Key Points
Simulations suggested that the dose reduction of rivaroxaban would decrease fatal events associated with major bleeding.
Dose optimization of FXa inhibitors might further enhance their therapeutic benefit.
Abstract
The noninferiority of direct oral factor Xa (FXa) inhibitors (rivaroxaban, apixaban, and edoxaban) in treatment of atrial fibrillation were demonstrated compared with warfarin by several large clinical trials; however, subsequent meta-analyses reported a higher risk of major bleeding with rivaroxaban than with the other FXa inhibitors. In the present study, we first estimated the changes of prothrombin time (PT) in 5 randomized trials based on reported population pharmacokinetic and pharmacodynamic models and then carried out a model-based meta-analysis to obtain models describing the relationship between PT changes and the event rates of ischemic stroke/systemic embolism (SE) and of major bleeding. By using the models, we simulated the optimal therapeutic doses for each FXa inhibitor. It was suggested that dose reduction of rivaroxaban from the current 20 mg/d to 10 mg/d would decrease patient deaths from major bleeding (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.64-0.74) with little increase in those for ischemic stroke/SE (HR, 1.11; 95% CI, 1.07-1.20). The overall decrease in the mortality caused by both events was estimated as 5.81 per 10 000 patient-years (95% CI, 3.92-8.16), with an HR of 0.87 (95% CI, 0.83-0.91). For apixaban and edoxaban, no distinct change in the overall mortality was simulated by dose modification. This study suggested that the current dose of rivaroxaban might be excessive and would need to be reduced to decrease the excess risk of major bleeding.
Introduction
Direct oral factor Xa (FXa) inhibitors, including rivaroxaban, apixaban, and edoxaban, also known as direct oral anticoagulants (DOACs), have emerged as a new choice that supersedes warfarin, which was the only available oral anticoagulant for the past 50 years.1,2 The efficacy and safety of FXa inhibitors have been examined by large, randomized, controlled trials (RCTs) including >10 000 atrial fibrillation (AF) patients, and the results showed that all the FXa inhibitors are noninferior or superior to warfarin in preventing stroke/systemic embolism (SE) and suppressing major bleeding.3-5
Although the large trials established the therapeutic benefits of FXa inhibitors over those of warfarin, it should be noted that the efficacy-safety profiles of FXa inhibitors observed in these trials differ somewhat between drugs. The incidence of major bleeding in phase 3 trials was comparable between rivaroxaban and warfarin (hazard ratio [HR], 1.04; 95% confidence interval [CI], 0.90-1.20),3 whereas it was significantly lower for apixaban (HR, 0.60; 95% CI, 0.60-0.80) and edoxaban 60 mg once daily (HR, 0.80; 95% CI, 0.71-0.91) than for warfarin.4,5 Higher bleeding frequencies with rivaroxaban have also been observed in several studies. Sharma et al conducted a meta-analysis of 19 RCTs regarding 4 DOACs (rivaroxaban, apixaban, edoxaban, and dabigatran) and reported that major bleeding risks were comparable between rivaroxaban and warfarin (HR, 0.81; 95% CI, 0.67-0.98), but significantly lower for apixaban (HR, 0.63; 95% CI, 0.51-0.77) and edoxaban 60 mg once daily (HR, 0.81; 95% CI, 0.67-0.98) regardless of age6 ; Nielsen et al analyzed the data of 61 678 patients with nonvalvular AF that were identified from 3 Danish nationwide databases and also reported that rivaroxaban had a risk of major bleeding comparable to that of warfarin (HR, 1.06; 95% CI, 0.91-1.23), whereas that of apixaban was significantly lower than the risk associated with warfarin (HR, 0.61; 95% CI, 0.49-0.75).7 Given the same mechanism of action of the 3 FXa inhibitors,8-11 we hypothesized that these variabilities would be attributed to the fact that their current doses are not fully optimized from the perspective of the risk-benefit balance.
