Key Points
Platelet-poor plasma clotting and fibrinolysis assays detect bleeding tendency in patients with factor XI deficiency.
Contact pathway inhibition with corn trypsin inhibitor increases sensitivity of these assays to bleeding tendency.
Abstract
Individuals with factor XI (FXI) deficiency have a variable bleeding risk that cannot be predicted from plasma FXI antigen or activity. This limitation can result in under- or overtreatment of patients and risk of bleeding or thrombosis. Previously, plasma clot fibrinolysis assays showed sensitivity to bleeding tendency in a small cohort of patients with severe FXI deficiency. Here, we determined the ability of plasma clot formation, structure, and fibrinolysis assays to predict bleeding tendency in a larger, independent cohort of patients with severe and partial FXI deficiency. Patients were characterized as nonbleeders or bleeders based on bleeding after tonsillectomy and/or dental extraction before diagnosis of FXI deficiency. Blood was collected in the absence or presence of the contact pathway inhibitor corn trypsin inhibitor (CTI). Clotting was triggered in platelet-poor plasma with tissue factor, CaCl2, and phospholipids in the absence and presence of thrombomodulin or tissue plasminogen activator. Clot formation and fibrinolysis were assessed by turbidity and confocal microscopy. CTI-treated plasmas from bleeders showed significantly reduced clot formation and decreased resistance to fibrinolysis compared with plasmas from controls or nonbleeders. Differences were enhanced in the presence of CTI. A model that combines activated partial thromboplastin time with the rate of clot formation and area under the curve in fibrinolysis assays identifies most FXI-deficient bleeders. These results show assays with CTI-treated platelet-poor plasma reveal clotting and clot stability deficiencies that are highly associated with bleeding tendency. Turbidity-based fibrinolysis assays may have clinical utility for predicting bleeding risk in patients with severe or partial FXI deficiency.
Introduction
Factor XI (FXI) deficiency is a rare, autosomal disorder. Homozygous or compound heterozygous patients have severe deficiency (≤15 IU/dL), whereas heterozygotes have partial deficiency (16-60 IU/dL).1,2 Spontaneous bleeding is rare; however, patients with all levels of FXI deficiency can present with bleeding following surgery or injury. Bleeding typically occurs at sites with high fibrinolytic activity (mouth, nose, genitourinary tract) and can also include heavy menstrual bleeding, intracerebral hemorrhage, and gastrointestinal bleeding.1-8
Interestingly, patients with comparable FXI antigen or activity exhibit variable bleeding tendencies even within families in which individuals share the same genotype.3-6 Many patients are asymptomatic even after trauma or surgery, whereas others report excessive bleeding. FXI antigen, FXI:clotting (FXI:C) activity, and activated partial thromboplastin time (APTT) do not correlate with hemorrhagic tendency.4-6,9 There is marked variability in the treatment of FXI deficiency worldwide; some physicians replace FXI in patients with severe deficiency only, whereas others treat all patients (severe and partial deficiency) with a significant bleeding history or unknown bleeding risk. Without tools to predict bleeding risk, FXI-deficient patients may be undertreated, increasing risk of hemorrhage, or overtreated, placing patients at risk of thrombosis (reported in some patients who have received FXI concentrate), volume overload (with fresh frozen plasma), transfusion-related acute lung injury, and development of FXI inhibitors (with both fresh frozen plasma and FXI concentrate).10-12 Assays that predict bleeding risk and reveal hemostatic mechanisms in FXI-deficient patients would have high clinical value and may lead to new management strategies for FXI-deficient patients and individuals with other bleeding disorders.
Recent attempts to measure FXI deficiency and differentiate FXI-deficient bleeders and nonbleeders have focused on “global coagulation assays.” Whole blood thromboelastography is sensitive to FXI, but fails to effectively distinguish bleeders from nonbleeders.13-15 Thrombin generation assays have shown promise in detecting FXI deficiency and bleeding risk.14,16-18 Rugeri et al16 detected prolonged lag times and reduced rates and peaks of thrombin generation in corn trypsin inhibitor (CTI)-treated platelet-rich plasmas (PRPs) from bleeders vs controls or nonbleeders. Pike et al17 compared thrombin generation characteristics in PRP and platelet-poor plasma (PPP) from a large cohort of FXI-deficient patients. They observed that thrombin generation measured in PPP, or PRP normalized to 150 × 109/L platelets, differentiated bleeders from controls and nonbleeders, and found that thrombin generation in CTI-treated PRP is best suited to identify clinical bleeding phenotype in FXI-deficient patients.17 However, thrombin generation assays require specialized substrates and fluorometers that are expensive and not widely available. In addition, platelet-based assays are particularly sensitive to preclinical variables including time from arm to assay and difficulty maintaining quiescent platelets during isolation; this is especially challenging when steps to normalize platelet count are incorporated into the protocol.19,20 Moreover, PRP is not amenable to transport between distal centers, limiting efforts to standardize and validate these assays.
Zucker et al21 tested the ability of plasma clot formation and fibrinolysis assays to differentiate FXI-deficient bleeders from nonbleeders. These assays are performed with CTI-treated PPP, triggered by tissue factor (TF) and recalcification, and monitored by turbidity. In a small cohort of patients with severe FXI deficiency, bleeders exhibited reduced clot formation and significantly decreased resistance to fibrinolysis than did controls or FXI-deficient nonbleeders.21 Because turbidity-based clotting assays can be performed using thawed, frozen PPP and widely available microplate spectrophotometers, these assays may be useful as predictive tools for identifying patients at risk for bleeding.
Herein, we measured clot formation, structure, and fibrinolysis in PPP from a large cohort of patients with severe or partial FXI deficiency. Our objectives were to: (1) validate the ability of clot formation and fibrinolysis assays to differentiate FXI-deficient bleeders from nonbleeders in an independent cohort of FXI-deficient patients, (2) test the sensitivity of these assays in patients with partial FXI deficiency, and (3) determine the value of contact pathway inhibition with CTI in these assays.
Materials and methods
Materials
CTI and rabbit lung thrombomodulin were from Haematologic Technologies, Inc. (Essex Junction, VT). Innovin (human TF) was from Siemens Healthcare Diagnostics (Newark, DE). Tissue plasminogen activator (tPA) was from Abcam (Cambridge, MA). Phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine were from Avanti Polar Lipids (Alabaster, AL). Large unilamellar phospholipid vesicles (41% phosphatidylcholine/44% phosphatidylethanolamine/15% phosphatidylserine) were made as described.21,22 AlexaFluor488-conjugated fibrinogen was from Molecular Probes (Eugene, OR).
Human subjects
Subjects were a subset of individuals from a previously described cross-sectional study.17 Briefly, subjects were 71 adults with FXI deficiency (FXI:C 0-60 IU/dL; supplemental Table 1) recruited from Manchester Royal Infirmary Haemophilia Centre. Controls were 50 healthy adults with no personal or family history of thrombosis or bleeding disorders and no relevant medications. All controls had normal APTT, prothrombin time, and fibrinogen and FXI:C levels. Regional research ethical committee approval was obtained for blood collection (#11/NW/0612), and all subjects gave written informed consent.
FXI-deficient individuals were characterized as nonbleeders or bleeders based on their experience with tonsillectomy and/or dental extraction performed before diagnosis of FXI deficiency (ie, without preoperative prophylaxis). Nonbleeders (N = 48) were those who underwent uneventful procedures (no excessive bleeding). Bleeders (N = 23) were those requiring blood product transfusion or return to surgery or dentist for resuturing or packing. Six patients (5 nonbleeders and 1 bleeder) underwent deciduous tooth extraction only; analysis was performed with and without these patients. Collectively, 57 patients had partial FXI deficiency (16-60 IU/dL, 42 nonbleeders and 15 bleeders, all heterozygous for FXI mutations) and 14 patients had severe FXI deficiency (≤15 IU/dL, 6 nonbleeders and 8 bleeders, all homozygous for FXI mutations).
Clinical coagulation and clotting factor testing
Complete blood counts, FXI:C, APTT, prothrombin time, and fibrinogen were measured as described.17
Plasma preparation
Blood was collected by venipuncture using a 21G butterfly needle (Becton Dickinson Co, Plymouth, United Kingdom) into S-Monovette tubes containing 0.106 M trisodium citrate (1:9, volume-to-volume ratio) (Sarstedt, Leicester, United Kingdom), alone or into CTI (20 µg/mL, final concentration in whole blood) to minimize contact activation. The first 5 mL were discarded. PPP was prepared by centrifugation (3000g, 15 minutes, twice), aliquoted, and immediately frozen at −80°C.
