Key Points
Transfusion during hospitalization reduces fatigability levels in patients with anemia after hospital discharge.
Fatigability can be a useful outcome measure for studying the effect of transfusion on both the symptom of fatigue and patient’s physical function.
Abstract
In patients with anemia, there is interest in understanding the impact of red blood cell (RBC) transfusion on patient-reported outcomes such as fatigue. However, data from previous studies are mixed as to whether transfusion improves fatigue. One explanation for this is that prior studies have not examined whether changes in fatigue from transfusion may also affect patient activity levels. This is important because if transfusion reduces fatigue, patients may become more active, which could increase their fatigue. Thus, testing whether transfusion affects patients’ fatigability, a measure of fatigue in the context of activity, may be more useful than testing the effect of transfusion on fatigue alone. The objective of this study was to test the effect of transfusion during hospitalization on patients’ fatigability 7 days postdischarge. This prospective observational study included hospitalized general medicine patients with hemoglobin levels <10 g/dL. Patient-reported fatigability was collected during hospitalization and by telephone 7 days after discharge. Multivariable linear regression was used to test the association between receipt of a transfusion and fatigability 7 days postdischarge. Among the 350 patients participating, larger reductions in fatigability were observed with more transfused RBCs. Receipt of 1 U of RBCs was associated with a smaller reduction in fatigability, whereas receipt of 2 to 3 U of RBCs was associated with reductions in fatigability nearly 1 standard deviation from baseline and 3 times greater than patients receiving 1 U of RBCs. In hospitalized patients with anemia, receipt of a transfusion is associated with reductions in fatigability 7 days after hospital discharge.
Introduction
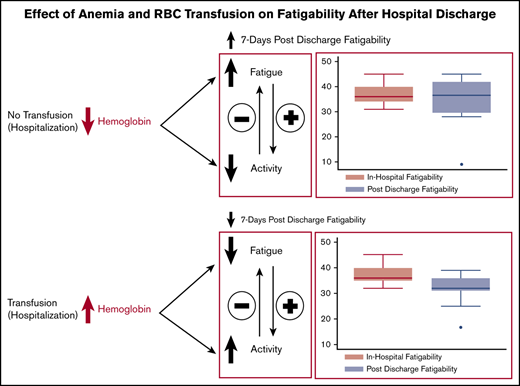
In patients with anemia, there is a renewed interest in understanding the impact of red blood cell (RBC) transfusion on patient-reported outcomes,1,2 such as fatigue. Fatigue is important because it is the primary symptom of anemia and is a significant concern to patients.3,4 It is also associated with reduced quality of life,1,3,5 decreased activity levels, deconditioning, and losses in functional status.6-8 Because fatigue is a physiological response to decreased oxygen delivery to the tissues of anemia, it is expected that transfusion, which increases oxygen delivery to the tissues, should reduce anemia-related fatigue. However, data are mixed from studies examining the effect of transfusion on anemia-related fatigue, with some studies showing a beneficial effect of transfusion on fatigue and other studies showing no effect.9-16 One reason why the data are inconclusive with respect to whether transfusion reduces fatigue may be that none of these studies accounted for patients’ activity levels when measuring fatigue.
Knowing a patient’s activity level or the activity context that provoked their self-reported fatigue level is critical for interpreting the effect of transfusion on fatigue because fatigue and activity can influence each other. For example, if transfusion reduces fatigue, it may result in increased activity that can then offset decreases in fatigue. By measuring fatigue alone, transfusion may seem unsuccessful if posttransfusion fatigue levels are the same or higher than pretransfusion levels, as the important changes in patient activity would not be appreciated. This example illustrates how previous studies that have measured fatigue without considering effects on a patient’s activity level can come to disparate conclusions about the effect of transfusion on fatigue. It also suggests that measuring the effect of transfusion on fatigue in the context of a known activity level, a measure known as fatigability, may be a better and more accurate way to capture the effect of transfusion.17-19
Fatigability is a measure of a patient’s self-reported fatigue in the context of a known activity level, with greater fatigability indicating more fatigue at any given level of activity. Fatigability standardizes self-reported fatigue to an objective measure of activity, making it possible to compare patients with similar fatigue levels but different levels of activity. This makes fatigability a potentially useful way to measure the effectiveness of transfusion, as it can capture changes in fatigue and/or activity that may occur after transfusion. Moreover, because fatigability includes a measure of patient activity, it also provides information about patients’ physical function, and improving physical function is often an ultimate aim of clinicians when considering transfusion in patients with symptoms from their anemia. Knowing the effect of transfusion on fatigability could address the fundamental clinical question of whether transfusion provides a net benefit for both the symptom of fatigue and patients’ functional capacity.
