Abstract
Visilizumab is a humanized anti-CD3 monoclonal antibody characterized by a mutated IgG2 isotype, lack of binding to Fcγ-receptors, and ability to induce apoptosis selectively in activated T cells. To test pharmacokinetics, safety, and immunosuppressive activity of visilizumab, 17 patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD) were enrolled in a phase 1 study. Six patients were given 7 doses of visilizumab (0.25 or 1.0 mg/m2) on days 1, 3, 5, 7, 9, 11, and 13. Because multiple doses of 1 mg/m2 caused delayed visilizumab accumulation and prolonged lymphopenia, the next 11 patients received a single dose of 3.0 mg/m2 on day 1. GVHD improved in all patients; 15 were evaluable through day 42. Multiple dosing resulted in 1 of 6 complete responses (CRs) and 5 partial responses (PRs), but all 6 patients died at a median of 87 days after starting visilizumab therapy. Single dosing resulted in 6 of 9 CRs, 3 PRs, and 7 of 11 patients surviving after 260 to 490 days (median, 359 days; P = .03). There were no allergic reactions and 3 grade 1 acute infusional toxicities. Plasma Epstein-Barr virus (EBV) DNA titers more than 1000 copies/mL and posttransplant lymphoproliferative disease (PTLD) developed in 2 of the first 7 patients. Based on rising EBV DNA titers, 5 of the next 10 patients were given the B cell–specific monoclonal antibody, rituximab. EBV DNA became undetectable and no overt PTLD developed. Visilizumab is well tolerated and has activity in advanced GVHD. A phase 2 study incorporating preemptive therapy for PTLD is warranted to determine the efficacy of visilizumab in GVHD.
Introduction
Acute graft-versus-host disease (GVHD) is mediated by donor T cells and remains a major barrier to successful hematopoietic cell transplantation despite prophylaxis using the best currently available drug combinations.1,2 Established acute GVHD is most frequently treated with systemic glucocorticoids, but disease manifestations persist in more than 60% of patients.3 There is no consistently effective therapy for patients with glucocorticoid-refractory GVHD, and survival has been poor.4-7
Recent advances in understanding the basic mechanisms for induction of central and peripheral T-cell tolerance to specific antigens have made it feasible to test the hypothesis that GVHD can be eliminated in humans by selective immunosuppression administered for a limited period of time. Transplantation tolerance can be facilitated by activation-induced apoptosis of peripheral T cells triggered by specific antigens.8 Signals through the T-cell receptor (TCR) are indispensable for induction of antigen-specific tolerance. In a murine model of GVHD, triggering of the TCR with non-Fcγ receptor (FcR)–binding anti-CD3 antibodies selectively induced apoptosis of donor T cells activated by recipient alloantigen in vivo and prevented the disease.9
Visilizumab (HuM291, Nuvion, Plymouth, MN) is a novel non–FcR-binding anti-CD3 monoclonal antibody (mAb) directed against the invariant CD3ε chain of the TCR.10 Its human IgG2 isotype confers the longest in vivo half-life among all human IgGs and reduces its ability to activate human complement or to interact with type I and III FcRs. Engineered mutations within the IgG2 Fc at amino acid residues 234 and 237 (Val→Ala) confer the inability to bind type II FcRs.11 Unlike the prototypic murine anti-CD3 mAbs, which induce T-cell activation by FcR binding and recruitment of antigen-presenting cells, non–FcR-binding anti-CD3 mAbs do not activate resting T cells and therefore induce less toxicity from cytokine release in vivo.10,12,13 We have shown that visilizumab induces apoptosis selectively in activated T cells and that it is much more effective in this regard than murine anti-CD3 mAbs. Visilizumab dissociates quickly from and causes minimal internalization of the TCR. Durable expression of the TCR allows sustained signaling by visilizumab leading to apoptosis.14We hypothesized that visilizumab might produce selective clonal deletion of activated pathogenic T cells and therefore serve as an ideal agent to treat GVHD. Here we report the initial study of safety, pharmacokinetics, and immunosuppressive activity of antibody visilizumab administered to patients with glucocorticoid-refractory acute GVHD.
Patients and methods
Patient selection
Patients were eligible if they developed grades II to IV acute GVHD 80 days or less after an allogeneic hematopoietic cell transplant during treatment with cyclosporine or tacrolimus and methylprednisolone. Eligibility criteria also required grades II to IV GVHD progressing after 3 days, grades III to IV persisting after 7 days, grade II persisting after 14 days of methylprednisolone at a dose of 2 mg/kg per day, or grades II to IV GVHD that recurred after tapering the dose of methylprednisolone. At least one of the following findings was required: evaluable rash, hyperbilirubinemia, diarrhea, or cramping abdominal pain. Treatment with antithymocyte globulin or other anti–T-cell mAbs during the preceding 45 days, mechanical ventilatory support, prior splenectomy, recurrent or secondary malignancy, or uncontrolled infections were criteria for exclusion.
Treatment plan and monitoring
Patients or their legal guardians gave written informed consent for antibody administration according to procedures and forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center and the U.S. Food and Drug Administration. Patients continued to receive treatment with cyclosporine or tacrolimus and methylprednisolone at 2 mg/kg per day or the dose of methylprednisolone at which recurrent GVHD became evident. Initially, cohorts of 3 patients were prescribed a multidose regimen of visilizumab: 0.25 or 1.0 mg/m2 daily dose was given on days 1, 3, 5, 7, 9, 11, and 13. Visilizumab was diluted in normal saline and administered intravenously over 3 to 5 minutes. Subsequently, after a protocol amendment, 11 patients were prescribed a single-dose regimen of visilizumab: 3.0 mg/m2 was administered over 15 minutes on day 1. Vital signs were monitored before and after visilizumab infusion at 15, 30, 45, and 60 minutes; then at 2, 3, 4, and 5 hours. The total cumulative visilizumab dose administered to patients was 1.44 to 13.65 mg (median, 4.53 mg) and the range of single doses administered was 0.5 to 6.15 mg (median, 2.37 mg).