Characterization of clot formation and lysis
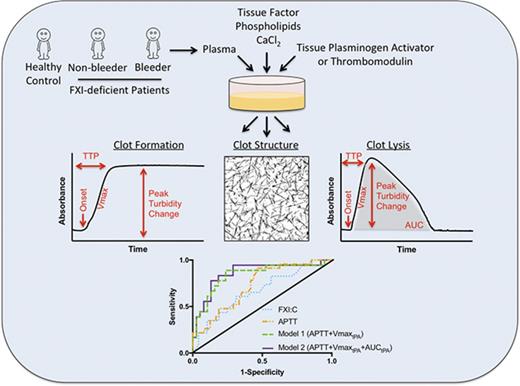
Clotting and fibrinolysis assays were performed immediately after samples were thawed at 37°C, as described elsewhere.21 Briefly, clotting was initiated by incubating recalcified (10 mM CaCl2, final) PPP with TF and phospholipids (1:30 000 dilution of Innovin and 4 µM, final, respectively) in the absence or presence of tPA (0.5 µg/mL, final) or thrombomodulin (5 nM, final). Final reaction volumes were 100 µL (85% PPP) in 96-well plates. Clot formation and lysis were monitored for 2 hours by turbidity at 405 nm (SpectraMax 384Plus plate reader, Molecular Devices, Sunnyvale, CA) at room temperature.
The onset of clot formation was the time to the inflection point before turbidity increase. Clotting rate was the slope of a line fitted to the maximum rate of turbidity increase (“Vmax”) using 5 to 10 points to determine the line (Softmax Pro 5.4, Molecular Devices). Time to plateau/peak (TTP) was the time to the turbidity plateau (absence of tPA) or maximum turbidity reached (presence of tPA). Peak turbidity change was the maximum clot turbidity less the starting turbidity. Area under the curve (AUC) was the sum of trapezoids formed by turbidity curves less a baseline established by the lowest measurement recorded (Kaleidagraph v4.5.0, Synergy Software, Reading PA). Lysis time was the difference between TTP and time the curve returned to baseline. For samples that did not clot, onset and TTP were recorded as 120 minutes, and Vmax, AUC, and lysis time were recorded as 0.
A subset of samples was not analyzed in the clot formation or fibrinolysis assays because of insufficient volume or lipemia that interfered with turbidity measurements. In total, 107 samples collected into CTI (49 controls, 40 nonbleeders, and 18 bleeders) and 118 samples collected in the absence of CTI (49 controls, 46 nonbleeders, and 23 bleeders) were analyzed.
Fibrin structure analysis
Plasmas were spiked with AlexaFluor488-congujated fibrinogen (80 µg/mL, final, 2.6% of total fibrinogen). Clots were formed with recalcified PPP, TF, and phospholipids (10 mM CaCl2, 1:30 000 dilution of Innovin, and 4 µM lipids, final, respectively) in Laboratory-Tek II chamber coverglass slides and imaged as described previously.23 Fibrin density was quantified by summing individual sections to create Z-projections and thresholding to visualize fibers and minimize noise (ImageJ, version 1.41o).23 The area covered by pixels above the threshold cutoff was determined using the ImageJ “Measure” function.
Statistical analyses
Descriptive statistics were summarized using means and standard deviations. Parameters were then compared between groups using Wilcoxon tests. A linear regression model was used to control for differences in FXI:C when comparing nonbleeders and bleeders. Receiver operating characteristic (ROC) analysis was used to assess the ability of individual and combined parameters to differentiate between groups. Logistic regression models were used to estimate the probability of the patient being a nonbleeder or bleeder, and the estimated probability was used to construct the ROC curve showing sensitivity and 1 minus specificity under different cutoffs of the estimated probabilities. Area under the ROC curve (AUROC) and 95% confidence intervals were calculated using the trapezoid method and Delong’s variance estimator. AUROC values >0.800 were considered good predictors of the outcome. Analyses and hypotheses testing were performed using GraphPad Prism v7.0 and IBM SPSS Statistics 24. All statistical tests were 2-sided and P < .05 was considered significant.
Results
Clinical laboratory characteristics
Subject characteristics and results from clinical coagulation tests are shown in supplemental Table 1 and supplemental Figure 1. Briefly, complete blood counts did not differ between controls, nonbleeders, and bleeders. Compared with healthy controls, both nonbleeders and bleeders had reduced FXI and prolonged APTT. FXI:C was lower and APTT was prolonged in bleeders compared with nonbleeders; however, these differences did not differentiate bleeders and nonbleeders with adequate sensitivity and specificity (AUROC, 0.668 and 0.728, respectively; supplemental Table 2). The prothrombin time was slightly longer and fibrinogen was slightly higher in nonbleeders and bleeders compared with controls, but both prothrombin time and fibrinogen were in the normal range and did not differ between nonbleeders and bleeders.
Clot formation and fibrinolysis assays performed with CTI-treated plasmas differentiate FXI-deficient bleeders from nonbleeders
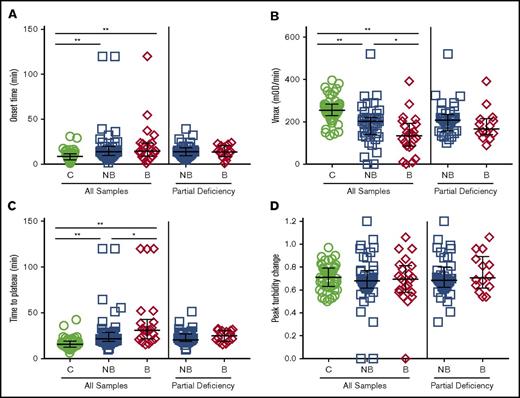
We first triggered clot formation in CTI-treated plasma samples from healthy controls and FXI-deficient patients by TF addition and recalcification and monitored clot formation by turbidity. Onset times to clot formation were prolonged nonsignificantly for bleeders compared with controls and nonbleeders (Figure 1A; Table 1). Compared with controls, both nonbleeders and bleeders had a significantly slower rate (Vmax) of clot formation, and bleeders had a slower Vmax than nonbleeders (Figure 1B; Table 1). Moreover, although controls and nonbleeders had similar TTP and peak turbidity changes, bleeders had prolonged TTP and decreased turbidity change (Figure 1C-D; Table 1) compared with either controls or nonbleeders. Differences in Vmax and turbidity change persisted even after controlling for the slight difference in FXI:C between nonbleeders and bleeders (Table 1) or omitting patients who had only undergone deciduous tooth extraction (data not shown). Vmax and peak turbidity change also differed between nonbleeders and bleeders when patients with severe FXI deficiency (≤15 IU/dL) were excluded (Figure 1B-D; Table 1).
Clot formation assays performed with CTI-treated plasmas differentiate FXI-deficient bleeders from nonbleeders. Clotting was triggered in CTI-treated plasmas from healthy individuals and FXI-deficient patients by recalcification and addition of tissue factor and phospholipids. Clot formation was monitored by turbidity. (A) Onset time, (B) rate (Vmax), (C) time to plateau, and (D) peak turbidity change for controls, all FXI-deficient patients, and only FXI-deficient patients with partial deficiency (16-60 IU/dL). Symbols represent plasmas from individual subjects; lines show median and interquartile range. *P < .05, **P < .005. B, bleeders; C, controls; mOD, milli optical density; NB, nonbleeders.
Clot formation assays performed with CTI-treated plasmas differentiate FXI-deficient bleeders from nonbleeders. Clotting was triggered in CTI-treated plasmas from healthy individuals and FXI-deficient patients by recalcification and addition of tissue factor and phospholipids. Clot formation was monitored by turbidity. (A) Onset time, (B) rate (Vmax), (C) time to plateau, and (D) peak turbidity change for controls, all FXI-deficient patients, and only FXI-deficient patients with partial deficiency (16-60 IU/dL). Symbols represent plasmas from individual subjects; lines show median and interquartile range. *P < .05, **P < .005. B, bleeders; C, controls; mOD, milli optical density; NB, nonbleeders.
