Abstract
Ruxolitinib is a recommended second-line treatment for the prevention of thrombosis in patients with polycythemia vera who become resistant or intolerant to hydroxyurea; however, evidence regarding its efficacy in terms of thrombosis reduction is uncertain. We searched Medline, Embase, and archives of abstracts from the European Hematology Association and the American Society of Hematology annual congresses from 2014 onward for randomized controlled trials comparing the treatment vs best available therapy (BAT). Our search retrieved 80 records; after screening of abstracts and full text, the total was reduced to 16. Evidence came from 4 randomized controlled trials, including 663 patients (1057 patients per year). We estimated a thrombosis risk ratio of 0.56 for ruxolitinib BAT, corresponding to an incidence of 3.09% and 5.51% patients per year, respectively. The number of thrombotic events reported with ruxolitinib was consistently lower than that with BAT in our sample, but, globally, the difference did not reach significance (P = .098). Hard evidence in favor of ruxolitinib is lacking; a clinical trial on selected patients at high risk of thrombosis would be warranted, but its feasibility is questionable.
Introduction
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm characterized by clonal expansion of an abnormal hematopoietic stem/progenitor cell that leads to an increased red blood cell mass as the main clinical feature; elevated white blood cell and platelet counts are also common.1 PV is almost always genetically characterized by the presence of an activating JAK2 mutation, primarily JAK2V617F, leading to a mis-regulated JAK-STAT signaling pathway. The natural history of PV is marked by arterial and venous thrombosis, bleeding tendency, and a propensity to transform into myelofibrosis or acute leukemia.
Hydroxyurea (HU) is the upfront recommended drug in high-risk patients, and a beneficial effect in reduction of thrombosis incidence is argued based on the results of a phase 2 study of the PVSG (Polycythemia Vera Study Group)2 and a propensity score matching analysis of a large cohort of patients included in the European Collaborative Low-dose Aspirin (ECLAP) study.3
Currently, ruxolitinib is recommended as a second-line drug in patients who become resistant or intolerant to HU or who are poor responders to HU.4 The drug achieves hematologic and molecular responses and can maintain the target hematocrit level without phlebotomy. However, currently, the evidence in favor of ruxolitinib for the prevention of cardiovascular events is uncertain, and estimates regarding the incidence of these complications are scattered over a series of different studies.
The goal of the current review was to systematically collect and pool the body of available evidence regarding the efficacy of ruxolitinib in terms of reduction of thrombosis incidence. We specifically selected randomized controlled trials (RCTs) that compared the treatment vs best available therapy (BAT).
Materials and methods
Database search and quality assessment
We searched medical databases (Medline, Embase, and PubMed Central) and archives of abstracts presented at European Hematology Association (EHA) and the American Society of Hematology (ASH) congresses from 2014 onward. The focus was on articles/abstracts reporting on randomized clinical trials and/or observational studies reporting an evaluation of thrombosis incidence in patients with PV treated with ruxolitinib. Search terms used included: “Polycythemia Vera,” “Thrombosis,” and “Ruxolitinib.” Literature was archived and flagged on the Rayyan web app for systematic reviews and meta-analysis.5
Quality and risk of bias of randomized clinical trials was assessed by using the Cochrane Risk of Bias 2 tool, and risk of bias was given a grade among “low,” “high,” or “some concerns.” We extracted the following data from selected studies: number of treated patients per arm, mean/median age, follow-up time, and number of thrombotic events. Whenever possible, we also collected information about the number of arterial and venous events and history of thrombosis.
Data extraction
The following data were extracted from the reports: number treated, median follow-up and/or total follow-up time, number of patients with thromboembolic events, median/mean age of patients, and number of patients with history of thrombosis. Whenever possible, the data were stratified according to treatment group.
Statistical analysis and reporting
Event counts, sample size, and study and patient characteristics were tabulated and reported, stratified according to drug treatment. Incidences per treatment with binomial 95% confidence intervals (CIs) are reported in forest plots; for comparative trials, estimates of the risk ratio are also reported.
The effect of treatment was evaluated by using a mixed effect Poisson model with random intercept and treatment effect to account for between-study heterogeneity. Intracluster correlation coefficients were used as heterogeneity measures.
The entire analysis was performed by using Stata software version 13.1.