Patient monitoring included complete blood counts, electrolytes and renal function tests daily, liver function tests every other day, and a chest x-ray once weekly for 6 weeks. Measurements recorded weekly after study entry through day 42 or until death included percent body surface area involved by rash, serum bilirubin level, 3-day averaged stool volume and frequency, presence of visible blood in stool, abdominal cramping, and daily oral caloric intake. Toxicities were classified and graded according to accepted modified criteria of the National Cancer Institute.
Staging of GVHD and definitions of clinical responses
Each organ (skin, liver, gut) was staged 1 through 4 for acute GVHD according to modified criteria based on the schema of Glucksberg et al15 and patients were also assigned a grade of acute GVHD (I through IV) based on overall severity.3 Responses to therapy were defined as follows: complete response (CR), the disappearance of symptoms in all organ systems; partial response (PR), the improvement of one or more organs with no worsening in other organs; mixed response (MR), the improvement of one or more organs with worsening in one or more; stable disease (SD), no significant change in any organ system; progressive disease (PD), progression in one or more organ system without improvement in other organs.
Study objectives
The primary objectives for the study were estimation of the maximally tolerated dose, pharmacokinetics, immunogenicity, and pharmacodynamic parameters of visilizumab. Secondary objectives were to assess potential efficacy of visilizumab and to correlate mAb levels, clinical response, and toxicity, if possible.
mAbs
Visilizumab was supplied by Protein Design Labs (Fremont, CA) and generation of the production cell line (HuM291) has been described by Cole et al.10 It was manufactured according to Good Manufacturing Practices (Protein Design Labs, Plymouth, MN). Rituximab (IDEC, San Diego, CA) is a chimeric IgG1 mAb against the CD20 antigen on mature B cells and was purchased commercially.16
Pharmacokinetic and immunogenicity assays
Serum levels of visilizumab were measured using a double-sandwich enzyme-linked immunosorbent assay (ELISA). A murine anti-id M291 mAb was used as the solid-phase capture reagent in Nunc-Immuno Maxisorp 96-well microplates (Nalge Nunc International, Rochester, NY). Serum samples were centrifuged and extracted with saturated ammonium sulfate before addition to precoated and blocked wells. Binding of visilizumab to a coated well was detected using an horseradish peroxidase (HRP)–conjugated sheep antihuman IgG2 reagent. Colorimetric measurement was performed on a THERMOmax plate reader (Molecular Devices, Sunnyvale, CA). Values obtained from serum samples were analyzed using SoftMax Pro software (Molecular Devices), fitting the data against a calibration curve using 4-parameter regression analysis. This assay was quantitative in the range of 12.5 to 300 ng/mL visilizumab.
The presence of serum antivisilizumab antibody formation was monitored using a nonsequential, double-antigen ELISA. Visilizumab antibody was used as the solid-phase capture reagent, and binding of antivisilizumab antibodies to the coated and blocked wells was detected using an HRP-conjugated visilizumab antibody. Colorimetric measurement and data analysis were performed as above. Regression of sample optical densities from a calibration curve using murine anti-id M291 results in a quantitative range of approximately 100 to 2500 ng/mL antivisilizumab idiotype equivalents.
Measurement of serum cytokines
Interleukin-4 (IL-4), IL-6, IL-10, tumor necrosis factor–α (TNF-α), and interferon-γ (IFN-γ) levels in serum samples were quantitated by ELISA as previously described.14 Capture and biotinylated secondary detector antibodies to each cytokine were obtained as matched pairs (Endogen, Boston, MA for IL-4, IL-10, and IFN-γ; R & D Systems, Minneapolis, MN for IL-6 and TNF-α). Standard curves were produced for each cytokine using recombinant human IL-4, IL-6, IL-10, TNF-α (R&D Systems) or IFN-γ (Biosource International, Camarillo, CA), respectively, in assay buffer. Interassay and intra-assay coefficients of variation were determined to be less than 10% with an assay limit of detection of 0.7 pg/mL for IL-4, 0.1 pg/mL for IL-6, 0.6 pg/mL for IL-10, 0.4 pg/mL for IFN-γ, and 0.5 pg/mL for TNF-α.
Flow cytometry
Peripheral blood leukocytes were isolated from anticoagulated blood samples before and after treatment with visilizumab according to standard methods. Cells were washed in phosphate-buffered saline (PBS) and stained with antibody pairs or triplets conjugated with fluorescein-isothiocyanate (FITC), phycoerythrin (PE), or peridinin chlorophyll protein (PerCP; Becton Dickinson, San Jose, CA) and analyzed as described previously.17 Because T-cell–bound visilizumab inhibited the binding of other anti-CD3 mAbs we identified T cells by the characteristic forward and light scatter and bright expression of CD5. Visilizumab-coated T cells were detected using FITC-conjugated F(ab′)2 goat antihuman IgG (Jackson Immunoresearch Labs, West Grove, PA). Saturation of visilizumab-binding sites achieved in vivo was evaluated by comparing the fluorescence intensity of cells that were incubated with a human IgG2 mAb of irrelevant specificity, washed, and stained with FITC-conjugated antiglobulin (bound CD3) with that of cells incubated with excess antibody visilizumab, washed, and stained with antiglobulin (total CD3). Free CD3 epitopes were detected by staining cells with FITC-conjugated anti-CD3 antibody visilizumab.
Epstein-Barr virus and human herpesvirus 6 quantitative polymerase chain reaction
DNA was extracted from sera using the Qiagen column (Qiagen, Santa Clarita, CA). For each specimen, 400 μL serum was used for the extraction and the DNA was eluted in 100 μL Tris 10 mM, of which 10 μL was used for polymerase chain reaction (PCR). Human herpesvirus 6 (HHV-6) and Epstein-Barr virus (EBV) DNA was detected using a real-time fluorescent probe quantitative PCR assay according to previously described methodology,18 and results of the PCR were analyzed using a Perkin Elmer-Applied Biosystems Sequence Detector 7700 (Foster City, CA).19 The limit of detection of the assays was 1 copy of viral DNA per reaction (40 μL serum assayed) or 25 copies/mL serum. In all reactions, noncompetitive internal control DNA (EXO) was used to ensure that negative results were not due to nonspecific inhibition of the PCR.