A model-based meta-analysis (MBMA) is a quantitative analytical approach that integrates multiple studies using mathematical models.12,13 Several studies successfully constructed useful exposure-response models by applying the MBMA to literature information on clinical trials.14-17 We, hence, first tried to develop exposure-response models for each FXa inhibitor regarding both ischemic stroke/SE and major bleeding in order to assess the appropriateness of the current doses by simulation. For FXa inhibitors, however, a small number of studies had a sample size statistically adequate to detect frequencies of rarely occurring serious events such as ischemic stroke and major bleeding.18 Furthermore, most of the large trials involved only 1-dose regimen.3-5,19,20
To address the issue, we performed a MBMA that integrates trials of rivaroxaban, apixaban, and edoxaban assuming that prothrombin time (PT) comparably predicts event risks for the 3 FXa inhibitors. PT (or PT international normalized ratio [PT-INR]) is a coagulation test widely used for dose adjustments of warfarin, and the correlations between PT and plasma concentrations of FXa inhibitors have been well analyzed and modeled.21 In the present study, time-dependent changes of PT in patient populations in each trial were simulated using the published population pharmacokinetic (PK) and pharmacokinetic/pharmacodynamic (PK/PD) models21-25 to analyze their relationships with incidences of ischemic stroke/SE and major bleeding. The constructed models were used to estimate the optimal dose regimens of FXa inhibitors.
Methods
Data collection
Clinical trials of the FXa inhibitors were identified using ClinicalTrials.gov. A trial search was carried out on 17 June 2016 with the following search formula: (“rivaroxaban” OR “xarelto” OR “bay 59-7939” OR “edoxaban” OR “lixiana” OR “du-176b” OR “apixaban” OR “eliquis” OR “BMS-562247-01”) AND (“ischemic stroke” OR “major bleeding”). Studies included in the analysis were double-blind RCTs of >1-year duration that enrolled patients aged >18 years diagnosed with AF and assessed the incidences of ischemic stroke/SE or major bleeding. Trials targeting patients undergoing surgeries or those scheduled for elective cardioversion were excluded. The studies identified from ClinicalTrials.gov were initially selected based on registration information, and their full-text articles were reviewed to verify that the inclusion criteria were fulfilled. After the review, all the necessary information including the incidences of ischemic stroke/SE and major bleeding and patient characteristics including sex, age, body weight, lean body mass, creatinine clearance, CHADS2 (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke or transient ischemic attack [doubled]) score, and percentages of patients with age >75 years, comorbidities (hypertension, diabetes mellitus, congestive heart failure, history of transient ischemic attack/SE), concomitant use of P-glycoprotein inhibitors, and prior use of vitamin K antagonist and aspirin were extracted from papers.
Population PK and PK/PD models
The population PKs of rivaroxaban and those of apixaban and edoxaban were described based on the 1- and 2-compartment models with first-order absorption, respectively.21-25 A population PK/PD model has been reported for rivaroxaban.21 In this study, a significant interindividual variability was observed with regard to the baseline value of PT but not the slope for the treatment effect. Therefore, it was possible to extend this model to apixaban and edoxaban by adjusting the slope for the treatment effect based on the prior reports.24,25 In extending the model, the effects of differences in sensitivity of thromboplastin reagents was corrected by additionally adjusting the slope parameters in each model such that the models represent the PT values of RecombiplasTin, which was the most sensitive reagent in accordance with a method reported by Gosselin et al.26 The details for the population PK and PK/PD models used in the analysis are provided in supplemental Methods and supplemental Table 1.
Model analysis
Estimation of PT.
Time courses of PT at steady state in patient populations of each treatment arm were estimated by simulation using the population PK and PK/PD models. PT is generally expressed as PT-INR, a ratio of PT to that of healthy person corrected by international sensitivity index, to compensate differences in coagulation activity of the reagents used in the laboratory. However, the international sensitivity index should not be applied to FXa inhibitors because it is determined based on anticoagulation activity of warfarin.27 In addition, PT increases induced by therapeutic concentrations of FXa inhibitor are small, thus variable compared with warfarin.21 Therefore, in the present analysis, PT ratio, a relative increase of PT from baseline of each patient, was used for the simulation analyses to avoid these difficulties. The detailed procedure of PT estimation is provided in supplemental Methods.
Model development.