Plasma clot formation and fibrinolysis parameters: presence of CTI
| . | All samples . | Partial FXI deficiency . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | C, mean ± SD . | NB, mean ± SD . | B, mean ± SD . | P, C vs NB* . | P, C vs B* . | P, NB vs B (adj)*† . | NB . | B . | P, NB vs B (adj)*† . |
| Clotting, N | 49 | 40 | 18 | 38 | 12 | ||||
| Onset time, min | 29.0 ± 24.7 | 25.2 ± 18.0 | 46.9 ± 41.9 | .888 | .118 | .079 (.036) | 25.4 ± 18.3 | 34.0 ± 30.3 | .517 (.195) |
| Vmax, mOD/min | 46.3 ± 20.4 | 37.1 ± 16.8 | 22.2 ± 21.6 | .004 | .000 | .002 (.009) | 36.9 ± 16.6 | 26.8 ± 22.1 | .035 (.062) |
| Time to plateau, min | 54.5 ± 27.2 | 62.4 ± 27.6 | 81.7 ± 34.7 | .142 | .006 | .033 (.060) | 62.5 ± 27.8 | 73.0 ± 33.3 | .237 (.236) |
| Peak turbidity change | 0.57 ± 0.17 | 0.62 ± 0.16 | 0.41 ± 0.26 | .108 | .010 | .001 (.002) | 0.62 ± 0.16 | 0.47 ± 0.21 | .009 (.012) |
| Clotting + TM, N | 49 | 40 | 18 | 38 | 12 | ||||
| Onset time, min | 58.7 ± 36.4 | 71.2 ± 41.2 | 84.6 ± 40.5 | .217 | .025 | .226 (.376) | 72.9 ± 41.8 | 84.4 ± 40.0 | .382 (.526) |
| Vmax, mOD/min | 23.8 ± 20.0 | 15.1 ± 16.6 | 12.8 ± 22.8 | .031 | .011 | .308 (.435) | 14.6 ± 16.4 | 10.2 ± 15.4 | .395 (.424) |
| Time to plateau, min | 89.8 ± 28.8 | 99.7 ± 27.6 | 106.4 ± 27.6 | .068 | .011 | .269 (.378) | 100.2 ± 27.3 | 107.2 ± 23.7 | .603 (.463) |
| Peak turbidity change | 0.38 ± 0.26 | 0.32 ± 0.30 | 0.18 ± 0.27 | .416 | .008 | .134 (.107) | 0.31 ± 0.30 | 0.17 ± 0.26 | .211 (.210) |
| Clotted samples, N (%)‡ | 39 (80) | 25 (63) | 9 (50) | .076 | .016 | .356 (.529) | 23 (61) | 6 (50) | .505 (.654) |
| Fibrinolysis, N | 49 | 39 | 18 | 37 | 12 | ||||
| Onset time, min | 27.7 ± 14.9 | 28.5 ± 21.8 | 55.9 ± 42.3 | .562 | .026 | .026 (.036) | 28.5 ± 22.2 | 44.6 ± 33.4 | .106 (.092) |
| Vmax, mOD/min | 67.7 ± 24.1 | 52.7 ± 23.7 | 27.3 ± 25.2 | .004 | .000 | .000 (.008) | 53.2 ± 23.8 | 33.8 ± 24.9 | .006 (.023) |
| Time to peak, min | 44.3 ± 16.7 | 46.2 ± 19.6 | 71.7 ± 37.6 | .684 | .011 | .022 (.026) | 46.4 ± 2.1 | 62.0 ± 3.7 | .114 (.068) |
| Peak turbidity change | 0.47 ± 0.13 | 0.41 ± 0.15 | 0.25 ± 0.19 | .125 | .000 | .001 (.010) | 0.42 ± 0.15 | 0.29 ± 0.16 | .010 (.026) |
| AUC | 577.7 ± 260.5 | 518.0 ± 304.7 | 260.1 ± 252.5 | .164 | .000 | .011 (.023) | 527.0 ± 310.3 | 295.9 ± 185.3 | .009 (.029) |
| Lysis time, min | 26.0 ± 8.4 | 23.4 ± 10.8 | 15.3 ± 11.6 | .086 | .002 | .029 (.151) | 23.6 ± 11.0 | 19.1 ± 9.1 | .214 (.280) |
| . | All samples . | Partial FXI deficiency . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | C, mean ± SD . | NB, mean ± SD . | B, mean ± SD . | P, C vs NB* . | P, C vs B* . | P, NB vs B (adj)*† . | NB . | B . | P, NB vs B (adj)*† . |
| Clotting, N | 49 | 40 | 18 | 38 | 12 | ||||
| Onset time, min | 29.0 ± 24.7 | 25.2 ± 18.0 | 46.9 ± 41.9 | .888 | .118 | .079 (.036) | 25.4 ± 18.3 | 34.0 ± 30.3 | .517 (.195) |
| Vmax, mOD/min | 46.3 ± 20.4 | 37.1 ± 16.8 | 22.2 ± 21.6 | .004 | .000 | .002 (.009) | 36.9 ± 16.6 | 26.8 ± 22.1 | .035 (.062) |
| Time to plateau, min | 54.5 ± 27.2 | 62.4 ± 27.6 | 81.7 ± 34.7 | .142 | .006 | .033 (.060) | 62.5 ± 27.8 | 73.0 ± 33.3 | .237 (.236) |
| Peak turbidity change | 0.57 ± 0.17 | 0.62 ± 0.16 | 0.41 ± 0.26 | .108 | .010 | .001 (.002) | 0.62 ± 0.16 | 0.47 ± 0.21 | .009 (.012) |
| Clotting + TM, N | 49 | 40 | 18 | 38 | 12 | ||||
| Onset time, min | 58.7 ± 36.4 | 71.2 ± 41.2 | 84.6 ± 40.5 | .217 | .025 | .226 (.376) | 72.9 ± 41.8 | 84.4 ± 40.0 | .382 (.526) |
| Vmax, mOD/min | 23.8 ± 20.0 | 15.1 ± 16.6 | 12.8 ± 22.8 | .031 | .011 | .308 (.435) | 14.6 ± 16.4 | 10.2 ± 15.4 | .395 (.424) |
| Time to plateau, min | 89.8 ± 28.8 | 99.7 ± 27.6 | 106.4 ± 27.6 | .068 | .011 | .269 (.378) | 100.2 ± 27.3 | 107.2 ± 23.7 | .603 (.463) |
| Peak turbidity change | 0.38 ± 0.26 | 0.32 ± 0.30 | 0.18 ± 0.27 | .416 | .008 | .134 (.107) | 0.31 ± 0.30 | 0.17 ± 0.26 | .211 (.210) |
| Clotted samples, N (%)‡ | 39 (80) | 25 (63) | 9 (50) | .076 | .016 | .356 (.529) | 23 (61) | 6 (50) | .505 (.654) |
| Fibrinolysis, N | 49 | 39 | 18 | 37 | 12 | ||||
| Onset time, min | 27.7 ± 14.9 | 28.5 ± 21.8 | 55.9 ± 42.3 | .562 | .026 | .026 (.036) | 28.5 ± 22.2 | 44.6 ± 33.4 | .106 (.092) |
| Vmax, mOD/min | 67.7 ± 24.1 | 52.7 ± 23.7 | 27.3 ± 25.2 | .004 | .000 | .000 (.008) | 53.2 ± 23.8 | 33.8 ± 24.9 | .006 (.023) |
| Time to peak, min | 44.3 ± 16.7 | 46.2 ± 19.6 | 71.7 ± 37.6 | .684 | .011 | .022 (.026) | 46.4 ± 2.1 | 62.0 ± 3.7 | .114 (.068) |
| Peak turbidity change | 0.47 ± 0.13 | 0.41 ± 0.15 | 0.25 ± 0.19 | .125 | .000 | .001 (.010) | 0.42 ± 0.15 | 0.29 ± 0.16 | .010 (.026) |
| AUC | 577.7 ± 260.5 | 518.0 ± 304.7 | 260.1 ± 252.5 | .164 | .000 | .011 (.023) | 527.0 ± 310.3 | 295.9 ± 185.3 | .009 (.029) |
| Lysis time, min | 26.0 ± 8.4 | 23.4 ± 10.8 | 15.3 ± 11.6 | .086 | .002 | .029 (.151) | 23.6 ± 11.0 | 19.1 ± 9.1 | .214 (.280) |
adj, adjusted; SD, standard deviation; TM, thrombomodulin.
P by Wilcoxon.
Adjusted for FXI:C.
P by comparison of proportions.
Compared with native clotting assays, assays performed in the presence of thrombomodulin showed prolonged onset time and TTP and reduced Vmax and peak turbidity change for all samples (Table 1). Whereas clotting parameters were generally similar between controls and nonbleeders, bleeders had significantly longer onset times and TTP and reduced Vmax and peak turbidity change compared with controls. However, none of these parameters were significantly different from nonbleeders (Table 1). Fewer plasma samples from bleeders were able to form clots in the presence of thrombomodulin (Table 1). These data are consistent with findings in patients with severe FXI deficiency,21 but suggest addition of thrombomodulin adds less differentiating ability in patients with partial deficiency.