Although fatigability has recently been used as an outcome measure in patients with anemia,19 the effect of transfusion on fatigability has not been previously studied. The objective of this study was to: (1) determine whether transfusion during hospitalization improves patient’s fatigability 7 days postdischarge; and (2) compare the effect of transfusion on fatigability vs the effect of transfusion on both fatigue and physical function as individual outcome measures in patients 7 days after hospital discharge. We hypothesized that transfusion would be associated with a larger reduction in fatigability 7 days after hospital discharge among patients with high baseline fatigability than among patients with low baseline fatigability.
Methods
Study design
This study was a prospective observational study of hospitalized general medicine patients with anemia at The University of Chicago Medical Center (UCMC). The study period was May 2018 through February 2019. The Institutional Review Board approved the study procedures, and all study subjects provided informed consent.
Study eligibility and inclusion criteria
All general medicine inpatients at UCMC were approached for consent to the University of Chicago Hospitalist Project (UCHP),20 an established inpatient clinical research infrastructure at UCMC. Patients consenting to the UCHP and with a hemoglobin (Hb) level <10 g/dL at any point during their hospitalization were eligible for participation in this study. We chose to study general medicine patients because they receive the highest proportion of transfusion in hospitalized patients,21,22 and the effect of transfusion on fatigability or fatigue has been not been thoroughly studied in a general medicine population. An Hb level <10 g/dL was chosen as an inclusion criterion because equipoise remains regarding the effect of transfusion on fatigue at any Hb level, and some clinicians still consider transfusion for patients with fatigue at Hb levels above restrictive transfusion thresholds. If patients were not eligible for inclusion at the time of consent for the UCHP, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb level was <10 g/dL. Proxies were sought to provide consent and answer questions for patients who failed the Short Portable Mental Status Questionnaire. The use of a proxy has been validated as an approach to assess quality of life outcomes and was preferred over the alternative of excluding patients who could not answer on their own.23
Patient demographic data collection
Hospital administrative data were used to determine patients’ age, sex, race/ethnicity, length of stay, patient comorbidities, and a Charlson Comorbidity Index score24 calculated by using International Classification of Diseases, Tenth Revision codes. Health Care Utilization Project diagnosis categories25 were used to identify patients with sickle cell anemia, gastrointestinal bleeding (GIB), and iron deficiency because these conditions are not included as part of the Charlson Comorbidity Index, and because patients with these conditions have transfusion practices that differ from those of other general medicine patients.
Measuring anemia
The first Hb level <10 g/dL during a patient’s hospitalization, making them eligible for study participation, was identified through manual review of the electronic health record. Additional Hb values during the patient’s hospitalization were obtained from the hospital’s clinical data repository. Each patients’ nadir Hb was used in the analysis because it is the measure of Hb most closely associated with the patient’s fatigue levels.26
Determining receipt of RBC transfusion while hospitalized
Whether patients received an RBC transfusion and the number of units transfused during hospitalization were obtained from the hospital’s clinical data repository.
Patient self-reported outcomes during hospitalization and 7 days postdischarge
Self-reported measures of fatigability, fatigue, and physical function were collected through an in-person interview either on the first day of hospital admission for patients eligible at admission, or the day the patient became eligible for the study for those who were not immediately eligible at hospital admission. All patients completing the inpatient interview were called 7 days after hospital discharge and were re-administered the fatigability, fatigue, and physical function instruments. The timing of the follow-up interview at 7 days postdischarge was selected to allow patients to have substantial experience after discharge to assess fatigability while still having Hb levels likely to be influenced by the transfusions they received during hospitalization.