Skin biopsies
For the last 11 patients in the study, if there was cutaneous involvement by GVHD, punch biopsies (4 mm) were performed 1 to 2 hours before administration of visilizumab and on study days 1 or 2, 7 or 8, and 42. Skin biopsies were frozen in OCT compound 4583 (Sakura Finetek, Torrance, CA) and stored at −75°C. Frozen sections were stained in Dr James Krueger's laboratory at Rockefeller University for CD3 and CD8, as described previously.20
T-cell proliferation assays
Peripheral blood mononuclear cells (PBMCs), frozen on day 0, just before, and on days 21 or 42 (or both) after visilizumab therapy, were thawed and compared to normal control PBMCs for mitogen responses to 0 to 10 μg/mL phytohemagglutinin (PHA; Sigma, St Louis, MO) or 0 to 8.0 μg/mL plate-bound anti-CD3 mAb BC321 with or without 1 μg/mL soluble anti-CD28 mAb 9.322 using standard methods. After 72 hours of incubation IL-2 produced in the supernatant of anti-CD3 and anti-CD28–stimulated cells was measured by ELISA. For PHA- and anti-CD3–stimulated cells thymidine incorporation was measured according to established methods. Data are presented as log counts per minute.
Statistical analysis
The 2-tail Fisher exact test was used to compare response data from the multidose and single-dose regimens, the log-rank test was used to compare survival, and the independent paired t test was used to compare mitogen response data.
Results
Study participants and compliance
Between June 1999 and December 2000, 17 patients met eligibility criteria and were entered into the study and, in 15 patients, the clinical diagnosis of GVHD was confirmed histopathologically. Patient characteristics are described in Table 1. All patients had received a myeloablative preparative regimen followed by T-cell–replete hematopoietic cell transplantation. Six patients were younger than 18 years old. All but one patient received methotrexate and cyclosporine or tacrolimus as part of GVHD prophylaxis. Methylprednisolone was contraindicated in one patient due to a recent history of aspergillosis. The distribution and grades of GVHD prior to therapy are shown in Table2. All but one patient had overall grades III to IV GVHD with visceral involvement. The patient with grade II acute GVHD had erythroderma occupying more than 90% of the total body surface area. One of 6 (17%) patients treated on the multidose regimen and 5 of 11 (45%) treated with the single-dose regimen had grade IV GVHD (P = .33). The 17 patients completed all scheduled doses of visilizumab.
Demographics of GVHD before treatment
| Characteristic . | Multidose . | Single dose . |
|---|---|---|
| No. of patients | 6 | 11 |
| Median age (y) | 40 | 24 |
| Range | 16-55 | 1-53 |
| Male/female | 6/0 | 4/7 |
| Diagnosis | ||
| ALL | 1 | 2 |
| AML/MDS | 2 | 6 |
| CML | 1 | 2 |
| NHL | 1 | 0 |
| Aplastic anemia | 1 | 0 |
| Osteopetrosis | 0 | 1 |
| Preparative regimen | ||
| Cy/TBI | 5 | 6 |
| Bu/Cy +/− ATG | 0 | 5 |
| Cy/ATG | 1 | 0 |
| GVHD prophylaxis | ||
| CSA/MTX | 6 | 9 |
| Tacrolimus/MTX | 0 | 1 |
| CSA/MP | 0 | 1 |
| Stem cell source | ||
| BM | 5 | 7 |
| PBSC | 1 | 3 |
| Cord blood | 0 | 1 |
| Donors | ||
| HLA-matched related | 3 | 1 |
| HLA-mismatched related | 1 | 1 |
| HLA-matched unrelated | 2 | 4 |
| HLA-mismatched unrelated | 0 | 5 |
| Days from transplantation until onset GVHD | ||
| Median | 16 | 17 |
| Range | 8-40 | 6-33 |
| Median days of MP therapy | 11 | 15 |
| Range | 0-13 | 5-50 |
| Days from transplantation until visilizumab therapy | ||
| Median | 37 | 31 |
| Range | 13-50 | 13-55 |
| Characteristic . | Multidose . | Single dose . |
|---|---|---|
| No. of patients | 6 | 11 |
| Median age (y) | 40 | 24 |
| Range | 16-55 | 1-53 |
| Male/female | 6/0 | 4/7 |
| Diagnosis | ||
| ALL | 1 | 2 |
| AML/MDS | 2 | 6 |
| CML | 1 | 2 |
| NHL | 1 | 0 |
| Aplastic anemia | 1 | 0 |
| Osteopetrosis | 0 | 1 |
| Preparative regimen | ||
| Cy/TBI | 5 | 6 |
| Bu/Cy +/− ATG | 0 | 5 |
| Cy/ATG | 1 | 0 |
| GVHD prophylaxis | ||
| CSA/MTX | 6 | 9 |
| Tacrolimus/MTX | 0 | 1 |
| CSA/MP | 0 | 1 |
| Stem cell source | ||
| BM | 5 | 7 |
| PBSC | 1 | 3 |
| Cord blood | 0 | 1 |
| Donors | ||
| HLA-matched related | 3 | 1 |
| HLA-mismatched related | 1 | 1 |
| HLA-matched unrelated | 2 | 4 |
| HLA-mismatched unrelated | 0 | 5 |
| Days from transplantation until onset GVHD | ||
| Median | 16 | 17 |
| Range | 8-40 | 6-33 |
| Median days of MP therapy | 11 | 15 |
| Range | 0-13 | 5-50 |
| Days from transplantation until visilizumab therapy | ||
| Median | 37 | 31 |
| Range | 13-50 | 13-55 |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; Cy, cyclophosphamide; TBI, total body irradiation; Bu, busulfan; ATG, antithymocyte globulin; CSA, cyclosporine; MTX, methotrexate; MP, methylprednisolone; BM, bone marrow; PBSC, peripheral blood stem cells.