We assumed that event risks after administration of FXa inhibitors varied dynamically along with the time-dependent change in PT, because there was a large fluctuation in the PT in patients receiving FXa inhibitors compared with those receiving warfarin.28-31 The dynamic relationships between PT ratio and probabilities of ischemic stroke/SE (PISSE) and major bleeding (PMB) were modeled using several functions, and the following functions were adopted in the final model: logit(PISSE) = E0ISSE × exp(θ1/θ2 × {exp[θ2 × (x − 1)] −1}) (equation 1) and logit(PMB) = E0MB + θ3 × ln x (equation 2), where x is the PT ratio, E0ISSE and E0MB are the placebo responses of ischemic stroke/SE and major bleeding, respectively; θ1 and θ2 are the exponent factors that represent the slope and degree of inflection of the PT ratio–event probability curve for ischemic stroke/SE, respectively; and θ3 is a change of the log odds ratio of major bleeding for every 1-unit change in the log of the PT ratio. Details of the model development are provided in supplemental Methods and supplemental Table 2.
In order to check the appropriateness of selection of the major bleeding in the analysis, we repeated the analysis by replacing the major bleeding with intracranial bleeding, major or nonmajor clinically relevant bleeding, or any bleeding. All event rates were then converted into their associated mortality and compared visually.
Simulation for dose optimization.
To estimate optimal doses of the 3 FXa inhibitors, we carried out simulations of treatment effects for various doses of each agent in virtual patient populations synthesized based on the weighted averages of all available patient characteristics data. The patient populations were synthesized using the same procedures as described previously. The incidence rate in the synthesized population was derived as the arithmetic mean of predictions for individual patients. The simulation was performed for both once-daily and twice-daily administrations assuming a perfect adherence to medication.
The optimality of the examined doses was assessed based on both estimated mortality and simple total incidence of ischemic stroke/SE and major bleeding. The mortality rate (Pdeath) was estimated according to the following equation: Pdeath = w1 × PISSE + w2 × PMB (equation 3), where PISSE and PMB are the incidences of ischemic stroke/SE and major bleeding, respectively, and w1 and w2 are weighting coefficients that correspond to the probabilities of death after the occurrence of each event. The weighting coefficients were determined based on outcomes of the clinical trials used in the model analysis.
Software.
NONMEM 7.3 (ICON Development Solutions, Ellicott City, MD) was used for the modeling analysis. R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for data preparation, performing simulations, and drawing figures.
Results
Clinical trial data
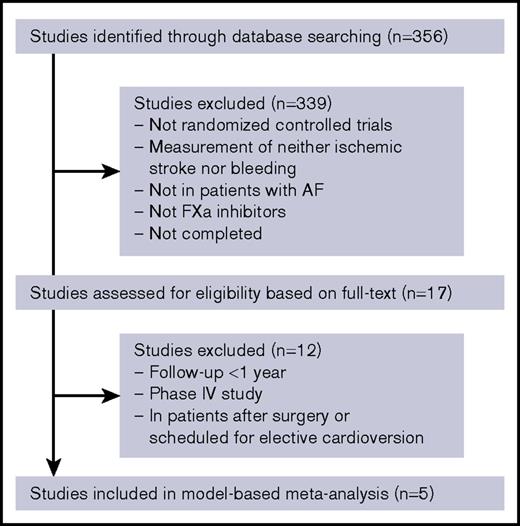
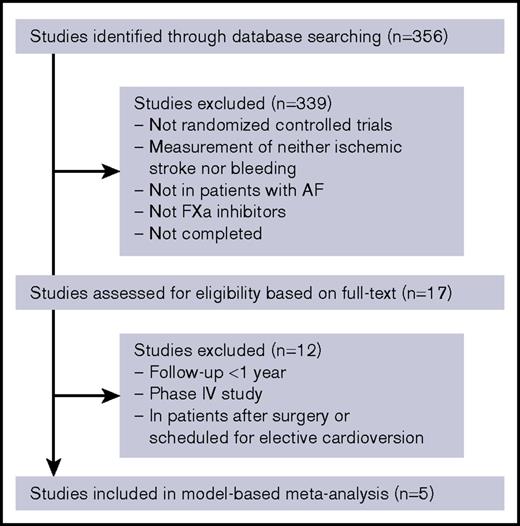
The flowchart of the study selection process is provided in Figure 1. A total of 5 studies including 57 655 patients fulfilled our inclusion criteria (Table 1). The final data set consists of 2 rivaroxaban studies (ROCKET AF, J-ROCKET AF), 2 apixaban studies (ARISTOTLE, AVERROES), and 1 edoxaban study (ENGAGE AF). The high- and low-dose groups in the ENGAGE AF trial were distinguished from each other. A control arm of the AVERROES trial was excluded from the analysis because it was the only group in which aspirin was used as an active control drug. All information extracted from studies are provided in supplemental Table 3.