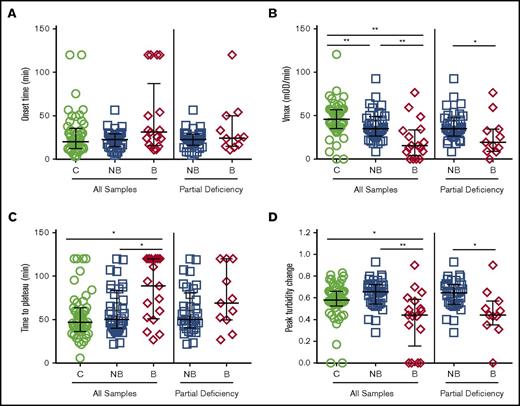
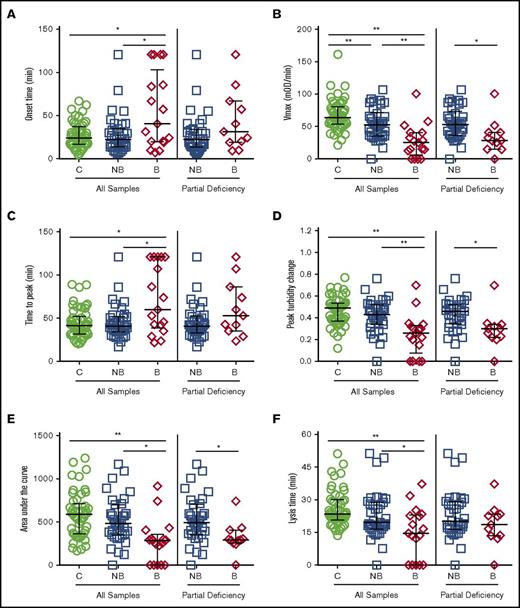
To assess clot resistance to fibrinolysis, we triggered clotting in the presence of tPA and monitored clot formation and subsequent fibrinolysis as an increase and decrease in turbidity, respectively. Controls and nonbleeders had similar onset times, TTP, peak turbidity change, AUC, and lysis times, but nonbleeders had a significantly slower Vmax than controls (Figure 2; Table 1). Compared with either controls or nonbleeders, bleeders had a significantly prolonged onset time and TTP, slower Vmax, decreased turbidity change and AUC, and shorter lysis times (Figure 2; Table 1). Differences persisted even after controlling for FXI:C (Table 1) or omitting patients who had only undergone deciduous tooth extraction (data not shown). Vmax, peak turbidity change, and AUC remained significantly different when patients with severe FXI deficiency were excluded (Figure 2; Table 1).
Fibrinolysis assays performed with CTI-treated plasmas differentiate FXI-deficient bleeders from nonbleeders. Clotting was triggered in CTI-treated plasmas from healthy individuals and FXI-deficient patients by recalcification and addition of tissue factor, phospholipids, and tissue plasminogen activator. Clot formation and lysis were monitored as an increase and subsequent decrease in turbidity. (A) Onset time, (B) rate (Vmax), (C) time to peak turbidity, (D) peak turbidity change, (E) area under the curve, and (F) lysis time for controls, all FXI-deficient, and only FXI-deficient patients with partial deficiency (16-60 IU/dL). Symbols represent plasmas from individual subjects; lines show median and interquartile range. *P < .05, **P < .005.
Fibrinolysis assays performed with CTI-treated plasmas differentiate FXI-deficient bleeders from nonbleeders. Clotting was triggered in CTI-treated plasmas from healthy individuals and FXI-deficient patients by recalcification and addition of tissue factor, phospholipids, and tissue plasminogen activator. Clot formation and lysis were monitored as an increase and subsequent decrease in turbidity. (A) Onset time, (B) rate (Vmax), (C) time to peak turbidity, (D) peak turbidity change, (E) area under the curve, and (F) lysis time for controls, all FXI-deficient, and only FXI-deficient patients with partial deficiency (16-60 IU/dL). Symbols represent plasmas from individual subjects; lines show median and interquartile range. *P < .05, **P < .005.
Collectively, these data are consistent with trends observed in the smaller cohort of patients with severe FXI deficiency,21 but were now powered to reveal significant differences in clot formation and resistance to fibrinolysis in CTI-treated plasmas from FXI-deficient nonbleeders and bleeders. Furthermore, these data show these differences can be detected in patients with only partial FXI deficiency (16-60 IU/dL).
Fibrin network structure correlates with bleeding tendency
Differences in clot formation parameters indicate differences in fibrin network structure,24 and plasma clots from bleeders with severe FXI deficiency have significantly reduced fibrin network density compared with controls or nonbleeders.21 We triggered clotting in a subset of CTI-treated plasmas from controls and FXI-deficient nonbleeders and bleeders. Plasmas were selected conservatively based on results from the clot formation assays: samples that were able to form a clot and for which peak turbidity change was closest to the mean peak turbidity change for each group. Following clot formation, we quantified fibrin network structure as described.21 Although fibrin network density in clots from controls, nonbleeders, and bleeders were not significantly different, clots from bleeders trended toward reduced density compared with controls or nonbleeders (1.76 ± 0.29, 1.76 ± 0.34, and 1.53 ± 0.25 arbitrary units, respectively; P = .15 and .13 for bleeders vs controls and nonbleeders, respectively; Figure 3).
Fibrin network structure correlates with bleeding risk in FXI-deficient patients. Clots were formed from CTI-treated plasmas by recalcification and addition of tissue factor and phospholipids in the presence of AlexFluor488-conjugated fibrinogen. Clots were scanned with a Zeiss LSM700 confocal laser scanning microscope (Carl Zeiss, Inc, Thornwood, NY) with a 63× oil immersion pan-apochromatic lens.42 Thirty optical sections (1024 × 1024 pixels) were collected at 0.36-μm intervals in the z-axis. Images were processed using 3-dimensional deconvolution algorithms (AutoQuant software × 3.0.1, Media Cybernetics Inc, Bethesda, MD). Fibrin network density analysis was performed as described in “Materials and methods.” (A) Representative confocal micrographs (z-projections of 30 individual slices) of plasma clots formed from a control individual and FXI-deficient nonbleeder and bleeder, as indicated. Images are 101.5 × 101.5 µm. (B) Fibrin network density (arbitrary units [A.U.]). Symbols represent plasmas from individual subjects; lines show median and interquartile range.
Fibrin network structure correlates with bleeding risk in FXI-deficient patients. Clots were formed from CTI-treated plasmas by recalcification and addition of tissue factor and phospholipids in the presence of AlexFluor488-conjugated fibrinogen. Clots were scanned with a Zeiss LSM700 confocal laser scanning microscope (Carl Zeiss, Inc, Thornwood, NY) with a 63× oil immersion pan-apochromatic lens.42 Thirty optical sections (1024 × 1024 pixels) were collected at 0.36-μm intervals in the z-axis. Images were processed using 3-dimensional deconvolution algorithms (AutoQuant software × 3.0.1, Media Cybernetics Inc, Bethesda, MD). Fibrin network density analysis was performed as described in “Materials and methods.” (A) Representative confocal micrographs (z-projections of 30 individual slices) of plasma clots formed from a control individual and FXI-deficient nonbleeder and bleeder, as indicated. Images are 101.5 × 101.5 µm. (B) Fibrin network density (arbitrary units [A.U.]). Symbols represent plasmas from individual subjects; lines show median and interquartile range.
Plasmas collected in the absence of CTI show fewer differences between bleeders and nonbleeders
To determine the effect of CTI on these assays, we also performed the clot formation and fibrinolysis assays using plasmas collected in the absence of CTI. In spite of being triggered with the same TF concentration, plasmas collected in the absence of CTI clotted faster (shorter onset times and TTP and higher Vmax) than CTI-treated plasmas, and almost all plasmas were able to form clots even in the presence of thrombomodulin (compare Tables 1 and 2). These observations indicate the contact pathway has a substantial effect on overall procoagulant activity, even in plasmas with reduced FXI.