Distribution of GVHD scores before therapy with visilizumab
| . | Stage or grade . | ||||
|---|---|---|---|---|---|
| . | 0 . | I . | II . | III . | IV . |
| Multidose regimen | |||||
| Skin | 4 | 0 | 1 | 1 | 0 |
| Liver | 2 | 1 | 0 | 3 | 0 |
| Gut | 0 | 0 | 1 | 4 | 1 |
| Overall | 0 | 0 | 0 | 5 | 1 |
| Single-dose regimen | |||||
| Skin | 4 | 0 | 4 | 3 | 0 |
| Liver | 7 | 2 | 0 | 2 | 0 |
| Gut | 2 | 0 | 0 | 5 | 4 |
| Overall | 0 | 0 | 1 | 5 | 5 |
| . | Stage or grade . | ||||
|---|---|---|---|---|---|
| . | 0 . | I . | II . | III . | IV . |
| Multidose regimen | |||||
| Skin | 4 | 0 | 1 | 1 | 0 |
| Liver | 2 | 1 | 0 | 3 | 0 |
| Gut | 0 | 0 | 1 | 4 | 1 |
| Overall | 0 | 0 | 0 | 5 | 1 |
| Single-dose regimen | |||||
| Skin | 4 | 0 | 4 | 3 | 0 |
| Liver | 7 | 2 | 0 | 2 | 0 |
| Gut | 2 | 0 | 0 | 5 | 4 |
| Overall | 0 | 0 | 1 | 5 | 5 |
The number of patients with the following symptoms of GVHD included 9 with skin rash, 8 with serum bilirubin level 2.0 mg/dL or higher, 12 with diarrhea, 11 with abdominal cramps, 12 with stool blood, and 13 with nausea or vomiting or both.
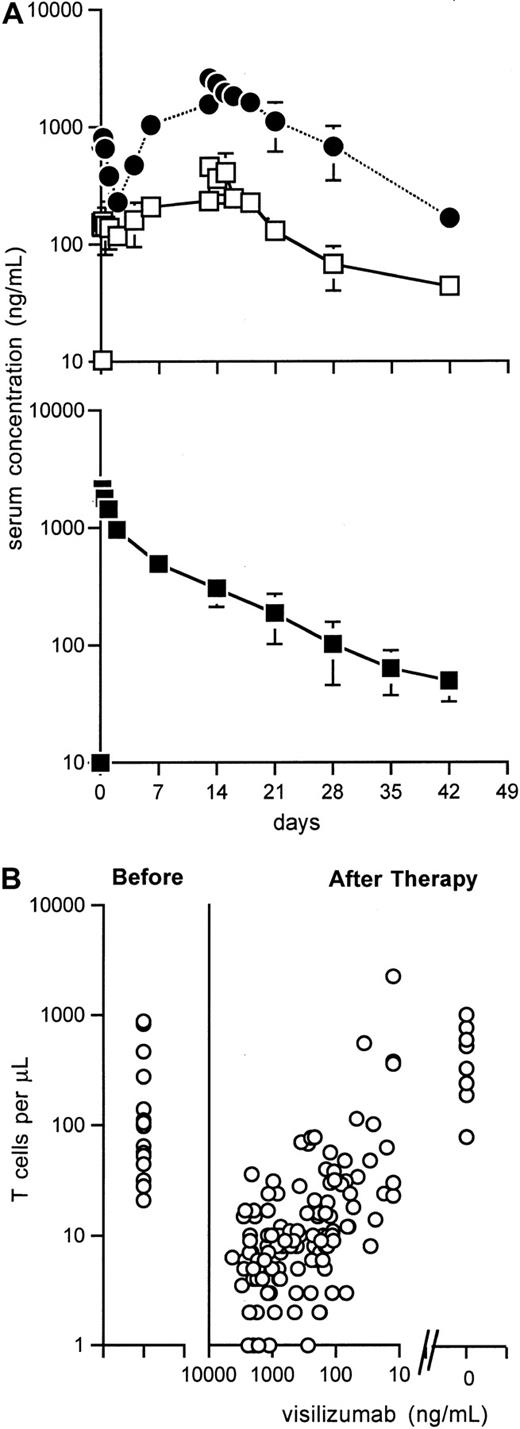
Pharmacokinetics of visilizumab
Mean serum concentrations of visilizumab over time for multidose and single-dose regimens are shown in Figure1A. The Cmax ± SEM at 1 hour after the first dose was 152 ± 23 and 791 ± 44 ng/ml, and average terminal elimination half-life was 103 and 177 hours, for patients treated, respectively, with 0.25 mg/m2 and 1.0 mg/m2. In the 3 patients treated at 1.0 mg/m2the number of doses correlated with trough drug levels (R2 = 0.69, 0.98, and 0.99), suggesting that 1 mg/m2 visilizumab saturates drug receptors in this patient population. Because of the observation that multiple doses of 1.0 mg/m2 led to visilizumab accumulation and delayed clearance, there was concern for potential increased toxicities. Subsequent study participants received a single 3-mg/m2dose, which resulted in a Cmax of 2217 ± 148 ng/mL. The terminal elimination half-life ± SEM was 162 ± 18 hours (range, 107-301 hours) and the mean systemic serum clearance ± SEM was 6.99 ± 1.23 L/m2 per hour (range, 1.95-16.1 L/m2 per hour). There was high intersubject variability but levels did not correlate with GVHD response rates.
Visilizumab pharmacokinetics and T-cell recovery.
(A) Mean ± SEM serum concentrations of visilizumab in patients treated with 7 doses of 0.25 mg/m2 (□, n = 3) or 1.0 mg/m2 (●, n = 3). Mean ± SEM serum concentrations of visilizumab in patients given a single dose of 3 mg/m2(■). Results are from days 0, 1 (n = 11), 2, 7, 14, 21 (n = 10), 28, 35 (n = 9), and 42 (n = 8). (B) Before therapy, absolute T-cell counts were 21 to 877/μL (median, 65/μL). After therapy, absolute T-cell counts increased as visilizumab serum levels decreased. Absolute T-cell counts were 80 to 1014/μL (median, 433/μL) at a median of 72 days (range, 42-81 days) following antibody clearance in 8 evaluable patients.