Estimation of PT
The time courses of PT ratio, as well as plasma drug concentrations, at steady state were successfully estimated for each treatment arm of FXa inhibitors by the published population models (supplemental Figure 1). All PT values were adjusted to represent the measurements when RecombiplasTin reagent is used. The estimated PT ratio in the trials of rivaroxaban have a large time-dependent variation and distributed in the higher range than that in the trial of the other 2 FXa inhibitors. In contrast, the PT ratio of apixaban was almost constant in the low range during a period of the administration interval.
MBMA
All the parameters for the finally adopted PT-response models were obtained with acceptable precision (Table 2). The log odds ratios of ischemic stroke/SE and major bleeding for the comparison of the placebo control and dose-adjusted warfarin were −1.49 (odds ratio = 0.23) and 0.796 (odds ratio = 2.22), respectively, which were consistent with the previously reported values.32-35 A statistically significant intertrial variability was found only in the placebo response of ischemic stroke/SE (% coefficient of variation = 7.28, P < .01). Although we explored covariates that can explain this between-trial variability, none of the covariates provided a statistically significant improvement in model fit. As a result, the constructed PT-response models successfully explained the incidence of ischemic stroke/SE and major bleeding in the large RCTs of the 3 FXa inhibitors (supplemental Figure 2).
Relationships between PT and event risks for FXa inhibitors
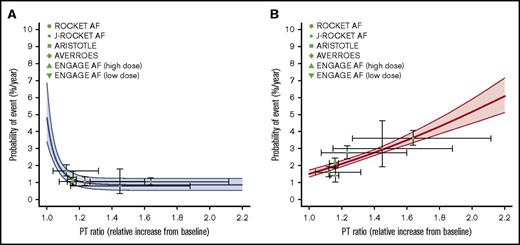
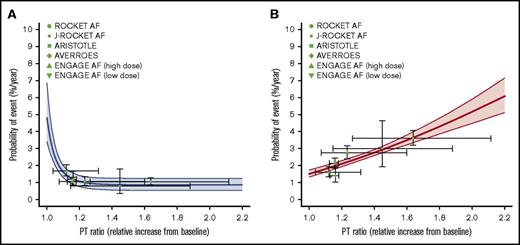
The model-predicted relationships between the probabilities of ischemic stroke/SE and major bleeding and the PT ratio in patients treated with FXa inhibitors are shown in Figure 2. The relationship between the anticoagulant activity of FXa inhibitors and event risk, which was derived from the data of the large clinical trials, was well characterized by the PT-response models. Additionally, the major bleeding risk in FXa inhibitors almost linearly increases with increasing PT ratio, whereas the suppression of ischemic stroke/SE reaches the maximum effect in the range where the increase in the anticoagulation activity is small (PT ratio <1.2). The estimated time-dependent variations in PT ratio (ie, the horizontal bars on the symbols) for the RCTs of rivaroxaban laid in a relatively high range.
Model-estimated relationships between PT ratio and event risks in FXa inhibitors estimated by a MBMA. (A) Ischemic stroke/SE. (B) Major bleeding. The solid curves and shaded areas represent the median estimates and 95% CIs, respectively. The symbols indicate observations in clinical trials vs the population means of the time-average of the PT ratio. The horizontal lines on the symbols represent the range between the trough and peak of the PT ratio as the population mean, and the vertical lines represent the 95% CI of the observations.