Plasma clot formation and fibrinolysis parameters: absence of CTI
| . | All samples . | Partial FXI deficiency . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | C, mean ± SD . | NB, mean ± SD . | B, mean ± SD . | P, C vs NB* . | P, C vs B* . | P, NB vs B (adj)*† . | NB . | B . | P, NB vs B (adj)*† . |
| Clotting, N | 49 | 46 | 23 | 40 | 15 | ||||
| Onset time, min | 9.2 ± 6.1 | 20.6 ± 23.0 | 22.0 ± 24.4 | .000 | .001 | .889 (.238) | 15.3 ± 7.4 | 14.1 ± 6.8 | .895 (.456) |
| Vmax, mOD/min | 258.2 ± 60.6 | 190.9 ± 94.3 | 143.2 ± 94.1 | .000 | .000 | .021 (.442) | 214.9 ± 74.8 | 190.8 ± 76.2 | .162 (.286) |
| Time to plateau, min | 16.7 ± 6.2 | 31.1 ± 26.2 | 42.4 ± 36.2 | .000 | .000 | .033 (.922) | 23.3 ± 8.1 | 24.7 ± 6.1 | .303 (.709) |
| Peak turbidity change | 0.71 ± 0.12 | 0.68 ± 0.22 | 0.69 ± 0.21 | .339 | .690 | .736 (.295) | 0.71 ± 0.17 | 0.75 ± 0.16 | .467 (.491) |
| Clotting + TM, N | 49 | 46 | 23 | 40 | 15 | ||||
| Onset time, min | 11.5 ± 5.8 | 27.9 ± 34.9 | 40.1 ± 56.4 | .000 | .000 | .959 (.999) | 20.2 ± 10.7 | 20.4 ± 10.0 | .932 (.941) |
| Vmax, mOD/min | 267.3 ± 72.8 | 200.2 ± 96.4 | 129.0 ± 79.1 | .000 | .000 | .001 (.023) | 224.7 ± 75.3 | 173.0 ± 51.6 | .006 (.007) |
| Time to plateau, min | 18.3 ± 6.2 | 38.5 ± 36.0 | 53.1 ± 51.4 | .000 | .000 | .081 (.908) | 27.8 ± 10.0 | 30.9 ± 11.2 | .400 (.380) |
| Peak turbidity change | 0.71 ± 0.16 | 0.67 ± 0.20 | 0.65 ± 0.30 | .594 | .923 | .664 (.446) | 0.70 ± 0.14 | 0.76 ± 0.16 | .192 (.158) |
| Clotted samples, N (%)‡ | 49 (100) | 44 (96) | 20 (87) | .160 | .011 | .170 (.864) | 40 (100) | 15 (100) | 1.00 (1.00) |
| Fibrinolysis, N | 49 | 46 | 23 | 40 | 15 | ||||
| Onset time, min | 9.5 ± 5.8 | 18.6 ± 25.9 | 35.9 ± 58.0 | .000 | .043 | .775 (.658) | 14.1 ± 8.3 | 13.7 ± 8.6 | .940 (.764) |
| Vmax, mOD/min | 260.2 ± 58.2 | 184.7 ± 86.3 | 131.2 ± 83.5 | .000 | .000 | .015 (.125) | 205.3 ± 71.3 | 177.3 ± 57.8 | .174 (.124) |
| Time to peak, min | 19.5 ± 6.9 | 33.2 ± 26.7 | 52.4 ± 53.4 | .000 | .000 | .198 (.624) | 26.8 ± 10.4 | 28.4 ± 10.2 | .478 (.676) |
| Peak turbidity change | 0.71 ± 0.13 | 0.71 ± 0.18 | 0.64 ± 0.30 | .607 | .461 | .314 (.240) | 0.73 ± 0.15 | 0.76 ± 0.19 | .977 (.622) |
| AUC | 3133 ± 1171 | 3035 ± 1197 | 2892 ± 1792 | .829 | .422 | .472 (.104) | 3165 ± 1144 | 3603 ± 1599 | .610 (.301) |
| Lysis time, min | 113.9 ± 33.5 | 115.4 ± 42.2 | 115.4 ± 52.3 | .663 | .291 | .756 (.884) | 122.3 ± 36.6 | 136.9 ± 23.6 | .307 (.192) |
| . | All samples . | Partial FXI deficiency . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | C, mean ± SD . | NB, mean ± SD . | B, mean ± SD . | P, C vs NB* . | P, C vs B* . | P, NB vs B (adj)*† . | NB . | B . | P, NB vs B (adj)*† . |
| Clotting, N | 49 | 46 | 23 | 40 | 15 | ||||
| Onset time, min | 9.2 ± 6.1 | 20.6 ± 23.0 | 22.0 ± 24.4 | .000 | .001 | .889 (.238) | 15.3 ± 7.4 | 14.1 ± 6.8 | .895 (.456) |
| Vmax, mOD/min | 258.2 ± 60.6 | 190.9 ± 94.3 | 143.2 ± 94.1 | .000 | .000 | .021 (.442) | 214.9 ± 74.8 | 190.8 ± 76.2 | .162 (.286) |
| Time to plateau, min | 16.7 ± 6.2 | 31.1 ± 26.2 | 42.4 ± 36.2 | .000 | .000 | .033 (.922) | 23.3 ± 8.1 | 24.7 ± 6.1 | .303 (.709) |
| Peak turbidity change | 0.71 ± 0.12 | 0.68 ± 0.22 | 0.69 ± 0.21 | .339 | .690 | .736 (.295) | 0.71 ± 0.17 | 0.75 ± 0.16 | .467 (.491) |
| Clotting + TM, N | 49 | 46 | 23 | 40 | 15 | ||||
| Onset time, min | 11.5 ± 5.8 | 27.9 ± 34.9 | 40.1 ± 56.4 | .000 | .000 | .959 (.999) | 20.2 ± 10.7 | 20.4 ± 10.0 | .932 (.941) |
| Vmax, mOD/min | 267.3 ± 72.8 | 200.2 ± 96.4 | 129.0 ± 79.1 | .000 | .000 | .001 (.023) | 224.7 ± 75.3 | 173.0 ± 51.6 | .006 (.007) |
| Time to plateau, min | 18.3 ± 6.2 | 38.5 ± 36.0 | 53.1 ± 51.4 | .000 | .000 | .081 (.908) | 27.8 ± 10.0 | 30.9 ± 11.2 | .400 (.380) |
| Peak turbidity change | 0.71 ± 0.16 | 0.67 ± 0.20 | 0.65 ± 0.30 | .594 | .923 | .664 (.446) | 0.70 ± 0.14 | 0.76 ± 0.16 | .192 (.158) |
| Clotted samples, N (%)‡ | 49 (100) | 44 (96) | 20 (87) | .160 | .011 | .170 (.864) | 40 (100) | 15 (100) | 1.00 (1.00) |
| Fibrinolysis, N | 49 | 46 | 23 | 40 | 15 | ||||
| Onset time, min | 9.5 ± 5.8 | 18.6 ± 25.9 | 35.9 ± 58.0 | .000 | .043 | .775 (.658) | 14.1 ± 8.3 | 13.7 ± 8.6 | .940 (.764) |
| Vmax, mOD/min | 260.2 ± 58.2 | 184.7 ± 86.3 | 131.2 ± 83.5 | .000 | .000 | .015 (.125) | 205.3 ± 71.3 | 177.3 ± 57.8 | .174 (.124) |
| Time to peak, min | 19.5 ± 6.9 | 33.2 ± 26.7 | 52.4 ± 53.4 | .000 | .000 | .198 (.624) | 26.8 ± 10.4 | 28.4 ± 10.2 | .478 (.676) |
| Peak turbidity change | 0.71 ± 0.13 | 0.71 ± 0.18 | 0.64 ± 0.30 | .607 | .461 | .314 (.240) | 0.73 ± 0.15 | 0.76 ± 0.19 | .977 (.622) |
| AUC | 3133 ± 1171 | 3035 ± 1197 | 2892 ± 1792 | .829 | .422 | .472 (.104) | 3165 ± 1144 | 3603 ± 1599 | .610 (.301) |
| Lysis time, min | 113.9 ± 33.5 | 115.4 ± 42.2 | 115.4 ± 52.3 | .663 | .291 | .756 (.884) | 122.3 ± 36.6 | 136.9 ± 23.6 | .307 (.192) |
P by Wilcoxon.
Adjusted for FXI:C.
P by comparison of proportions.
Compared with controls, plasmas from FXI-deficient patients (both nonbleeders and bleeders) had significantly prolonged onset times, decreased Vmax, and prolonged TTP in both the absence and presence of thrombomodulin and tPA (Figure 4A-C, Table 2). However, in contrast to CTI-treated plasmas, in the absence of CTI, only Vmax and TTP differed significantly between bleeders and nonbleeders (Figure 4; Table 2). Moreover, effects were reduced or lost when controlling for FXI:C and when patients with severe FXI deficiency were excluded (Figure 4; Table 2). These findings suggest contact inhibition with CTI enhances differences in plasma clotting potential between FXI-deficient bleeders and nonbleeders.