Visilizumab pharmacokinetics and T-cell recovery.
(A) Mean ± SEM serum concentrations of visilizumab in patients treated with 7 doses of 0.25 mg/m2 (□, n = 3) or 1.0 mg/m2 (●, n = 3). Mean ± SEM serum concentrations of visilizumab in patients given a single dose of 3 mg/m2(■). Results are from days 0, 1 (n = 11), 2, 7, 14, 21 (n = 10), 28, 35 (n = 9), and 42 (n = 8). (B) Before therapy, absolute T-cell counts were 21 to 877/μL (median, 65/μL). After therapy, absolute T-cell counts increased as visilizumab serum levels decreased. Absolute T-cell counts were 80 to 1014/μL (median, 433/μL) at a median of 72 days (range, 42-81 days) following antibody clearance in 8 evaluable patients.
Human antimouse antibody response
No human antibodies against visilizumab were detected in any of the 13 evaluable patients who survived until day 42 after initiation of visilizumab therapy.
Pharmacodynamics
Absolute numbers of T lymphocytes (CD5 bright, CD4+ or CD8+) lymphocytes were 21 to 877/μL (median, 65/μL) before treatment and 1 to 17/μL (median, 6/μL) at 2 hours after infusion of visilizumab; numbers stayed at 1 to 2 logs below baseline levels for at least 14 to 28 days after treatment (data not shown). A direct relationship was apparent between the time at which visilizumab decreased below 100 ng/mL in the serum and the time to achieve a peripheral blood T-cell count of more than 100 cells/μL (Figure 1B). Figure 2A illustrates the rapid depletion of CD8+ T cells from the skin of one representative patient after treatment with a single dose of visilizumab. Circulating peripheral blood T cells were also evaluated in this patient by PE-conjugated antibody specific for CD5, an antigen coexpressed on virtually all the T lymphocytes (Figure 2B). Double staining of T cells with FITC-conjugated antiglobulin specific for IgG2 indicated that small numbers of residual circulating T cells were coated with visilizumab in vivo for up to 21 days. Double staining of T cells with FITC-conjugated visilizumab showed that residual circulating T cells had a reduced number of free CD3 sites. During this time period the expression of total cell surface CD3 was partially maintained after therapy, indicating that visilizumab did not cause total internalization of the CD3 antigen in vivo.
Pharmacodynamics and T-cell mitogen responses after therapy with visilizumab.
(A) Skin biopsies from a representative patient with cutaneous GVHD documenting clearance of CD8+ dermal T-cell infiltrates (black dots) following a single dose of visilizumab on day 1. Original magnification × 100, immunoperoxidase staining. (B) Flow cytometric analysis of peripheral blood leukocytes from the same patient, before and after visilizumab. Details of antibody staining are described in “Patients and methods.” Lymphocytes were identified (left panels) by their characteristic forward and light scatter (R2). Thus set, the R2 gate contained more than 95% of the CD5 bright events in R1 (T cells). The right panels show T-cell surface CD3 molecules bound by visilizumab in vivo (dotted line) and total surface CD3 (solid line). The middle panels show free CD3 (solid line) or free CD3 blocked by exogenous visilizumab (dotted line). The relative amounts of free, bound, and total CD3 before and after administration of visilizumab are indicated by the respective histogram plots of peak fluorescence intensities of gated CD5+ T cells. (C) Patient T cells were frozen on study days 0, 21, and 42 when the peripheral blood T-cell counts were, respectively, 57/μL (range, 28-877/μL), 13/μL (range, 1-38/μL), and 32/μL (range, 22-1291/μL). Thawed T cells were compared to fresh healthy control T cells. Individual sample and median (—) mitogen responses to PHA, anti-CD3, and anti-CD3 + anti-CD28 as described in “Patients and methods” are shown. T-cell mitogen response is not significantly reduced by visilizumab therapy.
Pharmacodynamics and T-cell mitogen responses after therapy with visilizumab.
(A) Skin biopsies from a representative patient with cutaneous GVHD documenting clearance of CD8+ dermal T-cell infiltrates (black dots) following a single dose of visilizumab on day 1. Original magnification × 100, immunoperoxidase staining. (B) Flow cytometric analysis of peripheral blood leukocytes from the same patient, before and after visilizumab. Details of antibody staining are described in “Patients and methods.” Lymphocytes were identified (left panels) by their characteristic forward and light scatter (R2). Thus set, the R2 gate contained more than 95% of the CD5 bright events in R1 (T cells). The right panels show T-cell surface CD3 molecules bound by visilizumab in vivo (dotted line) and total surface CD3 (solid line). The middle panels show free CD3 (solid line) or free CD3 blocked by exogenous visilizumab (dotted line). The relative amounts of free, bound, and total CD3 before and after administration of visilizumab are indicated by the respective histogram plots of peak fluorescence intensities of gated CD5+ T cells. (C) Patient T cells were frozen on study days 0, 21, and 42 when the peripheral blood T-cell counts were, respectively, 57/μL (range, 28-877/μL), 13/μL (range, 1-38/μL), and 32/μL (range, 22-1291/μL). Thawed T cells were compared to fresh healthy control T cells. Individual sample and median (—) mitogen responses to PHA, anti-CD3, and anti-CD3 + anti-CD28 as described in “Patients and methods” are shown. T-cell mitogen response is not significantly reduced by visilizumab therapy.
Lymphocyte functional studies
The proliferative responses of patient T cells to PHA or plate-bound anti-CD3 antibody were reduced compared to T cells from healthy controls (Figure 2C). Before visilizumab therapy, patient T cells produced lower amounts of IL-2 in response to plate-bound anti-CD3 plus soluble anti-CD28 compared to control T cells. There was no suggestion that T-cell proliferative responses or IL-2 production were altered at 42 days after treatment with visilizumab.