Self-reported fatigability
Fatigability was measured by using the Pittsburgh Fatigability Scale (PFS).27 The PFS is the only self-reported instrument validated to measure fatigability. Although the PFS was originally validated in older adults, the questions on the PFS ask about fatigue in the context of a range of physical activities that are appropriate for adults of any age. Higher scores on the PFS reflect greater fatigability (higher fatigue at any activity level). Values for any missing data on the PFS were filled in by using a prorated score according to validated rules for missing data on this tool.28
Self-reported fatigue and physical function
Self-reported fatigue was measured by using the Patient-Reported Outcome Measurement Information Systems–Fatigue (PROMIS-F) instrument,29 and self-reported physical function was measured by using the PROMIS–Physical Function (PROMIS-PF) with mobility aid instrument. The PROMIS-PF measures self-reported physical activity performance based on whether a patient is able to ambulate 25 feet on a level surface. Both ambulatory and nonambulatory patients were enrolled in the current study, but our analysis included ambulatory patients only because the measure of fatigability requires patient activity. Higher scores on the PROMIS-F and PROMIS-PF represent higher levels of either patient fatigue or physical function, and values for any missing data were handled according to the validated HealthMeasures Scoring Service rules for missing data on the PROMIS.30
Statistical analysis
Statistical analysis was performed by using Stata statistical software (StataCorp, College Station, TX). Descriptive statistics were used to characterize patient demographic characteristics. Kruskal-Wallis and χ2 tests were used to compare differences in patient demographic characteristics based on the number of transfusions a patient had during hospitalization. To test the hypothesis that transfusion is most likely to benefit patients with high fatigability during hospitalization, an a priori decision was made to categorize patients as high or low fatigability by dividing the sample at the median inpatient fatigability level. Our primary model was a multivariable linear regression model with patient’s 7-day postdischarge fatigability level as the dependent variable, transfusion of RBCs as the primary predictor variable, and nadir Hb as an independent variable of interest. Receipt of a transfusion was modeled both as a continuous and categorical (0, 1 U, and 2 to 3 U) variable, and patient’s nadir Hb (Hb, 9-9.9 g/dL, 8-8.9 g/dL, 7-7.9 g/dL, and <7 g/dL) was analyzed as a categorical variable. Patients with ≥4-U transfusions were not analyzed because large-volume transfusions are given for high-volume bleeds or life-threatening illness and not for symptomatic improvement. The regression model controlled for patient age, sex, race, Charlson Comorbidity Index, and length of stay. We prespecified 2 sensitivity analyses based on this model: (1) additionally controlling for sickle cell anemia, GIB, and iron deficiency; and (2) adding the interaction between transfusion and nadir Hb into the model.
Our primary model was used to also test and compare whether transfusion was associated with reductions in 7-day postdischarge fatigue or physical function as dependent variables. In these models, patients were stratified into high baseline fatigue (fatigue model) or low physical function (physical function model) groups by dividing the sample at the median fatigue or physical function level; however, all independent variables remained the same as in our primary model. To test the effect of transfusion and fatigability on postdischarge fatigue, we added to the fatigue model the interaction between fatigability and transfusion, and compared it vs the interaction between fatigue and transfusion.