Plasmas collected in the absence of CTI show few significant differences in clotting between FXI-deficient nonbleeders and bleeders. Plasmas from healthy individuals and FXI-deficient patients that were not collected in the presence of CTI were clotted by recalcification and addition of tissue factor and phospholipids. Clot formation was monitored by turbidity. (A) Onset time, (B) rate (Vmax), (C) time to plateau, and (D) peak turbidity change for controls, all FXI-deficient patients, and only FXI-deficient patients with partial deficiency (16-60 IU/dL). Symbols represent plasmas from individual subjects; lines show median and interquartile range. *P < .05, **P < .005.
Plasmas collected in the absence of CTI show few significant differences in clotting between FXI-deficient nonbleeders and bleeders. Plasmas from healthy individuals and FXI-deficient patients that were not collected in the presence of CTI were clotted by recalcification and addition of tissue factor and phospholipids. Clot formation was monitored by turbidity. (A) Onset time, (B) rate (Vmax), (C) time to plateau, and (D) peak turbidity change for controls, all FXI-deficient patients, and only FXI-deficient patients with partial deficiency (16-60 IU/dL). Symbols represent plasmas from individual subjects; lines show median and interquartile range. *P < .05, **P < .005.
ROC analysis reveals parameters that associate with bleeding tendency in FXI-deficient patients
Given significant differences between clotting and fibrinolysis parameters in FXI-deficient nonbleeders and bleeders, we then used ROC analysis to determine overall sensitivity of these parameters to bleeding tendency. In CTI-treated plasmas, the Vmax had a good ability to distinguish bleeders from controls (AUROC, 0.807), but less ability to distinguish bleeders from nonbleeders (AUROC, 0.756; Table 3). In the presence of thrombomodulin, no parameters were effectively able to distinguish groups. In fibrinolysis assays, Vmax, peak turbidity change, and AUC had very good ability to differentiate bleeders from controls (AUROC, 0.898, 0.849, and 0.824, respectively); Vmax also differentiated bleeders from nonbleeders (AUROC, 0.800; Table 3). In the absence of CTI, several parameters were excellent at differentiating bleeders from controls (AUROC, >0.900); however, no parameters differentiated bleeders from nonbleeders (Table 3).
Area under receiver operator characteristic curve: individual parameters
| . | Presence of CTI . | Absence of CTI . | ||||
|---|---|---|---|---|---|---|
| . | C vs NB . | C vs B . | NB vs B . | C vs NB . | C vs B . | NB vs B . |
| Clotting | ||||||
| Onset time, min | 0.509 (0.387-0.630) | 0.625 (0.472-0.778) | 0.645 (0.479-0.811) | 0.776 (0.683-0.869) | 0.754 (0.625-0.884) | 0.511 (0.360-0.662) |
| Vmax, mOD/min | 0.680 (0.566-0.794) | 0.807 (0.670-0.945) | 0.756 (0.597-0.914) | 0.753 (0.653-0.853) | 0.862 (0.754-0.970) | 0.671 (0.532-0.810) |
| Time to plateau, min | 0.591 (0.472-0.710) | 0.721 (0.573-0.868) | 0.676 (0.512-0.839) | 0.812 (0.724-0.900) | 0.898 (0.825-0.972) | 0.658 (0.519-0.797) |
| Peak turbidity change | 0.599 (0.478-0.720) | 0.707 (0.557-0.857) | 0.777 (0.641-0.914) | 0.557 (0.440-0.675) | 0.529 (0.379-0.679) | 0.525 (0.377-0.672) |
| Clotting + TM | ||||||
| Onset time, min | 0.576 (0.455-0.696) | 0.677 (0.525-0.830) | 0.597 (0.438-0.755) | 0.768 (0.667-0.869) | 0.757 (0.614-0.900) | 0.504 (0.353-0.654) |
| Vmax, mOD/min | 0.632 (0.515-0.749) | 0.701 (0.553-0.849) | 0.581 (0.419-0.744) | 0.721 (0.619-0.822) | 0.911 (0.836-0.986) | 0.747 (0.626-0.868) |
| Time to plateau, min | 0.609 (0.491-0.728) | 0.697 (0.551-0.843) | 0.583 (0.423-0.744) | 0.816 (0.726-0.906) | 0.914 (0.839-0.990) | 0.629 (0.492-0.767) |
| Peak turbidity change | 0.550 (0.425-0.675) | 0.711 (0.564-0.858) | 0.619 (0.466-0.772) | 0.532 (0.414-0.650) | 0.507 (0.354-0.660) | 0.532 (0.382-0.683) |
| Fibrinolysis | ||||||
| Onset time, min | 0.536 (0.412-0.660) | 0.679 (0.508-0.849) | 0.685 (0.520-0.850) | 0.723 (0.617-0.829) | 0.649 (0.485-0.812) | 0.521 (0.358-0.685) |
| Vmax, mOD/min | 0.682 (0.565-0.799) | 0.898 (0.792-10.00) | 0.800 (0.663-0.936) | 0.784 (0.692-0.876) | 0.903 (0.824-0.982) | 0.681 (0.545-0.817) |
| Time to peak, min | 0.525 (0.403-0.648) | 0.705 (0.540-0.870) | 0.689 (0.520-0.859) | 0.785 (0.691-0.878) | 0.815 (0.702-0.929) | 0.595 (0.444-0.747) |
| Peak turbidity change | 0.595 (0.475-0.716) | 0.849 (0.720-0.978) | 0.768 (0.627-0.909) | 0.531 (0.413-0.648) | 0.554 (0.398-0.711) | 0.575 (0.422-0.728) |
| AUC | 0.587 (0.465-0.708) | 0.824 (0.697-0.952) | 0.777 (0.640-0.914) | 0.513 (0.396-0.630) | 0.559 (0.412-0.706) | 0.553 (0.403-0.703) |
| Lysis time, min | 0.607 (0.483-0.731) | 0.751 (0.602-0.899) | 0.682 (0.523-0.841) | 0.526 (0.406-0.646) | 0.578 (0.430-0.725) | 0.523 (0.375-0.671) |
| . | Presence of CTI . | Absence of CTI . | ||||
|---|---|---|---|---|---|---|
| . | C vs NB . | C vs B . | NB vs B . | C vs NB . | C vs B . | NB vs B . |
| Clotting | ||||||
| Onset time, min | 0.509 (0.387-0.630) | 0.625 (0.472-0.778) | 0.645 (0.479-0.811) | 0.776 (0.683-0.869) | 0.754 (0.625-0.884) | 0.511 (0.360-0.662) |
| Vmax, mOD/min | 0.680 (0.566-0.794) | 0.807 (0.670-0.945) | 0.756 (0.597-0.914) | 0.753 (0.653-0.853) | 0.862 (0.754-0.970) | 0.671 (0.532-0.810) |
| Time to plateau, min | 0.591 (0.472-0.710) | 0.721 (0.573-0.868) | 0.676 (0.512-0.839) | 0.812 (0.724-0.900) | 0.898 (0.825-0.972) | 0.658 (0.519-0.797) |
| Peak turbidity change | 0.599 (0.478-0.720) | 0.707 (0.557-0.857) | 0.777 (0.641-0.914) | 0.557 (0.440-0.675) | 0.529 (0.379-0.679) | 0.525 (0.377-0.672) |
| Clotting + TM | ||||||
| Onset time, min | 0.576 (0.455-0.696) | 0.677 (0.525-0.830) | 0.597 (0.438-0.755) | 0.768 (0.667-0.869) | 0.757 (0.614-0.900) | 0.504 (0.353-0.654) |
| Vmax, mOD/min | 0.632 (0.515-0.749) | 0.701 (0.553-0.849) | 0.581 (0.419-0.744) | 0.721 (0.619-0.822) | 0.911 (0.836-0.986) | 0.747 (0.626-0.868) |
| Time to plateau, min | 0.609 (0.491-0.728) | 0.697 (0.551-0.843) | 0.583 (0.423-0.744) | 0.816 (0.726-0.906) | 0.914 (0.839-0.990) | 0.629 (0.492-0.767) |
| Peak turbidity change | 0.550 (0.425-0.675) | 0.711 (0.564-0.858) | 0.619 (0.466-0.772) | 0.532 (0.414-0.650) | 0.507 (0.354-0.660) | 0.532 (0.382-0.683) |
| Fibrinolysis | ||||||
| Onset time, min | 0.536 (0.412-0.660) | 0.679 (0.508-0.849) | 0.685 (0.520-0.850) | 0.723 (0.617-0.829) | 0.649 (0.485-0.812) | 0.521 (0.358-0.685) |
| Vmax, mOD/min | 0.682 (0.565-0.799) | 0.898 (0.792-10.00) | 0.800 (0.663-0.936) | 0.784 (0.692-0.876) | 0.903 (0.824-0.982) | 0.681 (0.545-0.817) |
| Time to peak, min | 0.525 (0.403-0.648) | 0.705 (0.540-0.870) | 0.689 (0.520-0.859) | 0.785 (0.691-0.878) | 0.815 (0.702-0.929) | 0.595 (0.444-0.747) |
| Peak turbidity change | 0.595 (0.475-0.716) | 0.849 (0.720-0.978) | 0.768 (0.627-0.909) | 0.531 (0.413-0.648) | 0.554 (0.398-0.711) | 0.575 (0.422-0.728) |
| AUC | 0.587 (0.465-0.708) | 0.824 (0.697-0.952) | 0.777 (0.640-0.914) | 0.513 (0.396-0.630) | 0.559 (0.412-0.706) | 0.553 (0.403-0.703) |
| Lysis time, min | 0.607 (0.483-0.731) | 0.751 (0.602-0.899) | 0.682 (0.523-0.841) | 0.526 (0.406-0.646) | 0.578 (0.430-0.725) | 0.523 (0.375-0.671) |
Values show AUROC and 95% confidence intervals.