Drug-related toxicities
There were no allergic reactions. Three of 53 visilizumab infusions were followed by single transient grade I adverse events: facial flushing, upper limb weakness, and, in a third patient, low-grade fever (38°C) with mild chills at 1 hour after infusion. None of the17 patients had serum cytokine levels above 1 ng/mL and those without postinfusional adverse events did not have detectable peaks in cytokine release. Analyses of sera collected from the patient with transient postinfusional fever and chills indicated low levels, but well-defined peaks, in release of IFN-γ (23 pg/mL), TNF-α (224 pg/mL), IL-6 (134 pg/mL), and IL-10 (996 pg/mL) 1 to 2 hours after visilizumab administration compared to undetectable serum levels before infusion.
There was no consistent variation in chest x-ray findings or electrocardiograms after visilizumab therapy. For the multidose and single-dose regimens, respectively, the median serum creatinine values were 1.05 mg/dL (range, 0.6-1.4 mg/dL) and 0.5 mg/dL (range, 0.2-1.6 mg/dL) before treatment and 1.05 mg/dL (range, 0.6-1.9 mg/dL) and 0.5 mg/dL (range, 0.2-1.3 mg/dL) 14 days after the start of visilizumab therapy. For the multidose and single-dose regimens, respectively, the median serum bilirubin values were 3.95 mg/dL (range, 0.7-14.3 mg/dL) and 1.2 mg/dL (range, 0.5-12.0 mg/dL) before treatment and 3.05 (range, 0.6-9.8 mg/dL) and 0.85 mg/dL (range, 0.2-2.6 mg/dL) 14 days after the start of visilizumab therapy. Apart from one patient with liver GVHD whose serum aspartate aminotransaminase (AST) level fell from 1310 to 70 IU/mL by day 14 of the multidose regimen, there were no differences between values for serum AST, white blood cell count, or hematocrit before and up to 6 weeks after visilizumab therapy.
EBV reactivation
Because T-cell–depleted transplants and in vivo T-cell–depleting therapies have been associated with a high risk of EBV posttransplant lymphoproliferative disease (PTLD),23we prospectively monitored plasma specimens from study participants during the study observation period. At baseline, 16 of the 17 study participants had no detectable EBV DNA in the plasma. In 12 of 16 evaluable patients EBV DNA was detected after infusion of visilizumab. One of the last 10 patients died 54 hours after treatment with visilizumab and was not evaluable for EBV reactivation. Four patients had transient reactivation of up to 1800 copies plasma EBV DNA/mL lasting a median of 11 days (range, 1-39 days). In 3 of the first 7 patients progressive rises in plasma EBV DNA of more than 10 000 copies/mL were demonstrated, and 2 of these 3 patients developed rapidly fatal PTLD (Figure 3). The third patient died on day 33 of the study without diagnostic studies for PTLD obtained before death and without an autopsy. Five of the remaining 9 patients demonstrated serial rises in EBV DNA more than 1000 copies/mL and were treated preemptively with 1 or 2 doses (375 mg/m2) of the anti-CD20 monoclonal, rituximab. EBV DNA became undetectable in all 5 patients, no overt PTLD developed, and 3 patients survive. Subclinical but histologically confirmed PTLD was detected in one patient during a routine chest x-ray examination. PTLD had developed in the absence of measurable plasma EBV-DNA 1 month after the initial dose of rituximab; however, the peripheral blood T-cell count at that time remained less than 60/μL. PTLD resolved following 4 additional doses of rituximab and recovery of peripheral T-cell counts.
EBV reactivation following visilizumab.
Three individual patients (open symbols) developed high-level EBV reactivation and died (left panel); overt PTLD was documented in 2 patients (□, ○). The 5 remaining panels (solid symbols) show EBV-DNA copies/mL in individual patients who received preemptive therapy with rituximab (arrows) and did not develop PTLD. The far right panel shows an individual patient who developed asymptomatic PTLD 65 days after visilizumab therapy. The PTLD resolved after 4 additional doses of rituximab and concomitant recovery of T-cell numbers.
EBV reactivation following visilizumab.
Three individual patients (open symbols) developed high-level EBV reactivation and died (left panel); overt PTLD was documented in 2 patients (□, ○). The 5 remaining panels (solid symbols) show EBV-DNA copies/mL in individual patients who received preemptive therapy with rituximab (arrows) and did not develop PTLD. The far right panel shows an individual patient who developed asymptomatic PTLD 65 days after visilizumab therapy. The PTLD resolved after 4 additional doses of rituximab and concomitant recovery of T-cell numbers.
Response and survival after the multidose regimen
Among the 6 patients who received treatment with doses of 0.25 or 1.0 mg/m2 there were 3 CRs in the liver (median serum bilirubin, 3.95 mg/dL [range, 0.7-14.3 mg/dL] before and 1.5 mg/dL [range, 1.3-3.0 mg/dL] 42 days after therapy with visilizumab). Two patients had CRs in the gut and 4 had PRs. By study day 42, 1 of the 6 (17%) patients had a complete overall response, and 1 patient who died on day 33 was not evaluable. Two of 3 evaluable patients had chronic extensive GVHD at 80 days after transplantation. One patient received mycophenolate mofetil and another, antithymocyte globulin, as third-line systemic therapy for residual acute or chronic GVHD.
All 6 patients treated according to a multidose regimen, including 5 with ongoing GVHD, died within 33 to 346 days (median, 87 days) of visilizumab therapy. Causes of death were pulmonary aspergillosis in 2 patients and cytomegalovirus enteritis, EBV PTLD, respiratory syncytial virus pneumonia, multiorgan failure, and late relapse of leukemia in one patient each. Three patients were receiving methylprednisolone at least 2 mg/kg per day before visilizumab therapy and were unable to taper to less than 2 mg/kg per day before death. In one patient prednisone was tapered to 1 mg/kg per day. The longest survival occurred in a patient who was able to taper glucocorticoids.