Results
Study selection and characteristics
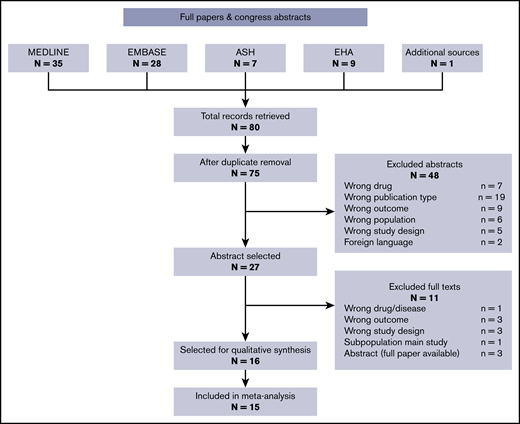
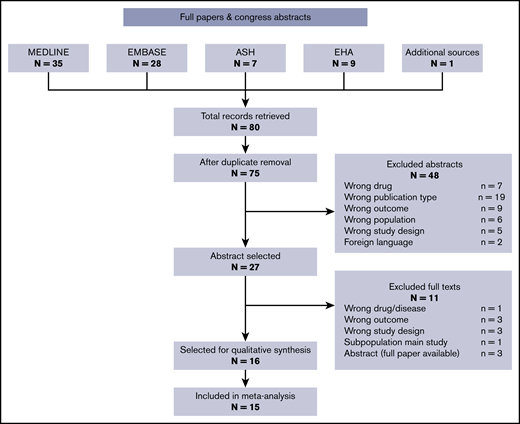
The search of the databases from the ASH and EHA congresses retrieved 16 records. Of those, 2 did not meet inclusion criteria in terms of outcome, drug, or study design, and 3 were noncomparative studies on ruxolitinib-treated patients; the latter are reported in this review, but they were not included in the analysis on efficacy. Three abstracts were excluded because they report the exact same data as full-text articles included in this review. The final abstract selection included 8 records.
The search of the medical databases retrieved 63 records (35 from Medline, 28 from Embase); the final total was 60 after removal of 3 duplicates. Two more were duplicate abstracts from an EHA congress, thus leaving 58 records. Based on abstract screening, 43 records were excluded, leaving 15 records for full-text screening. Eight full texts were excluded for the reasons reported in Figure 1. Four records were comparative studies of ruxolitinib vs BAT and were included in the comparative analysis on efficacy. One additional record was identified from reviews on the topic. The workflow is summarized in Figure 1 with number excluded and reasons. The final full-text selection included 8 records.
The final selection for the systematic review included 16 records (Table 1), of which 15 were included in the meta-analysis. The selected records included some reanalyses and follow-up studies on the same populations. In these cases, the most recent or informative record was kept, or, when feasible, we extracted information from multiple follow-ups and combined it in our database.
In particular, the body of evidence in terms of the effect of ruxolitinib came entirely from the following 4 RCTs: (1) RESPONSE (Study of Efficacy and Safety in Polycythemia Vera Subjects Who Are Resistant to or Intolerant of Hydroxyurea: JAK Inhibitor INC424 Tablets Versus Best Available Care), 9 records; (2) RESPONSE-2 (Ruxolitinib Efficacy and Safety in Patients With HU Resistant or Intolerant Polycythemia Vera vs Best Available Therapy), 4 records; (3) RELIEF (Randomized Switch Study From Hydroxyurea to Ruxolitinib for RELIEF of Polycythemia Vera Symptoms), 1 record; and (4) MAJIC, 2 records.
The RESPONSE trial6 is the oldest trial in our selection and had multiple updated follow-ups. The study was an open-label RCT comparing ruxolitinib vs BAT in patients with splenomegaly who were resistant or intolerant to HU. The study design included 2 preplanned analyses at 32 and 80 weeks and was subsequently updated at 5 years; after the 32-week cutoff date, patients from the BAT arm were allowed to cross over to the ruxolitinib arm, with most patients doing so.6,7 To have results as comparable as possible between the 2 groups, we only extracted outcomes up until before the time of crossover.
The RESPONSE-2 trial8 is an open-label RCT comparing ruxolitinib vs BAT in patients resistant or intolerant to HU without splenomegaly. The study design is essentially the same as RESPONSE. We retrieved 2 papers on the RESPONSE-2 trial covering different follow-ups.8,9 However, because the full follow-up time available for the ruxolitinib arm was not substantially different compared with BAT, we used full information about the ruxolitinib arm; for BAT, we only considered outcomes happening before the eventual crossover to ruxolitinib (28 weeks).
The RELIEF trial10 was the only double-blind RCT in our selection. It is also unique in that it is the only study in which ruxolitinib was administered to patients whose condition was generally well controlled with HU, although they still presented symptoms of the disease.
The MAJIC trial11 allowed comparisons on longer follow-up, as populations of both arms were followed up until 2.6 years at the time of this review. It is a randomized phase 2 trial of second-line administration of ruxolitinib vs BAT in essential thrombocythemia and PV. Although the trial includes patients with essential thrombocythemia, two subanalyses were recently presented12,13 that focused on comparing responses between the 2 arms in the PV group. Of note, all of these studies dealt with patients resistant or intolerant to first-line treatment, except for the RELIEF trial.
Quality assessment
Risk of bias.
The quality and risk of bias of the RCTs of ruxolitinib vs BAT were independently assessed by 2 independent reviewers (A.M. and A.F.) with the Cochrane Risk of Bias 2 tool.15 This tool is used to evaluate the risk of bias in 5 domains: randomization process, deviations from intended treatment, missing outcomes data, measurement of outcome, and selection of reported results.
Study quality was overall pretty high, and risk of bias was deemed low in the domains of missing data, measurement of outcome, and reporting of results; this assessment is also based on the fact that in our selection, thromboembolic events were reported as safety outcomes rather than as efficacy end points, which in our opinion makes bias in reporting less likely.
Some concerns were raised with regard to randomization process and deviations from intended intervention. In terms of randomization, patients’ characteristics were not always homogeneous between arms. In terms of deviations from intended interventions, it has been noted that, with the exception of the RELIEF study, all of the comparative trials in our selection were open-label, which is a potential source of bias by itself. However, these concerns are overall expected to be of small impact.
We did not apply formal instruments for assessing publication bias because of the very limited number of available studies, and because results did not actually show a strong effect of the experimental drug.
Efficacy and thromboembolic events
Qualitative synthesis of results.
Our search retrieved 3 noncomparative trials on ruxolitinib.16-18 Globally, they accounted for 261 patients. Based on 7 thromboembolic events, their annual incidence in this subselection of studies was 4.5% patients per year (supplemental Figure 1). However, these data were not included in our formal evaluation on efficacy, which was performed only on RCTs.
None of the comparative trials in our selection was aimed at examining hard efficacy primary end points such as thrombosis incidence, although they were usually reported as safety end points; instead, surrogate end points such as complete hematologic response (CHR) and molecular response (MR) were adopted. In the MAJIC substudy,13 MR was considered, and it was found that ruxolitinib is more efficient than BAT in obtaining MR and that MR correlates with inferior thrombosis risk. All of the 4 studies in the selection found a significant effect in terms of CHR.
However, some follow-up analyses on the RESPONSE trial did take thrombosis incidence into account after long-term treatment with ruxolitinib; namely, works by Alvarez-Larràn et al19 and Kiladjian et al.20 Alvarez-Larràn et al compared patients from the ruxolitinib arm of the RESPONSE trial vs propensity score–matched patients from a different cohort. They found an advantage of ruxolitinib both in terms of overall survival and thrombosis. Kiladjian et al, on the other hand, compared thrombosis incidence between patients with BAT and a 32-week follow-up vs patients who were assigned to ruxolitinib since the beginning and were followed up for 5 years; their analysis detected a difference in favor of ruxolitinib (1.2% vs 8.2% patients per year), although significance level was not reported. Thrombosis incidence under ruxolitinib treatment is reportedly the same after a 5-year follow-up. Interestingly, in the RESPONSE study, patients who were initially assigned to BAT but crossed over to ruxolitinib after the cutoff date had a thrombosis rate roughly between those of the 2 populations.
Meta-analysis of thrombosis incidence compared with BAT.
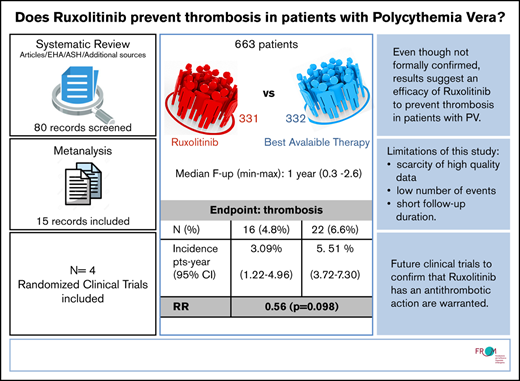
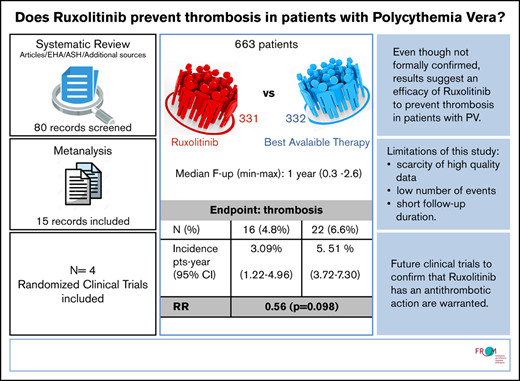
For all studies in the ruxolitinib selection, we recalculated thrombosis incidence and relative risk (RR) of thrombosis in the ruxolitinib vs BAT arms. To avoid potential bias due to having radically different follow-up times in the RESPONSE trial, patients from that cohort were only included up until the 32-week cutoff. In the end, the analysis accounted for 663 patients (1057 persons per year) and a total of 38 events. The patients’ characteristics were overall balanced between the BAT and ruxolitinib arms (weighted median age, 63.9 vs 63.3 years, respectively).