Model-estimated relationships between PT ratio and event risks in FXa inhibitors estimated by a MBMA. (A) Ischemic stroke/SE. (B) Major bleeding. The solid curves and shaded areas represent the median estimates and 95% CIs, respectively. The symbols indicate observations in clinical trials vs the population means of the time-average of the PT ratio. The horizontal lines on the symbols represent the range between the trough and peak of the PT ratio as the population mean, and the vertical lines represent the 95% CI of the observations.
Simulation for dose optimization
Based on the constructed PT-response models, treatment effects with various doses of each drug were simulated in a virtual population assuming typical AF patients. The weighting coefficient to calculate the mortality rate was set to 0.23 for ischemic stroke/SE and 0.07 for major bleeding, based on the mortality rates observed in the trials for the model development. The 95% CIs were calculated by repeating a series of simulation 1000 times (n = 10 000 per iteration) considering the uncertainty of parameters in the constructed models.
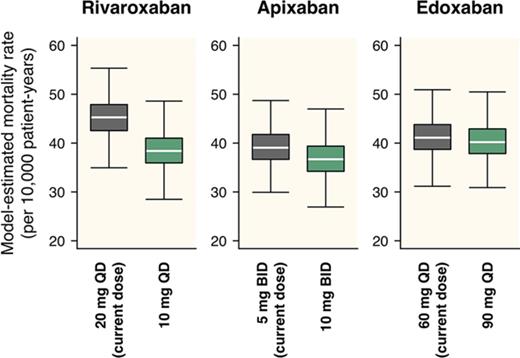
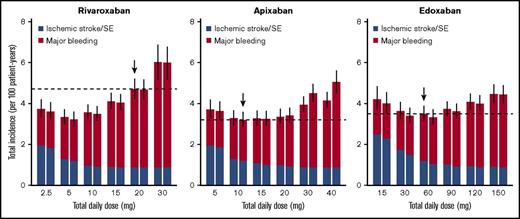
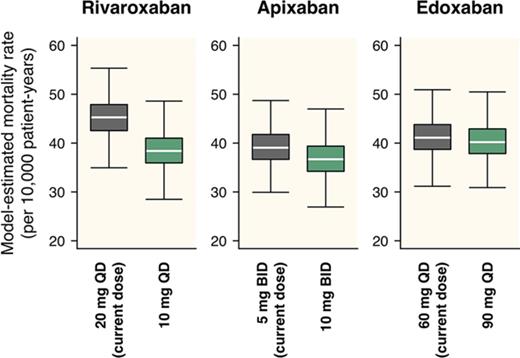
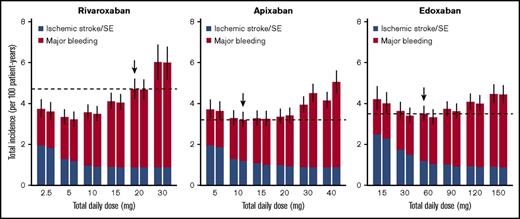
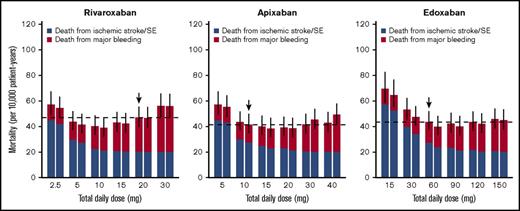
For rivaroxaban, although the current dose regimen is 20 mg once daily, a daily dose providing the lowest total incidence of ischemic stroke/SE and major bleeding was 5 mg/d, regardless of the frequency of administrations (Figure 3). An estimated decrease in the total incidence provided by dose reduction from the current 20 mg once daily to 5 mg once daily was 1.25 per 100 patient-years (95% CI, 0.89-1.71), with an HR of 0.73 (95% CI, 0.65-0.80; Table 3). On the other hand, the optimal dose that minimizes the mortality rate was estimated to be 10 mg/d (Figure 4), which was larger than that when assessed based on the total incidence of the events. An estimated decrease in mortality provided by the dose reduction from the current 20 mg once daily to 10 mg once daily was 5.81 per 10 000 patient-years (95% CI, 3.92-8.16), with an HR of 0.87 (95% CI, 0.83-0.91; Table 3). Especially in the case of mortality, the dose reduction of rivaroxaban decreased death from major bleeding (HR, 0.69; 95% CI, 0.64-0.74) with little increase in that for ischemic stroke/SE (HR, 1.11; 95% CI, 1.07-1.20).