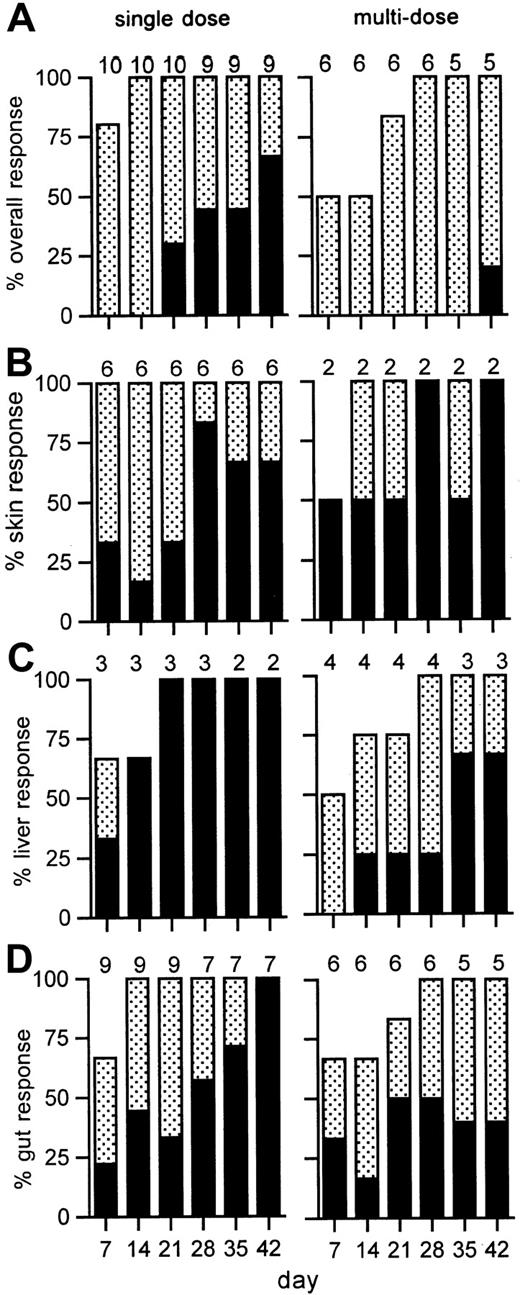
Response and survival after the single-dose regimen
Improvement in the manifestations of GVHD were noted within 1 to 4 days after antibody administration and continued for up to 42 days (Figure 4). At study day 42, 6 of 9 patients (67%; 95% CI, 30%-92%) had a CR to a single dose of 3 mg/m,2 including 3 of 5 patients who had grade IV GVHD. Two patients who died early (days 3, 31) were not evaluable at day 42. Six of 8 evaluable patients had chronic extensive GVHD at 80 days after transplantation. Four patients received mycophenolate mofetil and another, rapamycin, as third-line systemic therapy for residual chronic GVHD. These included 4 of the 6 patients who had CRs who were able to then proceed with tapering of glucocorticoids paced appropriately for chronic GVHD.
GVHD response to visilizumab.
Shown are the percentages of evaluable patients with a complete (solid bars) or partial (stippled bars) response to single (left) or multiple (right) doses of visilizumab at weekly time points. The overall response is shown in panel A, and organ responses are shown for skin (B), liver (C), or gut (D). The number of patients evaluated for response is indicated above each bar for each time point.
GVHD response to visilizumab.
Shown are the percentages of evaluable patients with a complete (solid bars) or partial (stippled bars) response to single (left) or multiple (right) doses of visilizumab at weekly time points. The overall response is shown in panel A, and organ responses are shown for skin (B), liver (C), or gut (D). The number of patients evaluated for response is indicated above each bar for each time point.
The median survival for the 11 patients treated with a single dose is more than 300 days, and 7 (64%) are alive after 260 to 490 days (median, 359 days; Figure 5). Four patients, including 2 with ongoing GVHD, died of infection at a median of 37 days after starting treatment with visilizumab. A patient with mental status changes before treatment with visilizumab, and whose baseline HHV6 DNA plasma viral load was shown retrospectively to be 3700 copies/mL, died 54 hours after treatment with visilizumab. At autopsy the cerebral cerebrospinal fluid specimen contained 3 × 105 copies/mL HHV6 DNA, consistent with a diagnosis of HHV6 encephalitis. The other causes of death were EBV PTLD, adult respiratory distress syndrome following CMV pneumonia, and cerebral aspergillosis, in one patient each.
Kaplan-Meier probability of overall survival of patients with acute glucocorticoid-refractory GVHD.
Survival after therapy with visilizumab is shown separately for patients treated on the single-dose (solid line, n = 11) and on multidose (striped line, n = 6) regimens. The difference between the 2 groups is significant at P = .03.
Kaplan-Meier probability of overall survival of patients with acute glucocorticoid-refractory GVHD.
Survival after therapy with visilizumab is shown separately for patients treated on the single-dose (solid line, n = 11) and on multidose (striped line, n = 6) regimens. The difference between the 2 groups is significant at P = .03.
Discussion
Acute GVHD improved in all patients treated with visilizumab, including those treated at the lowest dose level. We were particularly encouraged to see that 6 of 9 patients had CRs after treatment with a single 3-mg/m2 dose of visilizumab, although 8 of 11 patients required maintenance immunosuppression for management of subsequent chronic GVHD. Although there is no standard therapy for glucocorticoid-refractory acute GVHD, multiple doses of antithymocyte globulins have often been used. Survival has been less than 20% at 6 months and 6% to 12% at 1 year6,7,24 (and P.M., personal communication, September 19, 2001). At our institution, a single dose of the humanized anti-IL-2 receptor antibody, daclizumab, produced a response rate of 40% in 20 patients with glucocorticoid-refractory acute GVHD, but survival was only 20% at 180 days and 10% at 587 days.25 Subsequently, Przepiorka et al reported that 29% to 53% of patients with glucocorticoid-refractory acute GVHD survived at 120 days after receipt of 5 doses of daclizumab, but 40% of these patients subsequently required additional therapy with antithymocyte globulin at a median of 8 days.26 Forty percent to 50% of patients with glucocorticoid-refractory acute GVHD responded for at least 7 days, and survived at 180 days, after treatment with 0.1- to 0.2-mg/kg doses of an antihuman CD147 murine monoclonal antibody. Moderate to severe myalgia occurred in 28% to 60% of patients and was dose-limiting.27 Although patient numbers were limited, survival of patients treated with a single dose of visilizumab compares favorably to the recent experience with other agents.