Figure 2 shows the annual incidence of thrombosis per study per arm with binomial CIs and the corresponding calculated RRs. With the possible exception of data from the RESPONSE cohort, the data do not display a clear advantage of ruxolitinib in preventing thrombosis over the examined follow-up period.
RCTs on ruxolitinib (Rux). Annual incidence of thrombosis per study per arm with binomial CIs and corresponding calculated RRs.
RCTs on ruxolitinib (Rux). Annual incidence of thrombosis per study per arm with binomial CIs and corresponding calculated RRs.
Incidences under ruxolitinib and BAT were compared over the 4 studies by using a mixed effect Poisson model, with a random intercept and random treatment effect for study and a fixed effect for age. The model adjusts for the median age of patients in each arm of each study.
Heterogeneity as measured by using a random effect variance was virtually zero for random intercept, which was therefore removed from the model. Heterogeneity for the ruxolitinib effect was not zero, but a likelihood ratio test on the variance parameter was nonsignificant; that is, both baseline incidence and effect of ruxolitinib did not vary significantly among studies. We thus fitted a simple Poisson model with cluster-adjusted standard errors. None of the fixed effect estimates was significant; however, there was suspect evidence (P = .098) of an advantage of ruxolitinib (incidence rate ratio, 0.56; 95% CI, 0.28-1.11).
The overall thrombosis annual incidence rate, as calculated from the model, was 4.30% (95% CI, 3.00-5.60); the rate for BAT was 5.51% (95% CI, 3.72-7.30), and the rate for ruxolitinib was 3.09% (95% CI, 1.22-4.96).
Discussion
The goal of the current review was to collect and analyze the body of available evidence concerning efficacy in terms of thrombosis reduction with ruxolitinib, a drug currently proposed as second-line therapy in HU-resistant/intolerant patients. Our search was based on 80 candidate records retrieved from medical databases and archives of abstracts from congresses of the EHA and ASH. The end result was a selection of 28 records included in a systematic review, 15 of which included quantitative data suitable for our meta-analysis.
The bulk of evidence came from 4 RCTs: the RESPONSE, the RESPONSE-2, the RELIEF, and the MAJIC. Overall, study quality was high, and risk of bias was deemed low. Treatment arms were mostly balanced for concerns regarding the main thrombosis risk factors (age and thrombosis history). Median age ranged from 60 to 68 years, with a weighted average of 64.8 years, and was generally balanced between arms. Follow-up duration, however, varied remarkably from study to study and sometimes from arm to arm, and ranged from 0.3 to 2.6 years. A previous meta-analysis from Samuelson et al21 on ruxolitinib in PV and myelofibrosis included, among others, the RESPONSE study. The authors found it to be at high risk of bias due to the possibility of crossover. Although we agree that this issue is a potential source of bias, we addressed it by restricting our analysis window to the period before crossover.
All 4 studies adopted surrogate efficacy end points (ie, CHR and MR); for these end points, all of the studies found a distinct advantage of ruxolitinib over BAT. Nevertheless, all of the studies also reported thromboembolic events as safety end points, allowing for an evaluation of their incidence.
In that respect, Kiladjian et al20 did find an advantage of ruxolitinib over BAT in preventing thromboembolic events; however, the main limitation of their findings is the very large imbalance in follow-up duration between the ruxolitinib and BAT arms. Study patients not responding to BAT were allowed to cross over to ruxolitinib after a predefined cutoff time, and most of them actually did so; in a recent follow-up analysis on the RESPONSE cohort, the follow-up for the ruxolitinib arm was as long as 5 years, compared with <1 year available for BAT. Thus, in our meta-analysis, we chose to exclude data from the later follow-ups from the RESPONSE trial.
Based on a meta-regression of ruxolitinib treatment effect over 663 patients, the thrombosis annual incidence rate is 3.09 patients per year (95% CI, 1.22-4.96) for ruxolitinib, 5.51 (95% CI, 3.72-7.30) for BAT, and 4.30 (95% CI, 3.00-5.60) globally, with an RR for ruxolitinib vs BAT of 0.56. The evidence of an advantage of ruxolitinib is suspect (P = .098) but not significant. Despite the good balance in terms of age of the patients, we chose to include median age as an arm-specific covariate in our regression model to further reduce heterogeneity. Although effect of age was not significant in meta-regression, this is likely due to randomization ensuring that arms were balanced in that respect; our estimates nevertheless are expected to be affected by age in clinical practice.
Overall, despite circumstantial evidence in favor of ruxolitinib, the comparison vs BAT was inconclusive. However, study heterogeneity, as evaluated by using random intercept and slope variance, was negligible, adding to the robustness of our results. Remarkably, the incidence of thrombosis under BAT in this review was found to be higher than the 3.6% that was expected under HU treatment,22 whereas under ruxolitinib, it is slightly lower. This scenario is not surprising because ruxolitinib was usually (with the exception of the RELIEF study) administered as second-line treatment in patients resistant or intolerant to HU whose disease was therefore generally not well controlled with BAT. The fact that incidence under ruxolitinib overlaps with what is expected in well-controlled HU-treated patients may actually be evidence in favor of the former.
In the previous meta-analysis from Samuelson et al,21 an advantage of ruxolitinib in preventing thrombosis was evident, but the study was not limited to patients with PV and did not include the RELIEF, RESPONSE-2, and MAJIC trials, which were not yet published at the time of the review. Their results are therefore not comparable to ours.
The results presented in this review have clear limitations, mainly concerning number of events. Furthermore, our annual thrombosis rate estimates are limited to a short follow-up duration. However, analyzing a limited follow-up was necessary to make comparison between arms meaningful.
Overall, in our sample, the thrombosis rate was consistently lower with ruxolitinib compared with BAT, but the difference was not significant. It is expected that significance would be hard to achieve in a comparative trial of ruxolitinib vs any other cytoreductive drug focusing on thrombosis reduction as a primary end point. In fact, based on our incidence estimates, the amount of patients to be enrolled in such a trial would be in the thousands. RCTs this large have clear issues of feasibility. It is therefore advisable that combined or surrogate end points are adopted instead of thrombosis incidence.
An example of adoption of a combined end point is found in an ongoing phase 3 clinical trial (MITHRIDATE [A Multicenter International Study Comparing Ruxolitinib With Either Hydroxycarbamide or Interferon Alpha as First Line Therapy for High Risk Polycythemia Vera]; clinicaltrials.gov identifier NCT04116502) that uses as a primary end point event-free survival, including: major thrombosis/hemorrhage, death, transformation to myelodysplastic syndromes, acute myeloid leukemia, or post-PV myelofibrosis. However, such a combined end point may not be able to prove superiority/inferiority of ruxolitinib vs the comparators specifically in thrombosis prevention because the 3 drugs (ruxolitinib, HU, and interferon) are not expected to show a marked difference in performance under that aspect.
Currently, CHR was the most used end point in the studies included in our review; however, its validity as surrogate end point for thrombosis in phase 3 confirmatory trials has been questioned.23 Conversely, Curto-Garcia et al13 recently presented data on the correlation between MR and thrombosis in a subgroup of patients from the MAJIC cohort, in which they found a significant correlation between complete/partial MR and thrombosis occurrence (0 thromboses in responders vs 19.1% in nonresponders, over a 2.6-year median follow-up). These data, although promising, require further confirmation.
In terms of hematocrit control, the CYTO-PV (Cytoreductive Therapy in PV) trial30 showed that an aggressive therapy aimed at maintaining a target hematocrit <45% correlates with lower incidence of cardiovascular events. Hematocrit could therefore be a feasible surrogate end point for thrombosis. Hopefully, this approach could be applied to future analyses of treatments for thrombosis prevention in PV.
Authorship
Contribution: A.M. and A.F. performed the literature search and methodological evaluation; A.F. performed the data analysis; A.C. and A.G. supervised data analysis; A.M., A.F., and T.B. wrote the manuscript; and T.B. coordinated and supervised all the work.
Conflict-of-interest disclosure: T.B. is a member of the advisory board and has received research grant funding from Novartis and AOP (Orphan Drug Company). The remaining authors declare no competing financial interests.
Correspondence: Tiziano Barbui, FROM Research Foundation, c/o ASST–Papa Giovanni XXIII, Piazza OMS n. 1, 24127 Bergamo, Italy; e-mail: tbarbui@fondazionefrom.it.
References
Author notes
All data requests should be submitted to the corresponding author (Tiziano Barbui; e-mail: tbarbui@fondazionefrom.it).
The full-text version of this article contains a data supplement.