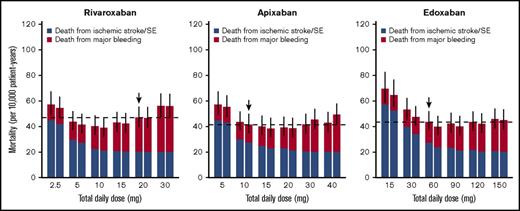
Event rates of ischemic stroke/SE and major bleeding simulated for various dose regimens of each FXa inhibitor. Left and right boxes for each daily dose represent once-daily and twice-daily administrations, respectively (ie, the box on the right side of X mg represents predictions when X/2 mg is administered twice a day). The horizontal dashed lines represent median predictions for the current dose regimens of each agent marked with downward arrows. The bars represent 95% CIs of the total incidence, derived from 1000 iterations.
Event rates of ischemic stroke/SE and major bleeding simulated for various dose regimens of each FXa inhibitor. Left and right boxes for each daily dose represent once-daily and twice-daily administrations, respectively (ie, the box on the right side of X mg represents predictions when X/2 mg is administered twice a day). The horizontal dashed lines represent median predictions for the current dose regimens of each agent marked with downward arrows. The bars represent 95% CIs of the total incidence, derived from 1000 iterations.
Mortality rates from ischemic stroke/SE and major bleeding simulated for various dose regimens of each FXa inhibitor. The figure is created in the same format as in Figure 3.
Mortality rates from ischemic stroke/SE and major bleeding simulated for various dose regimens of each FXa inhibitor. The figure is created in the same format as in Figure 3.
For apixaban, the current dose (10 mg/d twice daily) was nearly optimal when evaluated based on the total incidence of the events (Figure 3). Although it was insufficient to minimize mortality, the degree of decrease in the mortality was small even after doubling the dose (Figure 4). For edoxaban, the current dose (60 mg once daily) was nearly optimal with respect to both the total incidence and mortality (Figures 3 and 4). Intriguingly, the risk reduction associated with the increased number of administrations from once daily to twice daily was large compared with that of the other 2 drugs and was relatively noticeable especially when assessed based on mortality. As for rivaroxaban and apixaban, no distinct differences were found between the number of administrations in our simulation.
The dose of rivaroxaban in AF patients in Japan is being set lower to that in Western countries considering the larger drug exposure level of rivaroxaban in the Japanese.19,36,37 Thus, we simulated the total incidence and mortality at the estimated optimal dose by using the patient characteristics information of both ROCKET AF and J-ROCKET AF trials and compared them. As a result, no distinct difference was found in the estimated event rates for both the total incidence and mortality, although the Japanese population tended to have higher event rates than do Western populations (supplemental Figure 3).
Because bleeding definitions differ between each clinical trial, we examined the sensitivity of safety end point selection to the analysis by replacing the major bleeding with intracranial bleeding, major or nonmajor clinically relevant bleeding, or any bleeding (supplemental Figures 4-8). Briefly, the constructed models had a similar outline for any end point after conversion to mortality, suggesting that the selection of a safety end point little affected the results of dose optimization.
Discussion
To the best of our knowledge, this is the first study to analyze the quantitative relationship between the event risk and anticoagulant activity in AF patients treated with FXa inhibitors based on MBMA. The therapeutic utilities of FXa inhibitors have been established by large-scale RCTs and have been comprehensively evaluated by many subsequent analyses and clinical studies. In the present study, dose optimization was simulated based on the model constructed from MBMA of the large-scale RCTs, and the results showed that the current dose of rivaroxaban might need to be reduced to decrease the total incidence and/or estimated mortality caused by ischemic stroke/SE and major bleeding. The estimated time-dependent and interindividual fluctuations of the PT ratio in patients treated with the current regimen of rivaroxaban were almost within the range where the PT-response curve for ischemic stroke is flat. Consequently, the simulation indicated that the dose reduction of rivaroxaban would have little influence on the risk of ischemic stroke/SE. The estimated benefit in overall mortality was indicated by a noticeable decrease in the risk for major bleeding with little increase in that for ischemic stroke/SE.