Our pharmacokinetic data suggest that the size of the first dose of visilizumab affects treatment outcome more than the total cumulative dose. The incidence of CR and the duration of survival were superior after a single dose of 3 mg/m2 compared to 7 doses of 0.25 or 1.0 mg/m2 where the cumulative dose administered was 1.75 mg/m2 or 7 mg/m2. Patients treated with multiple doses of visilizumab did not have more severe GVHD or other characteristics that might predict for poorer responses to visilizumab compared to patients treated with single doses (Tables 1 and 2).
If rapid depletion of pathogenic activated donor T cells in target organs is important for effective control of acute GVHD, then a regimen that rapidly provides plasma concentrations of at least 2000 ng/mL might be optimal. We have previously reported that concentrations of visilizumab 200 ng/mL or more were necessary to induce maximal apoptosis in preactivated T cells14 or peripheral T cells from patients with acute GVHD.28 We speculate that a concentration of at least 2000 ng/mL would achieve desirable concentrations of visilizumab in lymph nodes, assuming typical lymph node–to-plasma concentration ratios.29 Whereas a single 3-mg/m2 dose of visilizumab immediately resulted in levels of more than 2000 ng/mL, such concentrations were not achieved until after the seventh dose (day 13) in patients who were administered 1.0 mg/m2 of antibody.
Compared to visilizumab, the immunosuppressive effects of prototypic wild-type murine anti-CD3 antibodies were not as marked. We presume that unlike visilizumab, murine anti-CD3 mAbs induce rapid internalization of CD3 molecules from the T-cell surface, which then limits activation-induced T-cell apoptosis.14,30Accordingly, peripheral blood T-cell lymphopenia has been transient after the administration of murine anti-CD3.31,32Similarly, a single dose of daclizumab did not deplete CD3+CD25+ peripheral blood T cells.26 Resolution of CD8+ dermal infiltrates in skin biopsies obtained from patients with skin GVHD suggests that visilizumab can induce in vivo T-cell depletion of activated pathogenic T cells.
The structural features of visilizumab involved humanization, choice of the IgG2 isotype, and non–FcR-binding mutations in the Fc tail. The clinical data reported here support the concept that rational antibody engineering can provide rapidly acting, effective, and tolerable immunosuppression with convenient single-dose administration.
A complication of GVHD therapy with visilizumab was reactivation of latent EBV and the development of fatal PTLD in 2 of the first 7 patients treated. The incidence of PTLD is highest 1 to 5 months after transplantation and the risk for developing PTLD is most strongly associated with ex vivo T-cell depletion of the graft and in vivo therapy with anti-CD3 monoclonal antibodies.33Our study modification to administer preemptive therapy for PTLD using rituximab was based on 2 prior observations. The incidence of PTLD is not increased when donor marrow has been depleted of both T and B cells.34-36 Second, early PTLD has been treated successfully with anti-B cell mAbs.37-39 EBV DNA can be detected in PBMCs of healthy volunteers but is generally absent from the plasma.40,41 Recipients of solid organ transplants may have EBV DNA in their plasma, but patients with EBV PTLD exhibited markedly elevated levels of plasma EBV DNA.42 Although absolute values of EBV DNA predictive of PTLD remain to be established, the previously reported experience, together with the data from this study, indicate that monitoring of EBV DNA and the early use of rituximab could reduce this complication of visilizumab.37Our 5 study patients who received rituximab experienced no adverse events related to the infusion. Following treatment with rituximab, the plasma EBV DNA titers declined to 0 to 50 copies/mL after a transient increase in plasma EBV DNA 12 to 18 hours after infusion of rituximab suggesting rapid lysis of B cells, including those containing EBV. In the nontransplant setting, rituximab induces significant depletion of CD20+ B cells, without eliminating early B-cell precursors or plasma cells. B-cell numbers recover by 9 to 12 months, and only 12% of patients have depressed serum immunoglobulin levels. These quantitative deficiencies have not been associated with an increase in documented clinical infections.16 It remains to be determined whether the combination of visilizumab and rituximab will significantly delay the reconstitution of serum immunoglobulins and thereby increase the risk of late infection. Mitogenic responses of residual peripheral blood T cells to PHA, anti-CD3, and anti-CD28 were not impaired by visilizumab therapy; however, this interpretation must be considered preliminary due to the small number of patients tested in these proliferation assays.
Our results provide basis for the design of phase 2 studies of visilizumab in steroid-refractory GVHD, a condition for which there is no current satisfactory therapy. In addition, there is considerable room for improvement in standard primary therapy of acute GVHD given that only 38% of patients have CRs and are alive at 6 weeks after treatment with glucocorticoids.3 We speculate that visilizumab might be even more effective in that setting. Whether visilizumab will have a steroid-sparing effect in patients initially treated for acute GVHD will need to be determined in future trials.
The authors are grateful to Sharon Chen for performing pharmacokinetic and immunogenicity assays on patient sera; Mark Kagen for performing immunocytochemical stains on frozen tissue; Jenny Lorenz and Lori Hubbard for the collection and management of clinical data; to physicians, physician assistants, nurses, and support staff who participated in the care of patients with GVHD; and to Mei-Li Huang, James Ferrenberg, Tracy Santo-Hayes, and Jared Castor who conscientiously provided timely viral PCR results.
Supported by grants CA18029, CA18221, HL36444, CA15704, AI33484, and AI40680 from the National Institutes of Health, Bethesda, MD; a grant from Protein Design Labs, Fremont, CA; and the Leukemia and Lymphoma Society (5039-00).
D.L. is an employee of the company that produces visilizumab. C.A. shares patent rights on visilizumab.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul A. Carpenter, Division of Clinical Research, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-290, Seattle, WA; e-mail: pcarpent@fhcrc.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal