMD sites on a continuum with the same risk factors but with CNS on the high end of the spectrum. Almost two-thirds of CNS-positive patients also had other sites of EMD, which supports the assumption that the ability of the disease to involve extramedullary sites is an intrinsic character of the specific leukemic phenotype, possibly related to expression of surface adhesion molecules.2,27
An intriguing issue regarding CNS involvement in AML is whether routine performance of an LP in every patient with AML, regardless of symptoms, will increase the rate of detection. The ECOG-ACRIN policy regarding routinely performing LP changed during the years from mandatory to possible (Tables 1 and 2), which enabled this issue to be evaluated. Our data suggest that a routine LP does not increase the detectable rate of CNS involvement. In contrast, a study from MDACC compared 1307 patients who did not undergo a routine LP during the course of AML treatment to 42 patients who underwent a routine LP at diagnosis and found an increase in the incidence of CNS involvement from 3.3% to 19%.10 However, it should be noted that neither this study nor the MDACC study used flow cytometry (FC) as part of the CSF assessment. In fact, an Italian study group reported that CNS involvement increased from 11 patients when conventional cytology was used to 33 patients when FC was used.12 It is unknown whether using more sensitive tools such as FC to detect blasts in the CSF would have an impact on outcome. This becomes even more challenging with the use of HiDAC as part of the consolidation therapy in AML, which penetrates the CSF and may eradicate the few residual blasts.28
Importantly, patients who are CNS positive do not have a worse OS compared with other patients who have EMD or those without EMD. Data from studies by the Italian study group12 and a Taiwanese study group11 support these findings, whereas the MDACC analysis10 reported a negative prognostic impact of CNS disease. It should be noted that in this study, other than the initial therapy at diagnosis (for example, adding IT MTX according to each protocol), the protocols did not permit for any significant deviations, such as administering more intensive therapy to patients with CNS involvement. Thus, the similar prognosis probably cannot be attributed to additional or more intensive therapy.
There are some obvious limitations to our study. It was a retrospective analysis that examined clinical trials that spanned 3 decades. Thus, intra-study comparisons of trials need to be cautiously interpreted. In addition, data about CNS imaging studies and BM cytogenetics are clearly limited, and FC was not part of the CSF assessment. Although few patients had CNS involvement in each of the 11 studies, the consistent pattern in this very large cohort of 3500 patients lends credence to the overall analysis and conclusions.
Hitherto, CNS involvement at diagnosis was perceived as a prognostically adverse factor. Some major clinical trials groups have excluded CNS-positive patients from standard clinical trials, such as in E3489, and even in the most contemporary ECOG-ACRIN AML clinical trial, E2906. Our data strongly suggest that, in general, patients with newly diagnosed AML who have CNS involvement should not be precluded from participating in clinical trials.
In conclusion, this retrospective analysis reported a low incidence of CNS involvement in patients with newly diagnosed AML and does not encourage routinely performing an LP. Assuming that prompt CNS-directed therapy is given, our data do not support a prognostic characterization or a recommendation for using a different systemic chemotherapy.
Acknowledgments
This study was supported by grants from the National Institutes of Health, National Cancer Institute (U10CA180820, U10CA180794, UG1CA189859, UG1CA233234, UG1CA180830, UG1CA233290, and UG1CA232760) and was coordinated by Peter J. O’Dwyer, and Mitchell D. Schnall, Group Co-Chairs of the ECOG-ACRIN Cancer Research Group.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Authorship
Contribution: C.G. designed the research , analyzed the data, and wrote the paper; J.-W.L. and D.D. analyzed the data; H.F.F., S.M.L., H.M.L., L.D.C., P.H.W., M.S.T., and M.R.L. performed the research; and J.M.R. and E.M.P. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chezi Ganzel, Hematology Department, Shaare Zedek Medical Center, 12 Schmuel Bait St, POB 3235, Jerusalem 9103102, Israel; e-mail: ganzelc@szmc.org.il
References
Author notes
For data sharing, please contact Chezi Ganzel via e-mail at ganzelc@szmc.org.il.