Some observational studies had conflicting outcomes. Gorst-Rasmussen et al identified 11 045 AF patients from the Denmark nationwide registries and showed that the frequency of all-cause mortality in patients treated with rivaroxaban was higher in the 15-mg group than in the 20-mg group.38 Similarly, in a recent postmarketing surveillance in Japan, the frequencies of both ischemic stroke and major bleeding were higher in AF patients receiving rivaroxaban daily at 10 mg, which is a reduced dose approved in Japan for patients with creatinine clearance <50 mL/min, than in those receiving the usual dose of 15 mg (ischemic stroke, 1.3%/y vs 0.7%/y; major bleeding, 1.6%/y vs 1.1%/y).39 However, it should be emphasized that the patients were considerably younger in the reduced-dose groups in these 2 studies: 82.8 years and 72.8 years in the Danish study and 77.7 years and 68.6 years in the Japanese postmarketing surveillance, for the normal and reduced dose groups, respectively. The mean CHADS2 score and the proportion of patients concomitantly receiving aspirin were also noticeably higher in the normal-dose groups in these studies.38,39 These are well-known risk factors for fatal events in AF patients, which would explain the higher mortality rate in patients receiving the reduced dose.40,41 In the Danish study, no distinct difference was observed between the 2 dose groups in the total incidence of stroke/SE/transient ischemic attack (6.0%/y vs 6.0%/y) and any bleeding (15 mg, 4.6%/y vs 20 mg, 4.2%/y).38 Therefore, it is unlikely that a dose reduction was directly associated with the increased mortality in this population. Currently, no RCT has been conducted that directly compares event frequencies among different dose regimens of FXa inhibitors in AF patients. In venous thromboembolism patients following orthopedic surgery, MBMA of DOACs has been reported recently.14
In the EINSTEIN CHOICE (Reduced-dosed Rivaroxaban in the Long-term Prevention of Recurrent Symptomatic Venous Thromboembolism) trial, the frequency of major bleeding decreased from 0.5% in the 20-mg group of rivaroxaban to 0.4% in the corresponding 10-mg group, but without statistical significance.42 The frequency of major bleeding in this study with venous thromboembolism patients was substantially lower than those in the large-scale RCTs with AF patients (eg, 3.6%/y in the ROCKET AF trial).3 The patients in the EINSTEIN CHOICE trial were considerably younger than those with AF in the large-scale RCTs. Although the simulation in the present study suggested that the dose reduction of rivaroxaban would decrease the risk of major bleeding, which consequently would lead to the overall decrease in mortality, it is limited to elderly AF patients with a relatively high risk of major bleeding.43,44
In the present study, in addition to doses, we examined the influence of the frequency of administration on event risks. Unlike warfarin, which has a significant lag-time to exert PD effects, the anticoagulant activity of FXa inhibitors fluctuates immediately because of changes in plasma drug concentrations.28-31 To take into account this characteristic, we modeled the dynamic relationship between PT and probabilities of events, assuming that event risks of FXa inhibitors fluctuated time dependently in accordance with the change in PT. In this model, the predicted event risks may differ between once-daily and twice-daily regimens for the same daily dose. The results suggested that an increase in the frequency of administration of edoxaban may contribute to the decreased event risks especially for ischemic stroke/SE. In contrast, for apixaban, which is currently the only FXa inhibitor administered as a twice-daily regimen, the estimated event risks were almost comparable between once-daily and twice-daily regimens for the same daily dose in our analysis. The reliability of our analysis is uncertain because only limited clinical trial has been conducted thus far to compare the efficacy or safety of the once-daily and twice-daily regimens of FXa inhibitors.45 However, the present analysis suggested that the frequency of administration may need to be considered to optimize the DOAC pharmacotherapy. Additionally, adherence should be considered to determine the frequency of administrations.1
The approval of FXa inhibitors is based on criteria focusing on superiority over warfarin that had been investigated in clinical trials with a large number of patients; this strategy seemed rational with respect to earlier introduction of effective FXa inhibitors to the market. However, as implicated in this study, doses of anticoagulant drugs may not have been fully optimized even if superiority over representative agents in the same field has been shown. Therefore, as a follow up, it would be important to clarify the most appropriate regimen for each drug and to enable selection of the optimal treatment of a patient based on his/her therapeutic needs. In a conventional framework, however, it is expected that a large-scale clinical trial be performed to achieve such objectives. Clinical trials that directly evaluate risk events as primary end points require a large number of subjects and long-term follow-up because of the efficacy of anticoagulants in further reducing less frequent serious events.3-5,19,20 Hence, appropriate biomarkers should be evaluated and model analysis used to optimize the efficiency of clinical trials in the future.