Results
Patient characteristics
Figure 1 describes the number of eligible and consented patients who completed both the inpatient and 7-day follow-up interviews. Table 1 describes the demographic characteristics of the 350 patients included in the analysis. Overall, 267 patients received no transfusion, 49 received a 1-U transfusion, and 34 received a 2- to 3-U transfusion. Patients receiving a transfusion (either 1 U or 2-3 U) had a lower nadir Hb (P < .01) and were more likely to have GIB (P < .01) than patients who did not receive a transfusion. Patients who did not receive a transfusion were more likely to have iron deficiency (P < .01) than those who received a transfusion. The overall median baseline (inpatient) fatigability level of the sample was 33 (interquartile range, 5-49), and 174 patients were classified as having “high fatigability,” and 176 patients were classified as having “low fatigability.” Among patients receiving no transfusion, 136 (51%) had low baseline fatigability, and 131 (49%) had high baseline fatigability. Among patients receiving a 1 U transfusion, 26 (53%) had low baseline fatigability, and 23 (47%) had high baseline fatigability. Among patients receiving a transfusion of 2 to 3 U, a total of 14 (41%) had low baseline fatigability and 20 (59%) had high baseline fatigability. There were no differences in transfusion according to baseline fatigability (high vs low) level (P = .52). There were also no differences in baseline characteristics between patients who completed only the inpatient interview (n = 520) and those who completed both the inpatient and the 7-day follow-up interview and were included in the final sample (n = 350) (supplemental Table 1). Supplemental Table 3 includes the individual comorbidities of patients in the sample who received no transfusion, a 1-U transfusion, or a 2- to 3-U transfusion.
Effect of RBC transfusion on fatigability
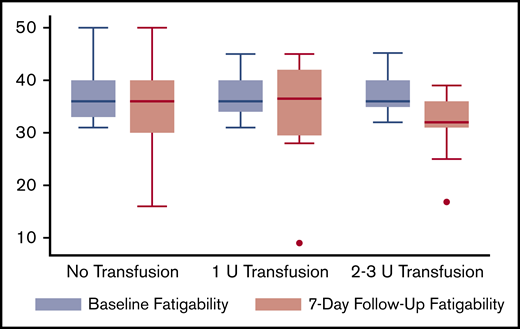
In patients with high fatigability during hospitalization, receipt of a transfusion was associated with decreased fatigability after hospital discharge. Figure 2 shows the unadjusted baseline and 7-day follow-up fatigability scores according to transfusion category. In the regression model, receipt of a transfusion was associated with lower fatigability levels 7 days after hospital discharge (β = –2.9; P = .03). Compared with patients receiving no transfusion, patients receiving either a 1 U (β = –2.6; P = .35) or 2 to 3 U (β = –7.3; P = .02) transfusion during hospitalization had lower fatigability levels 7 days after hospital discharge (Table 2). In patients receiving 2 to 3 U of RBCs, the reduction in fatigability levels at 7 days postdischarge was not only statistically significant, but the reduction in fatigability (β = –7.3) was nearly 3 times that of a 1 U transfusion, and almost a 1 standard deviation (SD = 9) decrease from baseline (inpatient) fatigability levels. Although lower Hb levels were associated with greater fatigability at 7 days postdischarge in patients with high fatigability, this effect did not reach statistical significance.
In our prespecified sensitivity analyses controlling for sickle cell anemia, iron deficiency, and GIB, the effect of transfusion on fatigability levels 7 days postdischarge was the same as in our primary model (supplemental Table 2), and there was no effect of the interaction between transfusion and Hb on 7-day fatigability levels. Because patients receiving a transfusion had a lower nadir Hb during hospitalization than patients not receiving a transfusion, we also performed a post hoc sensitivity analysis restricting the model to patients with a nadir Hb <8 g/dL during hospitalization (n = 75). In this analysis, the effect of transfusion on fatigability levels 7 days postdischarge was again the same as in our primary model. In contrast to patients with high fatigability, in patients with low baseline fatigability during hospitalization, there was no effect of transfusion on fatigability at 7 days postdischarge and no pattern of association between Hb level during hospitalization and fatigability 7 days postdischarge (Table 2).
Effect of RBC transfusion on fatigue and physical function
There was no effect of transfusion on either fatigue or physical function levels at 7 days after hospital discharge. Although receipt of any transfusion reduced fatigue levels 7 days postdischarge (β = –1.7; P = .28), the result was not statistically significant. Similarly, compared with patients receiving no transfusion, patients receiving either a 1 U (β = –3.3; P = .32) or 2 to 3 U transfusion (β = –7.0; P = .08) had lower fatigue levels, but the results were not statistically significant (Table 3). Lower Hb levels were associated with higher fatigue levels (positive coefficient) 7 days postdischarge, but the effect was not statistically significant, and there was no association between Hb level and physical function scores at 7 days postdischarge. Neither the interaction between transfusion and fatigue or transfusion and fatigability had an effect on fatigue levels 7 days postdischarge (Table 4). Receipt of a transfusion also had no effect on physical function 7 days after hospital discharge (β = 0.03; P = .98), and compared with patients receiving no transfusion, receipt of either a 1 U or 2 to 3 U transfusion was not associated with changes in physical function levels 7 days postdischarge (Table 5). When both the fatigue and the physical function models where restricted to patients with a nadir Hb <8 g/dL only, there was no effect of transfusion on fatigue (1 U transfusion, β = –2.8, P = .41; 2 to 3 U transfusion, β = –4.5, P = .19) or physical function (1 U transfusion, β = –3.6, P = .26; 2 to 3 U transfusion, β = 0.47, P = .90) 7 days after hospital discharge.
Discussion
This study shows that in hospitalized patients with anemia and high fatigability, receipt of a transfusion during hospitalization was associated with reductions in fatigability 7 days after hospital discharge. Compared with patients receiving no transfusion, receipt of a 1 U RBC transfusion was associated with a small reduction in fatigability, and although the effect was not statistically significant, the confidence intervals were wide and skewed largely negative. This result, plus the significant effect of transfusion on fatigability as a continuous variable, suggests that the increase in tissue oxygenation from a single unit of RBCs likely reduces fatigability but that our study was not powered to detect this difference compared with patients receiving no transfusion. In addition, patients with mild to moderate anemia who still experience significant fatigue would be the patients most expected to benefit clinically from a single unit transfusion with reduced fatigability and/or fatigue. However, in the current study, many of these patients did not undergo transfusion, likely because their Hb never fell below restrictive transfusion thresholds, limiting our ability to test this hypothesis. Receipt of 2 to 3 U of RBCs was, however, associated with clinically large and statistically significant reductions in fatigability. Patients receiving 2 to 3 U of RBCs had postdischarge fatigability levels almost 3 times lower than patients receiving 1 U of RBCs, and nearly an entire standard deviation lower than baseline fatigability levels. Overall, this pattern of larger reductions in fatigability with more units of transfused RBCs is suggestive of transfusion having a potential dose–response effect on reducing fatigability. Although our data cannot fully substantiate it, a potential dose–response effect should be further explored because it would be consistent with clinical and physiological reasoning, whereby increasing the oxygen delivery to the tissues from higher Hb levels would be expected to provide a greater reduction in patients’ symptoms. Importantly, the data from this study also confirm our hypothesis that the effect of transfusion on reducing fatigability is likely to be greatest for patients with high baseline fatigability. Indeed, in our study, transfusion did not improve fatigability levels for patients with low baseline fatigability, and there was no pattern of association between Hb levels during hospitalization and fatigability levels postdischarge for these patients. This suggests that in patients with low fatigability, compensatory physiological mechanisms have likely resulted in less severe fatigue relative to activity regardless of Hb level, and our data indicate that a transfusion is unlikely to benefit these patients. It is important to note therefore that Hb alone does not predict either patients’ fatigability levels during hospitalization or their likelihood of response to transfusion. Rather, measuring fatigability in combination with Hb is likely necessary to identify patients who have high fatigability and low Hb, and are most likely to benefit from transfusion with reduced fatigability after hospital discharge.
In addition to showing that transfusion reduces fatigability after hospital discharge, this study also compared the effect of transfusion on fatigability vs the effect of transfusion on fatigue and physical function alone. Fatigue has historically been used as an important patient-reported outcome measure in patients with anemia. Data from our study show that although transfusion reduces fatigue 7 days after hospital discharge in a pattern similar to that of fatigability, the effect is not statistically significant. The pattern similar to fatigability, of larger improvements in fatigue with more units of RBCs transfused, is not surprising because fatigue and fatigability are related constructs. Interestingly, the interaction between transfusion and fatigability predicted reductions in fatigue postdischarge, whereas the interaction between transfusion and fatigue had no association with postdischarge fatigue. This outcome suggests that fatigability may be more physiologically representative of the changes in fatigue and activity which patients experience as their Hb level changes, and that fatigue is a related but less modifiable phenotypic manifestation of those changes. The weaker association between transfusion and reductions in fatigue, compared with fatigability, is also likely because measuring fatigue without additionally appreciating a patient’s activity level can result in inaccurate estimates of the effect of transfusion. If fatigue is not standardized to activity (ie, fatigability), any measurement of fatigue or change in fatigue after transfusion will be imprecise because differences between patients who report similar levels of fatigue but have different activity levels (sedentary vs fully functional) are not accounted for. This can explain why past studies have produced inconsistent and paradoxical conclusions about the effect of transfusion on fatigue as a single outcome measure.
We also found no effect of transfusion on physical function alone. Physical function may be a too coarse and distal outcome measure for hospitalized patients with anemia. Although transfusion may ultimately have an effect on physical function, this effect is likely to occur first through subtle changes in fatigue as a precursor on the causal pathway toward changes in overall physical function.17 Therefore, fatigability because it accounts for patients’ activity level is a better and more accurate outcome measure than either fatigue or physical function alone, for evaluating the effect of transfusion on fatigue and activity.
There are several limitations to our study, including that it is a single-center observational study of general medicine patients. As such, our results may not generalize to other institutions, or to other patient populations (ie, cardiology, oncology) in which patient-reported outcomes after transfusion are important. In addition, as a small pilot study, we were not able to recruit every hospitalized patient with anemia into our study, and we were not able to reach every patient for the 7-day follow-up call. However, we found no differences in patients who completed and did not complete the 7-day follow-up interview. We also collected patient self-reported outcomes, but we were not able to collect Hb levels to correlate with self-reported measures at the 7-day follow-up time period. It is possible that patients’ fatigability, fatigue, and physical function levels are different than reported, or that there are other instruments which could better measure these outcomes after transfusion. In addition, as an observational study, we were not able to dictate the timing of when patients received transfusion during their stay. As such, the large effect of reduced fatigability in patients receiving a 2 to 3 U transfusion, compared with a 1 U transfusion, may be the result of these patients receiving transfusion closer to discharge and the follow-up interview time point; overall, however, this potential bias would favor our underlying hypothesis, that transfusion reduces fatigability, because it would suggest that the effect of a 1 U transfusion was underappreciated at the follow-up interview time point. Finally, to define high and low fatigability, we split the sample at the median fatigability level, but this method may not be the optimal way to define such groups and could have affected our results.
In summary, transfusion during hospitalization reduces fatigability levels in patients with anemia after hospital discharge. Fatigability can be a useful outcome measure in patients with anemia that can improve understanding of the effect of transfusion on both the symptom of anemia and the patient’s physical function. Future trials focused on the effect of transfusion on patient-reported outcomes should use fatigability as an important patient-centered outcome measure.
Requests for data sharing may be submitted to the corresponding author (Micah T. Prochaska; e-mail: mprochas@medicine.bsd.uchicago.edu).
Acknowledgments
The authors thank Eric Robinson and Dorothy Debiasse for their contributions.
This work was supported by a National Institutes of Health, National Heart, Lung, and Blood Institute K23 Patient-Oriented Research Career Development Award (K23HL140132) (M.T.P.).
Authorship
Contribution: M.T.P. and D.O.M. developed the idea and designed the study; M.T.P. and R.B. collected the data; M.T.P., H.Z., R.B., and D.O.M. analyzed the results and created the figures; and M.T.P. and D.O.M. wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Micah T. Prochaska, Department of Medicine, University of Chicago, 5841 S Maryland Ave, MC 5000, Chicago, IL 60637; e-mail: mprochas@medicine.bsd.uchicago.edu.
References
Author notes
The full-text version of this article contains a data supplement.