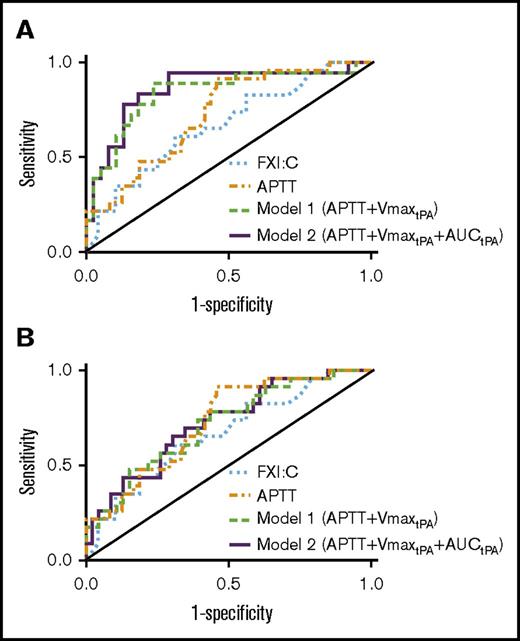
We also compared combinations of individual parameters with the highest AUROC values to identify a model with optimal sensitivity to bleeding tendency. Consistent with the apparent role for contact pathway inhibition in this assay, AUROC values were lower when using parameters from assays lacking CTI (Figure 5A-B; Table 4). Combining APTT with the CTI-treated plasma clot formation rate from fibrinolysis assays (VmaxtPA) increased the AUROC from 0.728 to 0.839 (model 1; Figure 5A; Table 4). Combining APTT, VmaxtPA, and AUC from fibrinolysis assays (AUCtPA) further increased the AUROC to 0.860 (model 2; Figure 5A; Table 4). Although the increases in AUROC did not reach significance, likely because of the sample size, the trends indicate model 2 has good-to-excellent ability to detect FXI-deficient bleeders. Importantly, model 2 demonstrated increased sensitivity and specificity for distinguishing nonbleeders and bleeders compared with FXI:C alone (AUROC, 0.860 vs 0.668, respectively, P < .05).
Receiver operating characteristic analysis. Receiver operating characteristic curve analysis of FXI:C alone, APTT alone, APTT+Vmax from fibrinolysis assays (model 1, APTT+VmaxtPA), and APTT plus Vmax and AUC from fibrinolysis assays (model 2, APTT+VmaxtPA+AUCtPA). FXI:C and APTT were measured without CTI. Models 1 and 2 show assay results from plasmas collected in the (A) presence or (B) absence of CTI.
Receiver operating characteristic analysis. Receiver operating characteristic curve analysis of FXI:C alone, APTT alone, APTT+Vmax from fibrinolysis assays (model 1, APTT+VmaxtPA), and APTT plus Vmax and AUC from fibrinolysis assays (model 2, APTT+VmaxtPA+AUCtPA). FXI:C and APTT were measured without CTI. Models 1 and 2 show assay results from plasmas collected in the (A) presence or (B) absence of CTI.
Area under receiver operator characteric curve: combined parameters
| . | +CTI, . | −CTI, . |
|---|---|---|
| Model . | AUROC (95% CI) . | AUROC (95% CI) . |
| FXI:C* | 0.668 (0.534-0.802) | |
| APTT* | 0.728 (0.607-0.849) | |
| VmaxtPA | 0.800 (0.663-0.936) | 0.681 (0.545-0.817) |
| AUCtPA | 0.777 (0.640-0.914) | 0.553 (0.403-0.703) |
| 1: APTT+VmaxtPA | 0.839 (0.715-0.963) | 0.713 (0.584-0.841) |
| 2: APTT+VmaxtPA+AUCtPA | 0.860 (0.744-0.975) | 0.721 (0.595-0.847) |
| . | +CTI, . | −CTI, . |
|---|---|---|
| Model . | AUROC (95% CI) . | AUROC (95% CI) . |
| FXI:C* | 0.668 (0.534-0.802) | |
| APTT* | 0.728 (0.607-0.849) | |
| VmaxtPA | 0.800 (0.663-0.936) | 0.681 (0.545-0.817) |
| AUCtPA | 0.777 (0.640-0.914) | 0.553 (0.403-0.703) |
| 1: APTT+VmaxtPA | 0.839 (0.715-0.963) | 0.713 (0.584-0.841) |
| 2: APTT+VmaxtPA+AUCtPA | 0.860 (0.744-0.975) | 0.721 (0.595-0.847) |
CI, confidence interval.
FXI:C and APTT are measured in the absence of CTI.
Using an optimal cutoff probability (25%) for detecting bleeding tendency by negative likelihood ratios, model 2 provides excellent sensitivity (0.944) and fair specificity (0.710) for detecting FXI-deficient bleeders, with positive and negative predictive values of 0.607 and 0.964, respectively. Thus, integrating APTT with 2 parameters from fibrinolysis assays in CTI-treated PPP (Vmax and AUC) identifies most FXI-deficient patients at risk for bleeding.
Discussion
Zucker et al21 previously tested the ability of plasma clot formation and fibrinolysis assays to distinguish FXI-deficient bleeders from controls and nonbleeders. In that small cohort of patients with severe FXI deficiency (average, ∼3 IU/dL FXI:C), several parameters were identified for which controls and nonbleeders had similar characteristics, but for which bleeders demonstrated impaired clotting characteristics. Findings from the current study validate and extend this work in important ways. First, our findings are now powered to reveal both parameters associated with FXI deficiency and parameters that are specifically and significantly associated with the bleeding phenotype. The ability to differentiate these parameters refines our understanding of the role of FXI in clot formation and hemostasis. Second, our data demonstrate the sensitivity of these assays to bleeding not only in patients with severe FXI deficiency, but also in patients with partial FXI deficiency. This advance broadens the potential utility of this assay to a population of FXI-deficient patients that are difficult to assess. Third, our results show CTI enhances sensitivity to differences between bleeders and nonbleeders. This finding advances the clinical development of this assay as a predictive tool and may also reveal mechanisms that promote bleeding in FXI-deficient patients.