The present study used PT as an explanatory biomarker to characterize the therapeutic effect, and not PK parameters or doses used in numbers in prior MBMA studies.15-17 This made it possible to integrate clinical trials of 3 different FXa inhibitors. PT was selected in this study based on the fact that the population models have been reported.21 Furthermore, 1 prior study showed the association of PT with an increased risk of bleeding complications.46 In contrast, it still remains controversial as to whether PT is the best predictive marker of event risks in patients receiving treatment with FXa inhibitors.46,47 Other clotting markers, such as activated partial thromboplastin time and anti-FXa activity, are also known to correlate with plasma concentrations of FXa inhibitors.26 The use of these clotting markers might be an alternative. Furthermore, thromboembolism events including ischemic stroke/SE, fibrin-related blood markers, such as soluble fibrin monomer complex and d-dimer,48-50 or thrombin generation markers, such as prothrombin fragment 1+2 and thrombin anti-thrombin complex,50,51 would be excellent explanatory biomarkers for event risk theoretically; however, no population models for the relationship between plasma concentrations of FXa inhibitors have been reported. The reliability of the PT-response model constructed in this study may need to be confirmed by future analysis adopting these biomarkers when they become available.
This study has several important limitations. First, outcomes of the present study are merely theoretical estimations based on the simulation, and thus the actual dose adjustments must be taken based on results of the clinical findings. Second, we hypothesized that for the 3 FXa inhibitors, the relationship between event risk and anticoagulant activity is the same. The direct oral FXa inhibitors, rivaroxaban, apixaban, and edoxaban, directly inhibit activated factor Xa with >10 000-fold selectivity vs other blood-related coagulation factors.8-11 Moreover, they have the same binding sites on FXa, and all of them inhibit both free FXa in plasma and FXa present on thrombus.8-11 However, the assumption has not been proved. Third, we adjusted population PK/PD models so that they estimate PT of the most sensitive thromboplastin reagent to all 3 FXa inhibitors. However, this would not completely eliminate bias. The findings presented here must be interpreted in light of such potential methodological limitations.
In conclusion, PT-response models of FXa inhibitors for ischemic stroke/SE and major bleeding were successfully constructed by the MBMA of large-scale RCTs in AF patients treated with rivaroxaban, apixaban, or edoxaban. In the simulation based on the constructed models, it was suggested that the current dose of rivaroxaban might need to be reduced to decrease the excess risk of major bleeding, and that the current doses of apixaban and edoxaban would be close to optimal.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by research funding from Grant-in-Aid for Scientific Research (C) (grant number JP17K08436) from the Japan Society for the Promotion of Science, and by the Research on Regulatory Science of Pharmaceuticals and Medical Devices (17mk0101090h0001) from Japan Agency for Medical Research and Development.
Authorship
Contribution: H.Y. and A.H. conceived the project and designed the analyses; H.Y. participated in analyses; H.Y. and A.H. wrote the manuscript; H.Y., H.H., H.S., and A.H. participated in manuscript preparation; and all authors edited and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akihiro Hisaka, Laboratory of Clinical Pharmacology and Pharmacometrics, Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba-shi, Chiba 260-8675, Japan; e-mail: hisaka@chiba-u.jp.