The clinical observation that bleeding risk is independent of FXI antigen or activity suggests an independent factor modifies the impact of FXI deficiency on hemostasis. That differences between bleeders and nonbleeders can be detected in PPP in vitro suggests this modifier is present in plasma and does not require platelets or other vascular cells. Moreover, findings that differences can be detected in both thrombin generation14,16-18 and clot formation (Zucker et al21 and present data) assays in the absence of fibrinolytic enzymes argue against a solely fibrinolytic-based mechanism. The identity of the modifying protein or activity in plasma remains unclear; however, there are several possibilities. First, FXI(a) cleaves factors IX, X, V, and VIII.25,26 Differences in these proteins may alter the ability of small amounts of FXI to promote thrombin generation. Previous studies have not detected differences in the levels of these proteins between bleeders and nonbleeders, but potential polymorphisms that may alter their functions have not been investigated. Second, contact pathway inhibition with CTI increases sensitivity of these assays to the clinical phenotype. When reactions are triggered with low TF in the presence of CTI, FXI activation is restricted to thrombin-mediated feedback mechanisms initiated by the extrinsic pathway.27 Thus, proteins within the TF pathway may modify the effect of FXI on clot formation and, consequently, bleeding risk. It has been speculated that some individuals have more robust factor VIIa/TF activity that reduces the impact of FXI deficiency.28 Because our assays were triggered with exogenous TF, differences in clot formation and fibrinolysis likely do not stem from TF activity directly, but may reflect differences in other proteins within, or activated by, this pathway. In addition, proteins that modify thrombin-mediated activation of FXI may become particularly important when FXI is low. Although polyphosphate can enhance this reaction,29 polyphosphate is labile and likely not present in frozen and thawed PPP; however, other activities may modify FXI activation or activity in plasma. It remains unclear whether 1 or a combination of these mechanisms modifies clot formation and bleeding risk in these patients.
Although determinants of bleeding can be detected in the absence of fibrinolysis, bleeding in FXI-deficient patients tends to occur at sites with high fibrinolytic activity. Reduced FXI leads to diminished thrombin generation, resulting in a reduced rate of fibrin formation, reduced activation of the thrombin-activatable fibrinolysis inhibitor, and increased susceptibility of clots to fibrinolysis.21,30-35 We observed enhanced sensitivity to bleeding phenotype in the presence of tPA, suggesting a fibrinolytic challenge augments the coagulation defect in these patients. Colucci et al36 detected both thrombin-dependent and thrombin-independent thrombin-activatable fibrinolysis inhibitor resistance in FXI-deficient plasmas. This activity, which was not identified, may differ between bleeders and nonbleeders and also contribute to the bleeding phenotype.
Development of plasma clotting assays that can identify patients with bleeding risk has important implications. First, an assay that enables prospective identification of FXI-deficient patients with bleeding risk may improve the standard of care of patients facing surgery by reducing bleeding incidence, as well as thrombotic complications.7,10-12,37 Second, in contrast to thrombin generation assays, clotting assays are less expensive and do not require a fluorometer or specialized software to analyze data. Thus, the accessibility of this technique increases its potential use across centers. Third, the ability to perform these assays with PPP reduces preclinical variables associated with platelet isolation and standardization, and enables shipment of frozen samples between centers for analysis and standardization. Fourth, fibrinolysis assays are sensitive to antifibrinolytic drugs, which may permit prospective dose optimization for individual patients and reduce unnecessary exposure to antifibrinolytics.38 Finally, because FXI deficiency is associated with decreased venous thromboembolism risk,8 there is substantial interest in FXI reduction strategies to reduce venous thromboembolism in at-risk patients. FXI knock-down has demonstrated efficacy in studies with nonhuman primates and with human patients undergoing elective total knee arthroplasty.39,40 Although limited experience suggests FXI deficiency does not increase bleeding risk after orthopedic surgery,7 FXI antagonism may increase bleeding in patients undergoing procedures involving the oropharynx or urinary tract or in women at risk for menorrhagia. An assay that can identify individuals with increased bleeding risk may be used in strategies to individually tailor anticoagulation therapy. Thus, our assay, which demonstrates high sensitivity for bleeding risk, may be useful in these clinical situations.
Our study has several advantages, including the large cohort that includes patients with severe and partial FXI deficiency, and defined criteria for characterizing patients as nonbleeders or bleeders based on personal history of bleeds. The study also has limitations. First, we lacked power to assess the effect of genotype on bleeding or investigate possible genomic modifiers of FXI deficiency on clot formation. Population-dependent differences in heterozygote frequencies41 may mediate FXI:C and phenotype in these patients. However, because the current study using a British cohort replicated findings previously noted in Israeli patients,21 the data suggest bleeding phenotype can be predicted from biochemical coagulation mechanisms and do not require genetic information. A second limitation stems from potential difficulties standardizing turbidity assays between laboratories. As with thrombin generation assays,19,20 clotting assays are affected by preanalytical variables and show relatively high assay variability. Now that clinically relevant conditions have been identified, efforts are ongoing to optimize these assays and minimize variability. For example, because CTI is consumed during contact activation, higher concentrations of CTI might further enhance sensitivity of this assay. PPP-based turbidity assays circumvent challenges associated with the use of platelets and therefore may be more readily standardized than other methods. Third, adoption of these assays for clinical use requires availability of an affordable CTI-containing blood collection tube, which is currently lacking. Finally, although our data suggest combining parameters (APTT+VmaxtPA+AUCtPA) provides better sensitivity and specificity for detecting bleeders than FXI:C, prospective analysis is needed to compare this model with current standard of care for estimating bleeding risk in FXI-deficient patients.
In summary, we have demonstrated the ability of turbidity assays to detect abnormal plasma clot formation and resistance to fibrinolysis in CTI-treated PPP from FXI-deficient patients. These assays are accessible and inexpensive and could be readily translated to clinical use. Finding that these assays have the potential to differentiate bleeding tendency not only in patients with severe21 but also partial FXI deficiency extends the application of this assay to a much larger, and difficult-to-manage, population. Additional work is necessary to identify the plasma determinants of clot formation and stability in FXI-deficient patients and characterize the relative contributions of abnormal clot formation and fibrinolytic susceptibility to bleeding risk.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Dougald M. Monroe for phospholipid vesicle preparations, Letonia Copeland-Hardin for technical assistance, and James R. Byrnes for reading the manuscript.
This study was supported by funding from the National Institutes of Health, National Heart, Lung, and Blood Institute (R56HL094740 and R01HL126974) (A.S.W.), Bayer Hemophilia Awards Program (G.N.G.), LFB Biotechnologies (G.N.G.), and a Wycherley fellowship grant, Manchester Royal Infirmary (G.N.G.).
Authorship
Contribution: G.N.G. and A.S.W. were responsible for overall study conception and design; L.A.H. performed experiments and analyzed and interpreted data; G.N.G., J.B., and P.H.B.B.-M. enrolled the patients, collected the clinical data, and performed initial analysis of patient and sample characteristics; F.-C.L. performed statistical analysis and interpreted data; A.S.W. and G.N.G. wrote the manuscript; and all authors edited the manuscript and gave approval of the final submitted version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 819 Brinkhous-Bullitt Building, CB #7525, Chapel Hill, NC 27599-7525; e-mail: alisa_wolberg@med.unc.edu.

![Figure 3. Fibrin network structure correlates with bleeding risk in FXI-deficient patients. Clots were formed from CTI-treated plasmas by recalcification and addition of tissue factor and phospholipids in the presence of AlexFluor488-conjugated fibrinogen. Clots were scanned with a Zeiss LSM700 confocal laser scanning microscope (Carl Zeiss, Inc, Thornwood, NY) with a 63× oil immersion pan-apochromatic lens.42 Thirty optical sections (1024 × 1024 pixels) were collected at 0.36-μm intervals in the z-axis. Images were processed using 3-dimensional deconvolution algorithms (AutoQuant software × 3.0.1, Media Cybernetics Inc, Bethesda, MD). Fibrin network density analysis was performed as described in “Materials and methods.” (A) Representative confocal micrographs (z-projections of 30 individual slices) of plasma clots formed from a control individual and FXI-deficient nonbleeder and bleeder, as indicated. Images are 101.5 × 101.5 µm. (B) Fibrin network density (arbitrary units [A.U.]). Symbols represent plasmas from individual subjects; lines show median and interquartile range.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/10/10.1182_bloodadvances.2017015123/3/m_advances015123f3.jpeg?Expires=1769080719&Signature=YBBm2xUVbYHqYr4j1pq127PMkJz0w5w76FW1d8DqEv93eqvJ~pwRagXi4lNLg7RHOpBs-QhkUaPPzUIrOwWyaYD27gSfmSrAUvnSXAMXBs8jlr2bFyA6Jog08VTG6UQ1uEWj~kpx7pmaVUe5WBABa71Mzaqd5FfmaApHUlp8Y4oJJZKZ15KSx7EdUIY~KRWHPLYeVOZvlpSy2SnGto4OVgAHAWkO0yf-YRt0yGsK-oo-oJVCb8bDwOItwcutO9QBDThRLJzOIpGe0n5ZUfHyaqFML8i15U6R9gnq3J-mnbE3~v0I1BrtlYS8ugm7WngfI0DOJIpCHnmYR-5f4c32qA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)